Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology
Abstract
:1. Introduction
2. Biodiversity of Licorice
3. Secondary Metabolites of Licorice
4. Ethnobotany of Licorice
Licorice Traditional and Modern Preparations
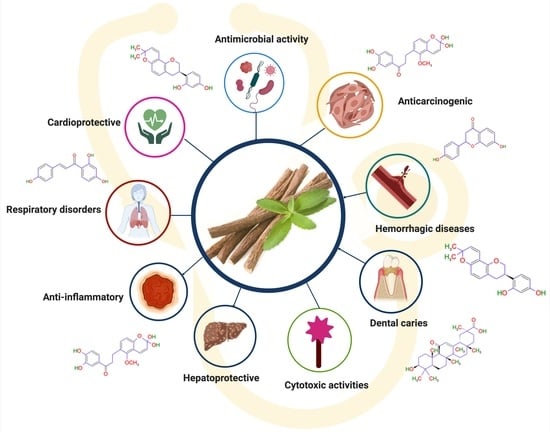
5. Effect of Licorice in Different Diseases
5.1. Anticancer Effect of Licorice
5.1.1. Effect on Human Cervical Cancer
5.1.2. ISL Effects on Breast Cancer
5.1.3. Effect on Hepatoma Cancer
5.1.4. Effect on Colon Cancer (CC)
5.1.5. Effect on Pancreatic Cancer
5.1.6. Effect on Prostate Cancer
5.2. Licorice in the Treatment of Respiratory Tract Infections
5.3. Licorice Effect on Cardiovascular System
5.4. Licorice Effect on Hepatoprotective System
5.5. Antimicrobial Activity
5.6. Anti-Inflammatory Activity
5.7. Dental Caries
5.8. Other Pharmacological Effects
6. Clinical Studies
7. Glycyrrhiza glabra Toxicological Effects
8. Conclusions and Future Recommendation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, N.A.; Egamberdieva, D. Phytochemical Constituents and Pharmacological Effects of Licorice: A Review. In Plant and Human Health; Springer International Publishing: Cham, Switzerland, 2019; Volume 3, pp. 1–21. ISBN 9783030044084. [Google Scholar]
- Hayashi, H.; Yokoshima, K.; Chiba, R.; Fujii, I.; Fattokhov, I.; Saidov, M. Field survey of Glycyrrhiza plants in Central Asia (5). Chemical characterization of G. bucharica Collected in Tajikistan. Chem. Pharm. Bull. 2019, 67, 534–539. [Google Scholar] [CrossRef]
- Sokolov, S.; Zamotayev, I. Directory of Medicinal Plants; Medicina: Moscow, Russia, 1985. (In Russian) [Google Scholar]
- Chevallier, A. The encyclopedia of medicinal plants. Choice Rev. Online 1997, 34, 34–3624. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Lutomski, J.; Nieman, C. Liquorice, Glycyrrhiza glabra L.-Composition, uses and analysis. Food Chem. 1990, 38, 119–143. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Hadian, J.; Nejad Ebrahimi, S.; Otto, L.G. Genetic structure and variation in Iranian licorice (Glycyrrhiza glabra L.) populations based on morphological, phytochemical and simple sequence repeats markers. Ind. Crop. Prod. 2020, 145, 112140. [Google Scholar] [CrossRef]
- Kang, M.R.; Park, K.H.; Oh, S.J.; Yun, J.; Lee, C.W.; Lee, M.Y.; Han, S.-B.; Kang, J.S. Cardiovascular protective effect of glabridin: Implications in LDL oxidation and inflammation. Int. Immunopharmacol. 2015, 29, 914–918. [Google Scholar] [CrossRef]
- Hui-yan, G.; Li-dong, G.; Jing-hua, Y. Measurement and comparison of glycyrrhizic acid contents in root of licorice (Glycyrrhiza uralensis Fisch.) from different cultivating areas. J. For. Res. 2002, 13, 141–143. [Google Scholar] [CrossRef]
- Hayashi, H.; Hattori, S.; Inoue, K.; Khodzhimatov, O.; Ashurmetov, O.; Ito, M.; Honda, G. Field Survey of Glycyrrhiza plants in Central Asia (3). Chemical characterization of G. glabra collected in Uzbekistan. Chem. Pharm. Bull. 2003, 51, 1338–1340. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “Essential Herbal Medicine”—Licorice: A Review of Phytochemicals and Its Effects in Combination Preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Xiaoying, W.; Han, Z.; Yu, W. Glycyrrhiza glabra (Licorice). In Sustained Energy for Enhanced Human Functions and Activity; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–250. ISBN 9780128093320. [Google Scholar]
- Rizzato, G.; Scalabrin, E.; Radaelli, M.; Capodaglio, G.; Piccolo, O. A new exploration of licorice metabolome. Food Chem. 2017, 221, 959–968. [Google Scholar] [CrossRef]
- Shibata, S. A Drug over the Millennia: Pharmacognosy, chemistry, and pharmacology of Licorice. Yakugaku Zasshi 2000, 120, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Tohma, H.S.; Gulçin, I. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int. J. Food Prop. 2010, 13, 657–671. [Google Scholar] [CrossRef]
- Alsayari, A.; Wahab, S. Genus Ziziphus for the treatment of chronic inflammatory diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Bai, H.Y.; Wu, Z.R.; Yang, Z.G. Phenolic compounds from cultivated Glycyrrhiza uralensis and their PD-1/PD-L1 inhibitory activities. Nat. Prod. Res. 2021, 35, 562–569. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Li, T.; Tong, Y.-G.; Chen, X.-J.; Chen, X.-P.; Wang, Y.-T.; Lu, J.-J. A Systematic review of the anticancer properties of compounds isolated from licorice (Gancao). Planta Med. 2015, 81, 1670–1687. [Google Scholar] [CrossRef] [Green Version]
- Pu, J.-Y.; He, L.; Wu, S.-Y.; Zhang, P.; Huang, X. [Anti-virus research of triterpenoids in licorice]. Bing du xue bao Chin. J. Virol. 2013, 29, 673–679. [Google Scholar]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Li, Y.-X.; Niu, Y.-T.; Zheng, J.; Wu, J.; Shi, G.-J.; Ma, L.; Niu, Y.; Sun, T.; Yu, J.-Q. Observing Anti-inflammatory and anti-nociceptive activities of glycyrrhizin through regulating COX-2 and pro-inflammatory cytokines expressions in Mice. Inflammation 2015, 38, 2269–2278. [Google Scholar] [CrossRef]
- Akamatsu, H.; Komura, J.; Asada, Y.; Niwa, Y. Mechanism of anti-inflammatory action of glycyrrhizin: Effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991, 57, 119–121. [Google Scholar] [CrossRef]
- Simmler, C.; Pauli, G.F.; Chen, S.-N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obolentseva, G.V.; Litvinenko, V.I.; Ammosov, A.S.; Popova, T.P.; Sampiev, A.M. Pharmacological and therapeutic properties of licorice preparations (A review). Pharm. Chem. J. 1999, 33, 427–434. [Google Scholar] [CrossRef]
- Graebin, C.S. The pharmacological activities of glycyrrhizinic acid (“Glycyrrhizin”) and glycyrrhetinic acid. In Reference Series in Phytochemistry; Nature Publishing Group: Berlin, Germany, 2018; pp. 245–261. [Google Scholar]
- Eiji, O.; Yasunori, M.; Sadahiro, W.; Junzo, S.; Noriyuki, S.; Yasuko, M.M.; Masanobu, M.; Kozo, U. Inhibition of phospholipase A2 and platelet aggregation by glycyrrhizin, an antiinflammation drug. Acta Med. Okayama 1983, 37, 385–391. [Google Scholar] [CrossRef]
- Gottlieb, D.; Shaw, P.D. (Eds.) Mechanism of Action; Springer: Berlin/Heidelberg, Germany, 1967; ISBN 978-3-642-46053-1. [Google Scholar]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Shi, J.; Li, H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-кβ and NLRP3 inflammasome pathways. Biomed. Pharmacother. 2018, 106, 976–982. [Google Scholar] [CrossRef]
- Mazur, J.; Roy, K.; Shigdar, S.; Kanwar, J.R. Efficacy of Promising Flavonoids from Festuca lonicera, and Acacia genera Against Glioblastoma Multiforme; Potential for the Dandenong Ranges. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–422. [Google Scholar]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Jung, H.-A.; Liu, Y.; Su, B.-N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; True, A.D.; Zhou, L.; Xiong, Y.L. Inhibition of lipid oxidation and rancidity in precooked pork patties by radical-scavenging licorice (Glycyrrhiza glabra) Extract. J. Food Sci. 2013, 78, C1686–C1694. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef] [Green Version]
