Recent Progress of Electrospun Herbal Medicine Nanofibers
Abstract
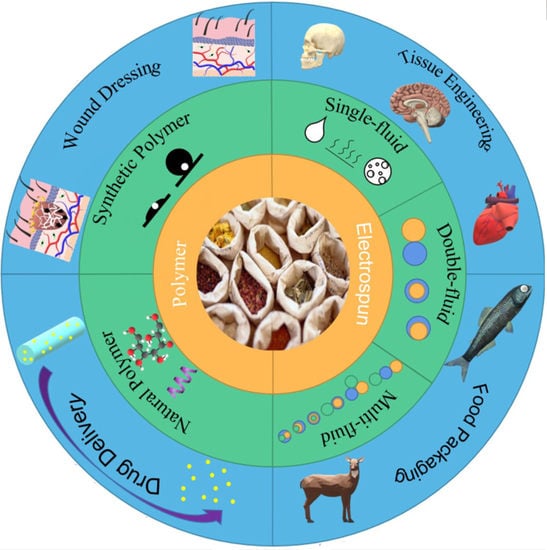
:1. Introduction
2. Electrospinning Technique
2.1. Introduction of Electrospinning Technology
2.2. Classification
2.2.1. Single-Fluid Electrospinning
2.2.2. Double-Fluid Electrospinning
2.2.3. Multi-Fluid Electrospinning
2.3. Influence Factors
2.4. Comparison of Electrospinning Technology with Other Nanofiber Technologies
3. Herbal Medicine Loaded into Electrospun Nanofibers
3.1. Synthesis of Polymer Loaded with Herbal Medicine
3.1.1. Hydrophobicity

3.1.2. Hydrophilicity
3.1.3. Other Herbal Medicine Polyester
3.2. Herbal Medicine Loaded Natural Polymer
3.2.1. Polysaccharides
Animal Polysaccharide
Plant Polysaccharide

Algal Polysaccharides
Fungal Polysaccharides
3.2.2. Proteins
4. Application of Herbal Medicine Loaded Nanofibers
4.1. Drug Delivery System
4.2. Wound Dressings
4.3. Tissue Engineering
4.4. Food Packaging
5. Conclusions and Outlook
- The synergistic actions of electrospun herbal medicines with other chemical active ingredients;
- The applications of electrospun complex nanostructures for manipulating the drug release behaviors of herbal medicines;
- The productions of electrospun herbal medicines on an industrial scale.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, W.W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lenaghan, S.C.; Wills, A.B.; Chen, Y.; Zhang, M. Evaluation of the nanofibrillar structure of dioscorea opposite extract for cell attachment. Colloid Surf. B 2011, 88, 425–431. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Alwahibi, M.S.; Dewir, Y.H.; Soliman, D.A.; Rizwana, H. Antibacterial activity of honey/chitosan nanofibers loaded with capsaicin and gold nanoparticles for wound dressing. Molecules 2020, 25, 4770. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Du, H.Z.; Hou, X.Y.; Miao, Y.H.; Huang, B.S.; Liu, D.H. Traditional Chinese medicine: An effective treatment for 2019 novel coronavirus pneumonia (NCP). Chin. J. Nat. Med. 2020, 18, 206–210. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Capcha, J.M.C.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef]
- Sharifi, M.; Bahrami, S.H.; Nejad, N.H.; Milan, P.B. Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing. J. Appl. Polym. Sci. 2020, 137, 49528. [Google Scholar] [CrossRef]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Boufi, S. Novel, multifunctional mucilage composite films incorporated with cellulose nanofibers. Food Hydrocolloid 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Samadian, H.; Moradi, N.; Izadi, Z.; Eftekhari, M.; Hamidi, M.; Shavandi, A.; Quéro, A.; Petit, E.; Delattre, C.; et al. Fabrication and characterization of nanocomposite hydrogel based on alginate/nano-hydroxyapatite loaded with Linum usitatissimum extract as a bone tissue engineering scaffold. Mar. Drugs 2022, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Lim, L.T. Release of allyl isothiocyanate from mustard seed meal powder entrapped in electrospun PLA–PEO nonwovens. Food Res. Int. 2015, 77, 467–475. [Google Scholar] [CrossRef]