- Vaya, J.; Belinky, P.A.; Aviram, M. Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free. Radic. Biol. Med. 1997, 23, 302–313. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Wang, W.; Hou, J. Identification and simultaneous determination of glycyrrhizin, formononetin, glycyrrhetinic acid, liquiritin, isoliquiritigenin, and licochalcone A in licorice by LC-MS/MS. Acta Chromatogr. 2014, 26, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Jiang, D.; Rasul, A.; Batool, R.; Sarfraz, I.; Hussain, G.; Mateen Tahir, M.; Qin, T.; Selamoglu, Z.; Ali, M.; Li, J.; et al. Potential anticancer properties and mechanisms of action of formononetin. BioMed Res. Int. 2019, 2019, 5854315. [Google Scholar] [CrossRef]
- De Simone, F.; Aquino, R.; De Tommasi, M.N.; Piacente, S.; Pizza, C. Chapter 8: Anti-HIV Aromatic Compounds from Higher Plants. In Bioactive Compounds from Biological Sources; Tringali, C., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 305–336. 704p. [Google Scholar]
- Hatano, T.; Yasuhara, T.; Okuda, T. Anti-human immunodeficiency virus phenolics from Licorice). Chem. Pharm. Bull. 1988, 36, 2286–2288. [Google Scholar] [CrossRef] [Green Version]
- Fukai, T.; Cai, B.S.; Maruno, K.; Miyakawa, Y.; Konishi, M.; Nomura, T. An isoprenylated flavanone from Glycyrrhiza glabra and rec-assay of licorice phenols. Phytochemistry 1998, 49, 2005–2013. [Google Scholar] [CrossRef]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural chalcones in Chinese Materia Medica: Licorice. Evid. Based Complement. Altern. Med. 2020, 2020, 3821248. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Belén, L.H.; Kaur, R.; Kregiel, D.; Uprety, Y.; Beyatli, A.; Yeskaliyeva, B.; Kırkın, C.; et al. Glycyrrhiza Genus: Enlightening Phytochemical Components for pharmacological and health-promoting abilities. Oxidative Med. Cell. Longev. 2021, 2021, 7571132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Wang, L.J.; Wang, J.J.; Feng, W.J.; Yang, Z.Q.; Ni, S.H.; Huang, Y.S.; Li, H.; Yang, Y.; Wang, M.Q.; et al. Hispaglabridin B, a constituent of liquorice identified by a bioinformatics and machine learning approach, relieves protein-energy wasting by inhibiting forkhead box O1. Br. J. Pharmacol. 2019, 176, 267–281. [Google Scholar] [CrossRef] [Green Version]
- Mitscher, L.A.; Park, Y.H.; Clark, D.; Beal, J.L. Antimicrobial agents from higher plants. antimicrobial isoflavanoids and related substances from Glycyrrhiza glabra L. var. typica. J. Nat. Prod. 1980, 43, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, S.; Chourasia, R.; Pandey, A.; Rai, A.K.; Sahoo, D. Chapter 2—Alzheimer’s disease: Ethanobotanical studies. In Naturally Occurring Chemicals Against Alzheimer’s Disease; Academic Press: Cambridge, MA, USA, 2021; pp. 11–28. ISBN 978-0-12-819212-2. [Google Scholar]
- Wahab, S.; Ahmad, I.; Irfan, S.; Siddiqua, A.; Usmani, S.; Ahmad, M.P. Pharmacological Efficacy and Safety of Glycyrrhiza glabra in the treatment of respiratory tract infections. Mini-Rev. Med. Chem. 2021, 21. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Haredy, S.A.; Niazy, R.M.; Linhardt, R.J.; Warda, M. Dose-dependent neuroprotective effect of oriental phyto-derived glycyrrhizin on experimental neuroterminal norepinephrine depletion in a rat brain model. Chem.-Biol. Interact. 2019, 308, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Allcock, E.; Cowdery, J. Hypertension induced by liquorice tea. Case Rep. 2015, 2015, bcr2015209926. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regeneration Res. 2017, 12, 660. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ara, I.; Mondal, M.S.A.; Kabir, Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 2021, 7, e07240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, J.; Wen, Y.; Chen, B.; Hou, J.; Peng, B.; Zhang, S.; Mi, H.; Jiang, Q.; Wu, X.; et al. Modified Sijunzi decoction in the treatment of ulcerative colitis in the remission phase: Study protocol for a series of N-of-1 double-blind, randomised controlled trials. Trials 2020, 21, 396. [Google Scholar] [CrossRef]
- Thakur, A.K.; Raj, P. Pharmacological perspective of Glycyrrhiza glabra Linn.: A Mini-Review. J. Anal. Pharm. Res. 2017, 5, 00156. [Google Scholar] [CrossRef] [Green Version]
- EMA/HMPC. Assessment report on Glycyrrhiza glabra L. and/or Glycyrrhiza inflata Bat. and/or Glycyrrhiza Uralensis Fisch ex D.C., radix; European Medicines Agency: Amsterdam, The Netherlands, 2011; Volume 44. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 2021. preprint. [Google Scholar] [CrossRef]
- Lim, T.K. Glycyrrhiza glabra. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2016; Volume 10, pp. 354–457. [Google Scholar]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Hani, U.; Begum, M.Y.; Wahab, S.; Siddiqua, A.; Osmani, R.A.M.; Rahmathulla, M. A comprehensive review of current perspectives on novel drug Delivery systems and approaches for lung cancer management. J. Pharm. Innov. 2021, 1–24. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-P.; Ohnuma, S.; Ambudkar, S.V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 609–620. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2015, 57, 1874–1905. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Hou, J.; Tian, S.; Liu, Y. Molecular mechanisms underlying the anticancer activities of licorice flavonoids. J. Ethnopharmacol. 2021, 267, 113635. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Xiu-Rong, Z.; Shi-Yao, W.; Wen, S.; Chao, W. Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2018, 18, 3429–3436. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Jiang, X.; Gao, H.Y.; Gao, S.H. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int. J. Oncol. 2017, 51, 1383–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.J.; Park, S.Y.; Kwon, G.T.; Lee, K.W.; Kang, Y.H.; Choi, M.S.; Yun, J.W.; Jeon, J.H.; Jun, J.G.; Park, J.H.Y. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev. Res. 2013, 6, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.N.; Oh, K.B.; Lee, M.H.; Seo, J.H.; Kim, E.; Yoon, G.; Cho, S.S.; Cho, Y.S.; Choi, H.W.; Chae, J.I.I.; et al. JAK2 regulation by licochalcone H inhibits the cell growth and induces apoptosis in oral squamous cell carcinoma. Phytomedicine 2019, 52, 60–69. [Google Scholar] [CrossRef]