- Mitra, S.; Mateti, T.; Ramakrishna, S.; Laha, A. A review on curcumin-loaded electrospun nanofibers and their application in modern medicine. JOM 2022, 74, 3392–3407. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, Y.; Lv, Z.J.; Wei, W.J.; Guan, X.; Guan, Q.L.; Leng, Z.Q.; Zhao, J.Y.; Miao, H.; Liu, J. Potential neurogenesis of human adipose-derived stem cells on electrospun catalpol-loaded composite nanofibrous scaffolds. Ann. Biomed. Eng. 2015, 43, 2597–2608. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Soleimani, M.; Vossoughi, M.; Ranjbarvan, P.; Hamedi, S.; Zamanlui, S.; Mahmoudifard, M. Study of epithelial differentiation and protein expression of keratinocyte-mesenchyme stem cell co-cultivation on electrospun nylon/B. vulgaris extract composite scaffold. Mater. Sci. Eng. C 2017, 75, 653–662. [Google Scholar] [CrossRef]
- Han, J.; Branford-White, C.; Zhu, L. Preparation of shikonin-loaded polyacrylonitrile nanofibers using electrospinning. Int. Conf. Adv. Fibers Polym. Mater. 2009, 1, 827–830. [Google Scholar]
- Ullah, A.; Saito, Y.; Ullah, S.; Haider, M.K.; Nawaz, H.; Duy-Nam, P.; Kharaghani, D.; Kim, I.S. Bioactive sambong oil-loaded electrospun cellulose acetate nanofibers: Preparation, characterization, and in-vitro biocompatibility. Int. J. Biol. Macromol. 2021, 166, 1009–1021. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Kirthika, S.; Keerthana, S.; Kumar, N.M.; Jeyanthi, J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 156, 430–437. [Google Scholar] [CrossRef]
- Mousavi, M.A.; Abdi, Z.; Khavasi, N.; Sardari, S.; Tofangchiha, S. Bromelain-ferula gum-loaded polyurethane nanofibers for bedsore healing in rats. Eur. J. Plast. Surg. 2021, 44, 563–568. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Hamedi, S.; Esmaeili, E.; Kabiri, M.; Babaie, A.; Soleimani, M.; Ardeshirylajimi, A. Mucoadhesive nanofibrous membrane with anti-inflammatory activity. Polym. Bull. 2019, 76, 4827–4840. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Kitmongkolpaisarn, S.; Boonyagul, S.; Koobkokkruad, T. Chemomechanical and morphological properties with proliferation of keratinocyte cells of electrospun poyhydroxyalkanoate fibers incorporated with essential oil. Polym. Adv. Technol. 2018, 29, 2364–2372. [Google Scholar] [CrossRef]
- Mouro, C.; Dunne, C.P.; Gouveia, I.C. Designing new antibacterial wound dressings: Development of a dual layer cotton material coated with poly(vinyl alcohol)_chitosan nanofibers incorporating Agrimonia eupatoria L. extract. Molecules 2021, 26, 83. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.J.; et al. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Agnes Mary, S.; Giri Dev, V.R. In vivo bioactivity of herbal-drug-incorporated nanofibrous matrices. J. Appl. Polym. Sci. 2015, 132, 42178. [Google Scholar] [CrossRef]
- Karami, Z.; Rezaeian, I.; Zahedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(ε-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Polym. Sci. 2013, 129, 756–766. [Google Scholar] [CrossRef]
- Ali, A.; Shahid, M.A.; Hossain, M.D.; Islam, M.N. Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int. J. Biol. Macromol. 2019, 138, 13–20. [Google Scholar] [CrossRef]
- Islam, M.A.; Begum, H.A.; Shahid, M.A.; Ali, A. Antibacterial electrospun nanofibers from poly (vinyl alcohol) and Mikania micrantha with augmented moisture properties: Formation and evaluation. J. Text Indust. 2021, 112, 1602–1610. [Google Scholar] [CrossRef]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial electrospun chitosan–polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydr. Polym. 2016, 140, 287–298. [Google Scholar] [CrossRef]