- Si, L.; Yan, X.; Hao, W.; Ma, X.; Ren, H.; Ren, B.; Li, D.; Dong, Z.; Zheng, Q. Licochalcone D induces apoptosis and inhibits migration and invasion in human melanoma A375 cells. Oncol. Rep. 2018, 39, 2160–2170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhou, S.; Shao, J.; Qu, X. Primary research on chinese medicine treatment of androgen-independent prostate cancer. Chin. J. Integr. Med. 2009, 15, 168–169. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Lebel, G.; Pellerin, G.; Lagha, A.B.; Grenier, D. Effects of the licorice isoflavans licoricidin and glabridin on the growth, adherence properties, and acid production of Streptococcus mutans, and assessment of their biocompatibility. Antibiotics 2021, 10, 163. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Chen, X.; Wang, Z.-Y.; Chai, K.; Wang, Y.-F.; Xu, X.-H.; Wang, X.-W.; Lu, J.-H.; Wang, Y.-T.; Chen, X.-P.; et al. Induction of C/EBP homologous protein-mediated apoptosis and autophagy by licochalcone A in non-small cell lung cancer cells. Sci. Rep. 2016, 6, 26241. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.-S.; Thakur, K.; Hussain, S.S.; Zhang, J.-G.; Xiao, G.-R.; Wei, Z.-J. Licochalcone A from licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem. Toxicol. 2018, 120, 407–417. [Google Scholar] [CrossRef]
- Bortolotto, L.F.B.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, T.; Zhang, W.; Zhou, L.; Yu, B.; Wang, W.; Yang, Z.; Liu, Z.; Zou, P.; Liang, G. Licochalcone A inhibits the proliferation of human lung cancer cell lines A549 and H460 by inducing g2/M cell cycle arrest and ER stress. Int. J. Mol. Sci. 2017, 18, 1761. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.J.; Wu, G.J.; Chen, R.J.; Chang, C.C.; Lien, L.M.; Chiu, C.C.; Tseng, M.F.; Huang, L.T.; Lin, K.H. Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 2018, 9, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tian, L.; Wang, L.; Li, W.; Xu, Q.; Xiao, X. Antitumor effects and the underlying mechanism of licochalcone A combined with 5-fluorouracil in gastric cancer cells. Oncol. Lett. 2017, 13, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Hsieh, T.C.; Guo, J.; Kunicki, J.; Lee, M.Y.W.T.; Darzynkiewicz, Z.; Wu, J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, M.; Liu, Q.; Wu, J.; Huang, W.; Li, X.; Du, B.; Xu, Q.; Duan, J.; Jiao, S.; et al. Sequential treatment with aT19 cells generates memory CAR-T cells and prolongs the lifespan of Raji-B-NDG mice. Cancer Lett. 2020, 469, 162–172. [Google Scholar] [CrossRef]
- Morsi, M.K.; El-Magoli, B.; Saleh, N.T.; El-Hadidy, E.M.; Barakat, H.A. Study of antioxidants and anticancer activity licorice Glycyrrhiza glabra extracts. Egypt. J. Nutr. Feeds 2008, 2, 177–203. [Google Scholar]
- Vlaisavljević, S.; Šibul, F.; Sinka, I.; Zupko, I.; Ocsovszki, I.; Jovanović-Šanta, S. Chemical composition, antioxidant and anticancer activity of licorice from Fruska Gora locality. Ind. Crop. Prod. 2018, 112, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Li, K.; Wang, H.; Kang, L.; Dang, C.; Zhang, Y. Discovery of glabridin as potent inhibitor of epidermal growth factor receptor in SK-BR-3 Cell. Pharmacology 2019, 104, 113–125. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, X.; Li, D.; Chen, H.; Jiang, J.; Wang, Z.; Sun, X.; Zheng, Q. Isoliquiritigen enhances the antitumour activity and decreases the genotoxic effect of cyclophosphamide. Molecules 2013, 18, 8786–8798. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the role of isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Wu, C.H.; Shieh, T.M.; Chen, H.Y.; Huang, T.C.; Hsia, S.M. The licorice dietary component isoliquiritigenin chemosensitizes human uterine sarcoma cells to doxorubicin and inhibits cell growth by inducing apoptosis and autophagy via inhibition of m-TOR signaling. J. Funct. Foods 2017, 33, 332–344. [Google Scholar] [CrossRef]
- Peng, F.; Tang, H.; Liu, P.; Shen, J.; Guan, X.; Xie, X.; Gao, J.; Xiong, L.; Jia, L.; Chen, J.; et al. Isoliquiritigenin modulates MIR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Sci. Rep. 2017, 7, 9022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, H.; Wang, Y.; Zheng, H.; Yu, W.; Chai, H.; Zhang, J.; Falck, J.R.; Guo, A.M.; Yue, J.; et al. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer. Toxicol. Appl. Pharmacol. 2013, 272, 37–48. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Peng, C.; You, J.; Shen, J.; Han, S.; Chen, J. Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via β-catenin/ABCG2 signaling. Carcinogenesis 2014, 35, 2544–2554. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.H.; Chiang, Y.F.; Shieh, T.M.; Chen, H.Y.; Shih, C.K.; Wang, T.H.; Wang, K.L.; Huang, T.C.; Hong, Y.H.; Li, S.C.; et al. Dietary compound isoliquiritigenin, an antioxidant from licorice, suppresses triple-negative breast tumor growth via apoptotic death program activation in cell and xenograft animal models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Cuendet, M.; Guo, J.; Luo, Y.; Chen, S.; Oteham, C.P.; Moon, R.C.; Van Breemen, R.B.; Marler, L.E.; Pezzuto, J.M. Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in Licorice. Cancer Prev. Res. 2010, 3, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Isoliquiritigenin induces apoptosis and cell cycle arrest through p53-dependent pathway in Hep G2 cells. Life Sci. 2005, 77, 279–292. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Ma, X.-f.; Zhou, X.; Gan, L.; Liu, Y.-y.; Wang, Z. hua Isoliquiritigenin enhances radiosensitivity of HepG2 Cells via disturbance of redox status. Cell Biochem. Biophys. 2013, 65, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Cuendet, M.; Oteham, C.P.; Moon, R.C.; Pezzuto, J.M. Quinone reductase induction as a biomarker for cancer chemoprevention. J. Nat. Prod. 2006, 69, 460–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Horinaka, M.; Takara, M.; Tsuchihashi, M.; Mukai, N.; Wakada, M.; Sakai, T. Combination of isoliquiritigenin and tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in colon cancer HT29 cells. Environ. Health Prev. Med. 2008, 13, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xu, J.; Yao, B.; Yin, H.; Cai, Q.; Shrubsole, M.J.; Chen, X.; Kon, V.; Zheng, W.; Pozzi, A.; et al. Inhibition of 11β-hydroxysteroid dehydrogenase type II selectively blocks the tumor COX-2 pathway and suppresses colon carcinogenesis in mice and humans. J. Clin. Investig. 2009, 119, 876–885. [Google Scholar] [CrossRef]
- Stewart, P.M.; Prescott, S.M. Can licorice lick colon cancer? J. Clin. Investig. 2009, 119, 760–763. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.K.; Park, K.K.; Lim, S.S.; Park, J.H.Y.; Chung, W.Y. Effects of the licorice extract against tumor growth and cisplatin-induced toxicity in a mouse xenograft model of colon cancer. Biol. Pharm. Bull. 2007, 30, 2191–2195. [Google Scholar] [CrossRef] [Green Version]
- Saeedifar, A.M.; Mosayebi, G.; Ghazavi, A.; Ganji, A. Synergistic evaluation of ginger and licorice extracts in a mouse model of colorectal cancer. Nutr. Cancer 2021, 73, 1068–1078. [Google Scholar] [CrossRef]
- Auyeung, K.K.W.; Ko, J.K.S. Novel herbal flavonoids promote apoptosis but differentially induce cell cycle arrest in human colon cancer cell. Investig. New Drugs 2010, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Takasuka, N.; Iigo, M.; Baba, M.; Nishino, H.; Tsuda, H.; Okuyama, T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004, 95, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-X.; Wink, M. Reversal of multidrug resistance in human colon cancer and human leukemia cells by three plant extracts and their major secondary metabolites. Medicines 2018, 5, 123. [Google Scholar] [CrossRef] [Green Version]
- Hani, U.; Osmani, R.A.M.; Siddiqua, A.; Wahab, S.; Batool, S.; Ather, H.; Sheraba, N.; Alqahtani, A. A systematic study of novel drug delivery mechanisms and treatment strategies for pancreatic cancer. J. Drug Deliv. Sci. Technol. 2021, 63, 102539. [Google Scholar] [CrossRef]
- Kasiappan, R.; Jutooru, I.; Mohankumar, K.; Karki, K.; Lacey, A.; Safe, S. Reactive oxygen species (ROS)-inducing triterpenoid inhibits rhabdomyosarcoma cell and tumor growth through targeting SP transcription factors. Mol. Cancer Res. 2019, 17, 794–805. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Chen, H.; Lee, M.H.; Li, H.; Ma, W.; Peng, C.; Song, N.R.; Lee, K.W.; Bode, A.M.; Dang, Z.; et al. Licochalcone A, a natural inhibitor of c-Jun N-terminal kinase 1. Cancer Prev. Res. 2014, 7, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Guru, S.K.; Jain, S.K.; Pathania, A.S.; Vishwakarma, R.A.; Bhushan, S.; Bharate, S.B. 3-(2,6-Dichloro-benzyloxy)-11-oxo-olean-12-ene-29-oic acid, a semisynthetic derivative of glycyrrhetic acid: Synthesis, antiproliferative, apoptotic and anti-angiogenesis activity. MedChemComm 2015, 6, 564–575. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nixon, D.W. Licorice and Cancer. Nutr. Cancer 2001, 39, 1–11. [Google Scholar] [CrossRef]
- Pirtskhalaishvili, G.; Hrebinko, R.L.; Nelson, J.B. The treatment of prostate cancer: An overview of current options. Cancer Pract. 2001, 9, 295–306. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, E.D.; Wang, J.; Panzhinskiy, E.E.; Tong, C.; Li, W.; Li, J. Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 841–847. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, D.Y.; Choi, H.J.; Jung, J.I.; Chung, W.Y.; Park, J.H.Y. Induction of cell cycle arrest in prostate cancer cells by the dietary compound isoliquiritigenin. J. Med. Food 2009, 12, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Alsayari, A.; Muhsinah, A.B.; Almaghaslah, D.; Annadurai, S.; Wahab, S. Pharmacological efficacy of Ginseng against respiratory tract infections. Molecules 2021, 26, 4095. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, M.F.; Hussain, A.; Usmani, S.; Shoaib, A.; Ahmad, W. Effectiveness of Azithromycin as add-on Therapy in COVID-19 Management. Mini-Rev. Med. Chem. 2021, 21, 2860–2873. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, I.; Usmani, S.; Ahmad, M.P. Efficacy of Dexamethasone for the Treatment of COVID-19 Infection: A Perspective Review. Curr. Drug Deliv. 2020, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, X.; Zhang, G.; Ming, Z.; Wang, T. Isoliquiritigenin inhibits cigarette smoke-induced COPD by attenuating inflammation and oxidative stress via the regulation of the Nrf2 and NF-кβ signaling pathways. Front. Pharmacol. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, J.H.; Yang, W.K.; Geum, J.H.; Kim, H.R.; Choi, S.Y.; Kang, Y.M.; An, H.J.; Lee, Y.C. Herbal combinational medication of Glycyrrhiza glabra, Agastache rugosa containing glycyrrhizic acid, tilianin inhibits neutrophilic lung inflammation by affecting cxcl2, interleukin17/stat3 signal pathways in a murine model of copd. Nutrients 2020, 12, 926. [Google Scholar] [CrossRef] [Green Version]
- Al-Jawad, F.; Al-Razzuqi, R.; Hashim, H.; Al-Bayati, N. Glycyrrhiza glabra versus Boswellia carterii in chronic bronchial asthma: A comparative study of efficacy. Indian J. Allergy Asthma Immunol. 2012, 26, 6. [Google Scholar] [CrossRef]
- Udupa, N.; Khan, S.; Polu, P.; Nayanabhirama, U. Herbal medicinal plants as an anticancer agents exploring newer pharmaceutical aids for safer, economic pharmaceutical dosage forms view project herbal medicinal plants as an anticancer agents. Ann. Phytomed. 2015, 4, 37–45. [Google Scholar]
- Gumpricht, E.; Dahl, R.; Devereaux, M.W.; Sokol, R.J. Licorice compounds glycyrrhizin and 18-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J. Biol. Chem. 2005, 280, 10556–10563. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evid. Based Complement. Altern. Med. 2018, 2018, 8438101. [Google Scholar] [CrossRef] [Green Version]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, J.; Lin, J.; Okunieff, P.; Zhang, L. Glycyrrhizic acid mitigates 2nd round radiotherapy-induced severe lung injury: A Case Report. J. Lung Dis. Treat. 2017, 3, 1000125. [Google Scholar] [CrossRef]
- Xie, Y.C.; Dong, X.W.; Wu, X.M.; Yan, X.F.; Xie, Q.M. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int. Immunopharmacol. 2009, 9, 194–200. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, A.B.; Karaman, O.; Erge, D.O.; Erbil, G.; Yilmaz, O.; Bagriyanik, A.; Uzuner, N. Glycyrrhizin and long-term histopathologic changes in a murine model of asthma. Curr. Ther. Res. Clin. Exp. 2011, 72, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Dogan, M.F.; Parlar, A.; Cam, S.A.; Tosun, E.M.; Uysal, F.; Arslan, S.O. Glabridin attenuates airway inflammation and hyperresponsiveness in a mice model of ovalbumin-induced asthma. Pulm. Pharmacol. Ther. 2020, 63, 101936. [Google Scholar] [CrossRef]
- Biological Effects of Quercetin in COPD; Case Medical Research; 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03989271 (accessed on 3 November 2021).
- Deutch, M.R.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. Bioactive Candy: Effects of licorice on the cardiovascular system. Foods 2019, 8, 495. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Jin, Z.; Jin, J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol. Med. Rep. 2013, 7, 1278–1282. [Google Scholar] [CrossRef] [Green Version]
- Meyer: Pseudohyperaldosteronismus-Lakritzverzehr mit Folgen—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Pseudohyperaldosteronismus:+Lakritzverzehr+mit+Folgen&author=Meyer,+R.&publication_year=2000&journal=Dtsch.+Arztebl.+Int.&volume=97&pages=A-596 (accessed on 23 July 2021).
- Rasool, M.; Iqbal, J.; Malik, A.; Ramzan, H.S.; Qureshi, M.S.; Asif, M.; Qazi, M.H.; Kamal, M.A.; Chaudhary, A.G.A.; Al-Qahtani, M.H.; et al. Hepatoprotective effects of Silybum marianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) in combination: A possible synergy. Evid. Based Complement. Altern. Med. 2014, 2014, 641597. [Google Scholar] [CrossRef] [Green Version]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as antiviral agent against Hepatitis C virus. J. Transl. Med. 2011, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Yoh, T.; Nakashima, T.; Sumida, Y.; Kakisaka, Y.; Nakajima, Y.; Ishikawa, H.; Sakamoto, Y.; Okanoue, T.; Mitsuyoshi, H. Effects of glycyrrhizin on glucocorticoid signaling pathway in hepatocytes. Dig. Dis. Sci. 2002, 47, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Wedemeyer, H.; Singer, A.; Khomutjanskaja, N.; Dienes, H.P.; Roskams, T.; Goldin, R.; Hehnke, U.; Inoue, H. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: Biochemical and histological effects after 52 weeks. J. Viral Hepat. 2012, 19, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-L.; Ma, T.-C.; Li, Y.-C.; Chen, J.-T.; Chang, Y.-S. Concurrent use of corticosteroids with licorice-containing TCM preparations in Taiwan: A National health insurance database study. J. Altern. Complement. Med. 2010, 16, 539–544. [Google Scholar] [CrossRef]
- Del Prete, A.; Scalera, A.; Iadevaia, M.D.; Miranda, A.; Zulli, C.; Gaeta, L.; Tuccillo, C.; Federico, A.; Loguercio, C. Herbal products: Benefits, limits, and applications in chronic liver disease. Evid. Based Complement. Altern. Med. 2012, 2012, 837939. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, B.; Wang, L.; Xue, P.; Deng, B.; Zhang, G.; Jiang, S.; Zhang, M.; Liu, M.; Pi, J.; et al. Curcumin protects human keratinocytes against inorganic arsenite-induced acute cytotoxicity through an NRF2-dependent mechanism. Oxidative Med. Cell. Longev. 2013, 2013, 412576. [Google Scholar] [CrossRef]
- Huang, X.; Qin, J.; Lu, S. Magnesium isoglycyrrhizinate protects hepatic L02 cells from ischemia/reperfusion induced injury. Int. J. Clin. Exp. Pathol. 2014, 7, 4755–4764. [Google Scholar] [PubMed]
- Liu, M.; Zheng, B.; Liu, P.; Zhang, J.; Chu, X.; Dong, C.; Shi, J.; Liang, Y.; Chu, L.; Liu, Y.; et al. Exploration of the hepatoprotective effect and mechanism of magnesium isoglycyrrhizinate in mice with arsenic trioxide-induced acute liver injury. Mol. Med. Rep. 2021, 23, 438. [Google Scholar] [CrossRef] [PubMed]
- Jian-ping, Y. Advances in Studies on The Synthesis of Glycyrrhizic acid, Glycyrrhetinic acid derivatives and their biological activities. Lishizhen Med. Mater. Med. Res. 2012, 23, 1174–1182. [Google Scholar]
- Tang, B.; Qiao, H.; Meng, F.; Sun, X. Glycyrrhizin attenuates endotoxin-induced acute liver injury after partial hepatectomy in rats. Braz. J. Med Biol. Res. 2007, 40, 1637–1646. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-C.; Chen, W.-K.; Liao, P.-H.; Yu, W.-C.; Lee, Y.-J. Synergistic effect of cadmium chloride and acetaldehyde on cytotoxicity and its prevention by quercetin and glycyrrhizin. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2001, 496, 117–127. [Google Scholar] [CrossRef]
- Rahman, S.; Sultana, S. Chemopreventive activity of glycyrrhizin on lead acetate mediated hepatic oxidative stress and its hyperproliferative activity in Wistar rats. Chem. Biol. Interact. 2006, 160, 61–69. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Sakamoto, M.; Matsushita, M.; Fujita, T.; Nishioka, K. Glycyrrhizin inhibits the lytic pathway of complement—Possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Microbiol. Immunol. 2000, 44, 799–804. [Google Scholar] [CrossRef]
- Roots, L.; Patil, S.M.; Patil, M.B.; Sapkale, G.N. Antimicrobial activity of Glycyrrhiza Glabra. CAB Direct 2009, 7, 585–591. [Google Scholar]
- Shinwari, Z.K.; Khan, I.; Naz, S.; Hussain, A. Assessment of antibacterial activity of three plants used in Pakistan to cure respiratory diseases. Afr. J. Biotechnol. 2009, 8, 7082–7086. [Google Scholar] [CrossRef]
- Ayfer Atefi, D.; Turgay Erdo, Ö. Antimicrobial activities of various medicinal and commercial plant extracts T›bbi ve Ticari Amaçl› Kullan›lan Baz› Bitki Ekstraktlar›n›n Antimikrobiyal Etkileri. Turk. J. Biol. 2003, 27, 157–162. [Google Scholar]
- Irani, M.; Sarmadi, M.; Bernard, F.; Ebrahimi, G.H.; Bazarnov, H.S. Leaves antimicrobial activity of Glycyrrhiza glabra L. Iran. J. Pharm. Res. 2010, 9, 425–428. [Google Scholar] [CrossRef]
- Karahan, F.; Avsar, C.; Ozyigit, I.I.; Berber, I. Antimicrobial and antioxidant activities of medicinal plant Glycyrrhiza glabra var. glandulifera from different habitats. Biotechnol. Biotechnol. Equip. 2016, 30, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D.; et al. Antimicrobial potential of Glycyrrhiza glabra roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef]
- Shirazi, M.H.; Ranjbar, R.; Eshraghi, S.; Sadeghi, G.; Jonaidi, N.; Bazzaz, N.; Izadi, M.; Sadeghifard, N. An Evaluation of antibacterial activity of Glycyrrhiza glabra Extract on the growth of Salmonella, Shigella and ETEC E. coli. J. Biol. Sci. 2007, 7, 827–829. [Google Scholar] [CrossRef] [Green Version]
- Sedighinia, F.; Safipour Afshar, A.; Soleimanpour, S.; Zarif, R.; Asili, J.; Ghazvini, K. Antibacterial activity of Glycyrrhiza glabra against oral pathogens: An in vitro study. Avicenna J. Phytomed. 2012, 2, 118–124. [Google Scholar] [CrossRef]
- Jafari-Sales, A.; Bolouri, P. Evaluation of the antimicrobial effects of Glycyrrhiza glabra L. on some gram positive and gram negative pathogenic bacteria in laboratory conditions. Jorjani Biomed. J. 2018, 6, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Geetha, R.V.; Anitha, R. In vitro evaluation of anti mycotic activity of ethanolic extract of Glycyrrhiza glabra. Asian J. Pharm. Clin. Res. 2013, 6, 205–206. [Google Scholar]
- Iqbal, Z. Antioxidant and antibacterial activity of organic extracts of roots of Glycyrrhiza glabra Linn. Plant 2017, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, P.; Wahab, S.; Dera, A.; Chandramoorthy, H.; Irfan, S.; Patel, A.; Abullias, S.; Zaman, G.; Ahmad, I. Anti-cancer activity of ethanolic leaf extract of Salvia officinalis against oral squamous carcinoma cells in vitro via caspase mediated mitochondrial apoptosis. Pharmacogn. Mag. 2020, 16, 554. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Su, X.; Liu, X.; Ren, K.; Ning, C.; Zhang, Q.; Zhang, S. Daphnes Cortex and its licorice-processed products suppress inflammation via the TLR4/NF-кβ/NLRP3 signaling pathway and regulation of the metabolic profile in the treatment of rheumatoid arthritis. J. Ethnopharmacol. 2021, 283, 114657. [Google Scholar] [CrossRef]
- Vasanth, M.P.; Purushotham, K.G.; Sathish, M.; Vimal Raj, D.; Venkatesh, M. In-vitro anti-inflammatory activity of liquorice (Glycyrrhiza glabra) using aqueous extract. Int. J. Res. Pharm. Sci. 2020, 11, 657–662. [Google Scholar] [CrossRef]
- Shin, E.M.; Zhou, H.Y.; Guo, L.Y.; Kim, J.A.; Lee, S.H.; Merfort, I.; Kang, S.S.; Kim, H.S.; Kim, S.; Kim, Y.S. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008, 8, 1524–1532. [Google Scholar] [CrossRef]
- Yu, X.; Bao, Y.; Meng, X.; Wang, S.; Li, T.; Chang, X.; Xu, W.; Yang, G.; Bo, T. Multi-pathway integrated adjustment mechanism of licorice flavonoids presenting anti-inflammatory activity. Oncol. Lett. 2019, 18, 4956–4963. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Guan, E.; Zhang, Y.; Shu, Z.; Wang, B.; Wu, X.; Chen, J.; Liu, J.; Fu, X.; Sun, W.; et al. Chemical profile and anti-inflammatory activity of total flavonoids from Glycyrrhiza uralensis fisch. Iran. J. Pharm. Res. 2018, 17, 726–734. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Zhang, M.; Yin, T.; Xu, R.; Xiao, W.; Wu, J.; Deng, B.; Gao, X.; Gong, W.; et al. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxidative Med. Cell. Longev. 2018, 2018, 7161592. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.-Q.; Xie, L.; Wu, J.; Xu, K.; Xiao, J.; Chen, D.-Q. Corrigendum: Isoliquiritigenin protects against pancreatic injury and intestinal dysfunction after severe acute pancreatitis via Nrf2 signaling. Front. Pharmacol. 2019, 10, 788. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C.; Wang, Y.; Zhang, J.; Wang, F.; Li, L.; Meng, X.; Li, G.; Li, Y.; Wang, L. Isoliquiritigenin attenuates LPS-induced AKI by suppression of inflammation involving NF-кβ pathway. Am. J. Transl. Res. 2018, 10, 4141–4151. [Google Scholar] [PubMed]
- Zhao, H.; Zhang, N.; Ho, V.; Ding, M.; He, W.; Niu, J.; Yang, M.; Du, X.L.; Zorzi, D.; Chavez-MacGregor, M.; et al. Adherence to treatment guidelines and survival for older patients with stage II or III colon cancer in Texas from 2001 through 2011. Cancer 2018, 124, 679–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Tan, R.Z.; Li, J.C.; Liu, T.T.; Zhong, X.; Yan, Y.; Yang, J.K.; Lin, X.; Fan, J.M.; Wang, L. Isoliquiritigenin attenuates uuo-induced renal inflammation and fibrosis by inhibiting mincle/syk/ nf-kappa b signaling pathway. Drug Des. Dev. Ther. 2020, 14, 1455–1468. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.; Hu, W.; Ye, S.-T.; Tan, Y.-S. Isoliquiritigenin alleviated the Ang II-induced hypertensive renal injury through suppressing inflammation cytokines and oxidative stress-induced apoptosis via Nrf2 and NF-кβ pathways. Biochem. Biophys. Res. Commun. 2018, 506, 161–168. [Google Scholar] [CrossRef]
- Abdel Maksoud, H.A.; Abdel Magid, A.D.; Mostafa, Y.M.; Elharrif, M.G.; Sorour, R.I.; Sorour, M.I. Ameliorative effect of liquorice extract versus silymarin in experimentally induced chronic hepatitis: A biochemical and genetical study. Clin. Nutr. Exp. 2019, 23, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Leutz, A.; Doerr, H.W.; Cinatl, J. Glycyrrhizin exerts antioxidative effects in H5N1 Influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE 2011, 6, e19705. [Google Scholar] [CrossRef] [Green Version]
- Hassan Khattab, H.A.R.; Abdel-Dayem, U.A.; Abdulsalam Jambi, H.; Tallat Abbas, A.; Ahmead Abdul-Jawad, M.T.; Fouad El-Shitany, N.A.E.A. Licorice (Glycyrrhizza glabra) extract prevents production of th2 cytokines and free radicals induced by ova albumin in mice. Int. J. Pharmacol. 2018, 14, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Jia, T.; Qiao, J.; Guan, D.; Chen, T. Anti-inflammatory effects of licochalcone A on IL-1β-stimulated human osteoarthritis chondrocytes. Inflammation 2017, 40, 1894–1902. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.P.; Patruno, A.; De Lutiis, M.A.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological effect of licochalcone C on the regulation of PI3K/Akt/eNOS and NF-кβ/iNOS/NO signaling pathways in H9c2 Cells in response to LPS stimulation. Int. J. Mol. Sci. 2017, 18, 690. [Google Scholar] [CrossRef]
- Allukian, M.; Horowitz, A.M. Oral Health. In Social Injustice and Public Health; Oxford University Press: Oxford, UK, 2005; pp. 357–377. ISBN 9780199865352. [Google Scholar]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Ahmad, I.; Irfan, S.; Abohashrh, M.; Wahab, S.; Abullais, S.S.; Javali, M.A.; Nisar, N.; Alam, M.M.; Srivastava, S.; Saleem, M.; et al. Inhibitory Effect of Nepeta deflersiana on climax bacterial community isolated from the oral plaque of patients with periodontal disease. Molecules 2021, 26, 202. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Rathee, M.; Sapra, A. Dental Caries; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Peters, M.C.; Tallman, J.A.; Braun, T.M.; Jacobson, J.J. Clinical reduction of S. mutans in pre-school children using a novel liquorice root extract lollipop: A pilot study. Eur. Arch. Paediatr. Dent. Off. J. Eur. Acad. Paediatr. Dent. 2010, 11, 274–278. [Google Scholar] [CrossRef]
- Segal, R.; Pisanty, S.; Wormser, R.; Azaz, E.; Sela, M.N. Anticariogenic activity of licorice and glycyrrhizine I: Inhibition of in vitro plaque formation by Streptococcus mutans. J. Pharm. Sci. 1985, 74, 79–81. [Google Scholar] [CrossRef]
- Gedalia, I.; Stabholtz, A.; Lavie, A.; Shapira, L.; Pisanti, S.; Segal, R. The effect of glycyrrhizin on in vitro fluoride uptake by tooth enamel and subsequent demineralization. Clin. Prev. Dent. 1986, 8, 5–9. [Google Scholar]
- Deutchman, M.; Petrou, I.D.; Mellberg, J.R. Effect of fluoride and glycyrrhizin mouthrinses on artificial caries lesions in vivo. Caries Res. 1989, 23, 206–208. [Google Scholar] [CrossRef]
- Edgar, W.M. Reduction in enamel dissolution by liquorice and glycyrrhizinic acid. J. Dent. Res. 1978, 57, 59–64. [Google Scholar] [CrossRef]
- Lee, P.H.; Chu, P.M.; Hsieh, P.L.; Yang, H.W.; Chueh, P.J.; Huang, Y.F.; Liao, Y.W.; Yu, C.C. Glabridin inhibits the activation of myofibroblasts in human fibrotic buccal mucosal fibroblasts through TGF-β/smad signaling. Environ. Toxicol. 2018, 33, 248–255. [Google Scholar] [CrossRef]
- Seino, H.; Arai, Y.; Nagao, N.; Ozawa, N.; Hamada, K. Efficient percutaneous delivery of the antimelanogenic agent glabridin using cationic amphiphilic chitosan micelles. PLoS ONE 2016, 11, e0164061. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef]
- Ravanfar, P.; Namazi, G.; Atigh, M.; Zafarmand, S.; Hamedi, A.; Salehi, A.; Izadi, S.; Borhani-Haghighi, A. Efficacy of whole extract of licorice in neurological improvement of patients after acute ischemic stroke. J. Herb. Med. 2016, 6, 12–17. [Google Scholar] [CrossRef]
- Yang, E.J.; Min, J.S.; Ku, H.Y.; Choi, H.S.; Park, M.-k.; Kim, M.K.; Song, K.S.; Lee, D.S. Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2012, 421, 658–664. [Google Scholar] [CrossRef]
- Yang, E.J.; Park, G.H.; Song, K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. NeuroToxicology 2013, 39, 114–123. [Google Scholar] [CrossRef]
- Ming, L.J.; Yin, A.C.Y. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013, 8, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.H.; Zhang, H.D.; Xu, C.J.; Bian, Y.J.; Xu, X.J.; Xie, Q.M.; Zhang, R.H. Neuroprotective effects of flavonoids extracted from licorice on kainate-induced seizure in mice through their antioxidant properties. J. Zhejiang Univ. Sci. B 2013, 14, 1004–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Jiang, Y.; Zhang, Z.; Hou, J.; Tian, S.; Liu, Y. The anti-diabetic activity of licorice, a widely used Chinese herb. J. Ethnopharmacol. 2020, 263, 113216. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Yamashita, Y.; Kishida, H.; Nakagawa, K.; Ashida, H. Licorice flavonoid oil enhances muscle mass in KK-Ay mice. Life Sci. 2018, 205, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kataya, H.H.; Hamza, A.A.; Ramadan, G.A.; Khasawneh, M.A. Effect of licorice extract on the complications of diabetes nephropathy in rats. Drug Chem. Toxicol. 2011, 34, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Kishida, H.; Nakagawa, K.; Yoshioka, Y.; Ashida, H. Liquorice flavonoid oil suppresses hyperglycaemia accompanied by skeletal muscle myocellular GLUT4 recruitment to the plasma membrane in KK-A y mice. Int. J. Food Sci. Nutr. 2019, 70, 294–302. [Google Scholar] [CrossRef]
- Mustafa, S.B.; Akram, M.; Muhammad Asif, H.; Qayyum, I.; Hashmi, A.M.; Munir, N.; Khan, F.S.; Riaz, M.; Ahmad, S. Antihyperglycemic activity of hydroalcoholic extracts of selective medicinal plants Curcuma longa, Lavandula stoechas, Aegle marmelos, and Glycyrrhiza glabra and their polyherbal preparation in alloxan-induced diabetic mice. Dose Response 2019, 17, 1559325819852503. [Google Scholar] [CrossRef] [Green Version]
- Bardhan, K.D.; Cumberland, D.C.; Dixon, R.A.; Holdsworth, C.D. Clinical trial of deglycyrrhizinized liquorice in gastric ulcer. Gut 1978, 19, 779–782. [Google Scholar] [CrossRef]
- Hajiaghamohammadi, A.A.; Ziaee, A.; Samimi, R. The efficacy of licorice root extract in decreasing transaminase activities in non-alcoholic fatty liver disease: A randomized controlled clinical trial. Phytother. Res. 2012, 26, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Son, D.-H.; Chung, T.-H.; Lee, Y.-J. A Review of the Pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Akbar, F.; Hasebe, A.; Horiike, N.; Onji, M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J. Gastroenterol. 2003, 38, 962–967. [Google Scholar] [CrossRef]
- Miyaji, C.; Miyakawa, R.; Watanabe, H.; Kawamura, H.; Abo, T. Mechanisms underlying the activation of cytotoxic function mediated by hepatic lymphocytes following the administration of glycyrrhizin. Int. Immunopharmacol. 2002, 2, 1079–1086. [Google Scholar] [CrossRef]
- Hung, C.-H.; Kee, K.-M.; Chen, C.-H.; Tseng, P.; Tsai, M.-C.; Chen, C.-H.; Wang, J.-H.; Chang, K.-C.; Kuo, Y.-H.; Yen, Y.-H.; et al. A Randomized controlled trial of glycyrrhizin plus Tenofovir vs. Tenofovir in chronic Hepatitis B with severe acute exacerbation. Clin. Transl. Gastroenterol. 2017, 8, e104. [Google Scholar] [CrossRef]
- Kumada, H. Long-term treatment of chronic hepatitis C with glycyrrhizin [Stronger Neo-Minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology 2002, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, T.G.J.; Vulto, A.G.; Hop, W.C.J.; Brouwer, J.T.; Niesters, H.G.M.; Schalm, S.W. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: A double-blind, randomized, placebo-controlled phase I/II trial. J. Gastroenterol. Hepatol. 1999, 14, 1093–1099. [Google Scholar] [CrossRef]
- Van Rossum, T.G.J.; Vulto, A.G.; Hop, W.C.J.; Schalm, S.W. Glycyrrhizin-induced reduction of alt in european patients with chronic hepatitis C. Am. J. Gastroenterol. 2001, 96, 2432–2437. [Google Scholar] [CrossRef]
- Nakamura, T.; Fujii, T.; Ichihara, A. Enzyme leakage due to change of membrane permeability of primary cultured rat hepatocytes treated with various hepatotoxins and its prevention by glycyrrhizin. Cell Biol. Toxicol. 1985, 1, 285–295. [Google Scholar] [CrossRef]
- Neumann-Haefelin, C.; Timm, J.; Spangenberg, H.C.; Wischniowski, N.; Nazarova, N.; Kersting, N.; Roggendorf, M.; Allen, T.M.; Blum, H.E.; Thimme, R. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology 2008, 47, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Arase, Y.; Kobayashi, M.; Saitoh, S.; Someya, T.; Hosaka, T.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Suzuki, F.; et al. A Long-Term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: A Cohort study of 1249 Patients. Dig. Dis. Sci. 2006, 51, 603–609. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Moon, B.S.; Kim, H.; Lim, H.C.; Lee, Y.C.; Lee, G.; Kim, S.H.; Park, M.; Kim, J.B. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann. Lab. Med. 2013, 33, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajiaghamohammadi, A.A.; Zargar, A.; Oveisi, S.; Samimi, R.; Reisian, S. To evaluate of the effect of adding licorice to the standard treatment regimen of Helicobacter pylori. Braz. J. Infect. Dis. 2016, 20, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Wittschier, N.; Faller, G.; Hensel, A. Aqueous extracts and polysaccharides from Liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Ethnopharmacol. 2009, 125, 218–223. [Google Scholar] [CrossRef]
- Steinberg, D.; Sgan-Cohen, H.D.; Stabholz, A.; Pizanty, S.; Segal, R.; Sela, M.N. The anticariogenic activity of glycyrrhizin: Preliminary clinical trials. Isr. J. Dent. Sci. 1989, 2, 153–157. [Google Scholar]
- Omar, H.R.; Komarova, I.; El-Ghonemi, M.; Fathy, A.; Rashad, R.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Helal, E.; Camporesi, E.M. Licorice abuse: Time to send a warning message. Ther. Adv. Endocrinol. Metab. 2012, 3, 125–138. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- Albermann, M.E.; Musshoff, F.; Hagemeier, L.; Madea, B. Determination of glycyrrhetic acid after consumption of liquorice and application to a fatality. Forensic Sci. Int. 2010, 197, 35–39. [Google Scholar] [CrossRef]
- Celik, M.M.; Karakus, A.; Zeren, C.; Demir, M.; Bayarogullari, H.; Duru, M.; Al, M. Licorice induced hypokalemia, edema, and thrombocytopenia. Hum. Exp. Toxicol. 2012, 31, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Johns, C. Glycyrrhizic acid toxicity caused by consumption of licorice candy cigars. Can. J. Emerg. Med. 2009, 11, 94–96. [Google Scholar] [CrossRef] [Green Version]
- Caradonna, P.; Gentiloni, N.; Servidei, S.; Perrone, G.A.; Greco, A.V.; Russo, M.A. Acute myopathy associated with chronic licorice ingestion: Reversible loss of myoadenylate deaminase activity. Ultrastruct. Pathol. 1992, 16, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Glycyrrhiza glabra (Licorice): A Review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef]
- Farese, R.V.; Biglieri, E.G.; Shackleton, C.H.L.; Irony, I.; Gomez-Fontes, R. Licorice-Induced Hypermineralocorticoidism. N. Engl. J. Med. 1991, 325, 1223–1227. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kawai, H.; Aoyama, R.; Watanabe, H.; Suzuki, K.; Suga, N.; Kitagawa, W.; Miura, N.; Nishikawa, K.; Imai, H. Torsades de Pointes induced by a combination of garenoxacin and disopyramide and other cytochrome P450, family 3, subfamily A polypeptide-4-influencing drugs during hypokalemia due to licorice. Clin. Exp. Nephrol. 2010, 14, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jang, J.Y.; Choi, B.-I.; Baek, I.J.; Yon, J.M.; Hwang, B.Y.; Park, D.; Jeon, J.H.; Nam, S.Y.; Yun, Y.W.; et al. Licorice extract does not impair the male reproductive function of rats. Exp. Anim. 2008, 57, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Fraunfelder, F.W. Ocular side effects from herbal medicines and nutritional supplements. Am. J. Ophthalmol. 2004, 138, 639–647. [Google Scholar] [CrossRef]
- Dobbins, K.R.B.; Saul, R.F. Transient visual loss after licorice ingestion. J. Neuro Ophthalmol. 2000, 20, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Santaella, R.M.; Fraunfelder, F.W. Ocular adverse effects associated with systemic medications. Drugs 2007, 67, 75–93. [Google Scholar] [CrossRef]
- Hall, R.C.; Clemett, R.S. Central retinal vein occlusion associated with liquorice ingestion. Clin. Exp. Ophthalmol. 2004, 32, 341. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Li, W.; Wang, W.; Wang, S.X.; Lu, J.; Chang, Z.F. Adverse reaction induced by licorice preparations: Clinical analysis of 93 cases. China J. Chin. Mater. Med. 2013, 38, 3768–3772. [Google Scholar] [CrossRef]








| Compound Name | Structure | Phytochemistry | Mechanism of Action | Reference |
|---|---|---|---|---|
| Glycyrrhizin |  | The main constituents are triterpene, saponins, and flavonoids. | Inhibited the prostaglandin, specifically prostaglandin E2 and cyclooxygenase activity as well as platelet aggregation. | [25,28,29] |
| Glycyrrhetinic acid |  | Active phytoconstituents are 18β-glycyrrhetinic acid, isoflavones, glabrin A and B, and glycyrrhizin. | Glycyrrhetinic acid has shown anti-inflammatory activity and inhibited 11β-hydroxysteroid dehydrogenase | [27] |
| Glabridin |  | Glabridin is an isoflavane, a type of isoflavonoid. This product is part of a more prominent family of plant-derived molecules, the natural phenols. | Glabridin inhibited melanogenesis by two mechanisms (1) inhibited the production of ROS (2) inhibited tyrosine. | [25,28,30] |
| Quercetin |  | Plant-derived flavonoid. | How flavonoids inhibited enzymes is not known. It inhibits lipoxygenase and cyclooxygenase activities and decreases the production of inflammatory metabolites. | [31] |
| Liquiritigenin |  | Phenolic compounds, | It is inhibited through the pathways NLRP3 and NF-кβ. | [32] |
| Isoliquiritigenin |  | Phenolic compounds, | Reduce the inflammatory response of macrophages via the inhibition of the activation of AP-1, NF-кβ, and AP-1. | [33,34,35,36,37,38,39] |
| Licochalcone C |  | Phenolic compounds, | Electron transport in the bacterial respiratory chain is inhibited. | [40] |
| Formononetin |  | Bioactive isoflavones | They were arresting the cell cycle, inducing apoptosis, stopping metastasis via targeting numerous pathways. | [41] |
| Licopyranocoumarin |  | Coumarins | Without sny cytotoxicity, it inhibited the production of cells in HIV-infected cell cultures. | [42,43] |
| Glabrocoumarin |  | Coumarins | Without causing any cytotoxicity, it inhibited the formation of cells in HIV-infected cell cultures. | [42,43] |
| Kanzonol Y |  | Chalcone | Inhibitory activity against Bacillus subtilis H17 | [44] |
| Paratocarpin B |  | Chalcone | Peroxynitrite antioxidant assay has shown the antioxidant property. It is the most potent antioxidant agent. | [35,45,46] |
| Glycyglabrone |  | chalcone | It exhibited potent free radical scavenging activity. | [35,45,46] |
| Mannopyranosyl-D glucitol |  | Mannose | Not reported | [47] |
| Glabridin |  | Isoflavones | Inhibitor of tyrosine. | [48] |
| Hispaglabridin B |  | Isoflavones | It is the most potent antioxidant agent. FoxO1 transcriptional activity was inhibited via the expression of muscle-specific E3 ubiquitin ligases MuRF1 and Atrogin1 were decreased. | [35,49] |
| 4-O-Methylglabridin |  | Isoflavans | Possess significant antimicrobial activity in vitro. | [50] |
| Microbe | Methods | Antibacterial Effect | Extract Used | References |
|---|---|---|---|---|
| Staphylococcus aureus, B. cereus, Pseudomonas aeruginosa | Cell culture | Inhibited the growth | G. glabra | [157] |
| Oral pathogens | In vitro | Inhibited the growth of oral pathogens | G. glabra | [160] |
| Mycobacterium tuberculosis H(37)Ra and H(37) Rv strains | In vitro | Inhibited both Gram-positive and Gram-negative bacteria | G. glabra | [158] |
| Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa and Escherichia coli | In vitro | Inhibited growth of pathogens | G. glabra | [161] |
| Salmonella typhi, S. paratyphi B, Shigella sonnei, S. flexneri, and enterotoxigenic E. coli. | In vitro | Inhibited growth | G. glabra | [159] |
| Candida albicans, Aspergillus niger, Aspergillus fumigates, Mucor spp and Penicilium marneffei | In vitro | Inhibited growth of micro-organisms | G. glabra | [162] |
| Staphylococcus aureus and Escherichia coli | In vitro | Mild antibacterial effect | G. glabra | [163] |
| Participants | Interventions | Comparisons | Outcomes | Study Design | References/NCT Number |
|---|---|---|---|---|---|
| 96 patients with gastric ulcers | Deglycyrrhizinated licorice | They were randomly allocated to treatment either with deglycyrrhizinised licorice or placebo. | No differences were found between the treatment groups in the proportions with complete healing. | A randomized, double-blind, placebo-controlled trial. | [206] |
| 12(Apparent Mineralocorticoid Excess) | Dietary Supplement: Licorice | Participants of the single-arm study will ingest licorice candy, and their blood, saliva, and urine samples will be collected. | No result posted | Interventional | NCT02939144 |
| 252 | Extractum Liquiritiae Fluidum, 1 g diluted in 30 cc water, gargle the solution for 60 s without swallowing it starting preoperatively, 3 times a day until postoperative day 2. | Randomized allocation Licorice & Sugar water | Licorice gargling will be deemed better than sugar-water only if found non-inferior on both opioid consumption and pain score and superior on at least one of the two. | A Randomized, Double-blind Study | NCT02968823 |
| 60 (Oral lichen planus) | Licorice & Triamcinolone Acetonide | Triamcinolone mucoadhesive film & licorice mucoadhesive film | No result posted | Randomized by double-blind clinical trial | NCT02453503 |
| 63 (High Caries Risk Patients) | Arabic, Gum, Licorice Root, Chlorhexidine | Arabic gum and licorice root extracts compared to Chlorhexidine | No result posted | Randomized, Parallel Assignment, | NCT03684993 |
| 236 (Sore Throat) | Licorice Versus Sugar-water Gargle | Licorice solution, sugar solution | Licorice gargling halved the incidence of sore throat. | Randomized, Double-blind Comparison | [12] |
| 66 (NAFLD) | 2 g aqueous licorice root extract for 2 months | 2 g aqueous licorice root extract and placebo | A significant drop in liver enzymes following administration of licorice root extract. | Double-blind randomized | [207] |
| 60 (with SAE of CHB) | Tenofovir plus intravenous glycyrrhizin | Tenofovir plus intravenous glycyrrhizin and Tenofovir | Early introduction of glycyrrhizin can be safe and helpful for patients with SAE of CHB. | Randomized | [211] |
| 57(hepatitis C patients) | Glycyrrhizin | Glycyrrhizin, or placebo | 240 mg dose of glycyrrhizin thrice-weekly does not affect HCV-RNA levels and lowers the serum ALT during treatment, and it is well-tolerated and safe. | Randomized | [213] |
| 69 (chronic hepatitis C) | Glycyrrhizin | Glycyrrhizin, or placebo | In individuals with chronic hepatitis C, glycyrrhizin therapy causes a substantial reduction in ALT. No major side effects were observed. | Randomized | [214] |
| 1249 (chronic hepatitis with or without cirrhosis) | Intravenous glycyrrhizin injection | The treated and untreated group | Glycyrrhizin injection therapy significantly decreased the incidence of hepatocellular carcinoma. | Retrospective study | [217] |
| 120 (dyspepsia either with peptic ulcer disease) | Licorice | Clarithromycin-based triple regimen, and study group that received licorice | Licorice enhances the eradication of H. pylori, particularly in the presence of peptic ulcer disease | Randomized controlled clinical trial | [219] |
| 21 dental students | Glycyrrhizin | Glycyrrhizin and placebo | Glycyrrhizin has the potential to inhibit tooth plaque | Pilot study | [221] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. https://doi.org/10.3390/plants10122751
Wahab S, Annadurai S, Abullais SS, Das G, Ahmad W, Ahmad MF, Kandasamy G, Vasudevan R, Ali MS, Amir M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants. 2021; 10(12):2751. https://doi.org/10.3390/plants10122751
Chicago/Turabian StyleWahab, Shadma, Sivakumar Annadurai, Shahabe Saquib Abullais, Gotam Das, Wasim Ahmad, Md Faruque Ahmad, Geetha Kandasamy, Rajalakshimi Vasudevan, Md Sajid Ali, and Mohd Amir. 2021. "Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology" Plants 10, no. 12: 2751. https://doi.org/10.3390/plants10122751