- Hani, N.M.; Torkamani, A.E.; Azarian, M.H.; Mahmood, K.W.; Ngalim, S.H. Characterisation of electrospun gelatine nanofibres encapsulated with Moringa oleifera bioactive extract. J. Sci. Food Agric. 2017, 97, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Kharat, Z.; Sadri, M.; Kabiri, M. Herbal extract loaded chitosan/PEO nanocomposites as antibacterial coatings of orthopaedic implants. Fiber. Polym. 2021, 22, 989–999. [Google Scholar] [CrossRef]
- Reinhardt, L.S.; Henn, J.G.; Morás, A.M.; de Moura Sperotto, N.D.; Ferro, M.B.; Cao, Z.; Roehe, A.V.; Petry, A.U.S.; Nugent, M.; Moura, D.J. Plantago australis hydroethanolic extract-loaded formulations: Promising dressings for wound healing. Rev. Bras Farmacogn. 2021, 31, 91–101. [Google Scholar] [CrossRef]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, R.; Sugumar, M.; Swaminathan, E.; Micheal Raj, A.P.; Dharmalingam, S. Design and fabrication of electrospun Morinda citrifolia-based nanofibrous scaffold as skin wound dressing material: In vitro and in silico analysis. Biomed. Mater. 2021, 16, 045014. [Google Scholar] [CrossRef]
- Ashjazadeh, M.A.; Jahandideh, A.; Abedi, G.; Akbarzadeh, A.; Hesaraki, S. Histopathology and histomorphological study of wound healing using Clove extract nanofibers (eugenol) compared to zinc oxide nanofibers on the skin of rats. Arch. Razi Inst. 2019, 74, 267–277. [Google Scholar] [PubMed]
- Kharat, Z.; Amiri Goushki, M.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO nanofibers containing Calendula officinalis extract: Preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminezhad, K.; Zare, M.; Lashkarara, S.; Yousefzadeh, M.; Aghazadeh Mohandesi, J. Fabrication of althea officinalis loaded electrospun nanofibrous scaffold for potential application of skin tissue engineering. J. Appl. Polym. Sci. 2020, 137, 48587. [Google Scholar] [CrossRef]
- Avci, M.O.; Muzoglu, N.; Yilmaz, A.E.; Yarman, B.S. Antibacterial, cytotoxicity and biodegradability studies of polycaprolactone nanofibers holding green synthesized Ag nanoparticles using atropa belladonna extract. J. Biomater. Sci. Polym. E 2022, 33, 1157–1180. [Google Scholar] [CrossRef]
- Rongthong, W.; Niamnont, N.; Srisuwannaket, C.; Paradee, N.; Mingvanish, W. Electrospun gelatin fiber mats mixed with C. carandas extract and its enhanced stability and bioactivity. J. Pharm. Sci. 2021, 110, 2405–2415. [Google Scholar] [CrossRef]
- Kouadri, I.; Satha, H. Extraction and characterization of cellulose and cellulose nanofibers from Citrullus colocynthis seeds. Ind. Crops Prod. 2018, 124, 787–796. [Google Scholar] [CrossRef]
- Shen, S.F.; Zhu, L.F.; Liu, J.; Ali, A.; Zaman, A.; Ahmad, Z.; Chen, X.; Chang, M.W. Novel core-shell fiber delivery system for synergistic treatment of cervical cancer. J. Drug Deliv. Sci. Technol. 2020, 59, 101865. [Google Scholar] [CrossRef]
- Zhi, K.; Sun, Y.; Zhao, H.; Zhang, C.; Peng, H.; Yang, X. Self-assembled supramolecular material derived from traditional Chinese medicine: Injectable self-assembled natural product gel for drug delivery with biological activity. Mater. Today Commun. 2020, 23, 101149. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Ashraf, S.S. Cinnamon extract loaded electrospun chitosan/gelatin membrane with antibacterial activity. Int. J. Biol. Macromol. 2021, 173, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Kotroni, E.; Simirioti, E.; Kikionis, S.; Sfiniadakis, I.; Siamidi, A.; Karalis, V.; Vitsos, A.; Vlachou, M.; Ioannou, E.; Roussis, V.; et al. In vivo evaluation of the anti-inflammatory activity of electrospun micro/nanofibrous patches loaded with Pinus halepensis bark extract on hairless mice skin. Materials 2019, 12, 2596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Xu, J.; Duan, X.; Lu, L.; Hu, D.; Zhang, L.; Nie, T.; Brown, K.B. Controllable synthesis of multi-walled carbon nanotubes/poly(3,4-ethylenedioxythiophene) core-shell nanofibers with enhanced electrocatalytic activity. Electrochim. Acta 2014, 137, 518–525. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Nada, A.A.; Ali, E.A.; Shalaby, A.S.G.; Soliman, A.A.F.; Emam, M.; El Raey, M.A. Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nano-forms: Multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol. 2019, 135, 407–421. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Adomavičiūtė, E.; Jankauskaitė, V.; Marksa, M.; Barsteigienė, Z.; Bernatoniene, J. Formation and investigation of electrospun Eudragit E100/oregano mats. Molecules 2019, 24, 628. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Ning, Z.; Song, Z.; Wang, C.; Liu, Y.; Wan, X.; Peng, S.; Liu, Z.; Lu, A. The effects of vinegar processing on the changes in the physical properties of frankincense related to the absorption of the main boswellic acids. Molecules 2019, 24, 3453. [Google Scholar] [CrossRef] [Green Version]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-coated poly(lactic acid) nanofibres loaded with essential oils for wound healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; He, L.; Chen, N.; Ramakrishna, S.; So, K.F.; Mo, X. Lycium barbarum polysaccharide encapsulated poly lactic-co-glycolic acid nanofibers: Cost effective herbal medicine for potential application in peripheral nerve tissue engineering. Sci. Rep. 2018, 8, 8669. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, O.M.; Iacob, A.T.; Mignon, A.; Van Vlierberghe, S.; Baican, M.; Danu, M.; Ibănescu, C.; Simionescu, N.; Profire, L. Design, preparation and in vitro characterization of biomimetic and bioactive chitosan/polyethylene oxide based nanofibers as wound dressings. Int. J. Biol. Macromol. 2021, 193, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. Effect of incorporating Elaeagnus angustifolia extract in PCL-PEG-PCL nanofibers for bone tissue engineering. Front. Chem. Sci. Eng. 2019, 13, 108–119. [Google Scholar] [CrossRef]
- Wang, Z.C.; Li, K.H.; Sun, H.J.; Wang, J.; Fu, Z.D.; Liu, M.Z. Icariin promotes stable chondrogenic differentiation of bone marrow mesenchymal stem cells in self-assembling peptide nanofiber hydrogel scaffolds. Mol. Med. Rep. 2018, 17, 8237–8243. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Jiang, H.; Lu, X.; Zhu, Y.; Chen, B. New strategy of photodynamic treatment of TiO2 nanofibers combined with celastrol for HepG2 proliferation in vitro. Nanoscale 2011, 3, 3115–3122. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, C.; Wang, J.; Song, F.; Yue, W.; Zhong, W. A simply triggered peptide-based hydrogel as an injectable nanocarrier of tanshinone IIA and tanshinones. Chem. Commun. 2017, 53, 529–532. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P. Electrospun novel nanocomposite comprising polyurethane integrated with ayurveda amla oil for bone tissue engineering. An. Acad. Bras. Cienc. 2020, 92, e20180369. [Google Scholar] [CrossRef]
- Suwantong, O.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing asiaticoside or Centella asiatica crude extract and the release characteristics of asiaticoside. Polymer 2008, 49, 4239–4247. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Pang, L.; Liu, Y.; Lin, Y.; Xiang, T.; Li, J.; Liao, S.; Jiang, Y. Astragalus polysaccharides/PVA nanofiber membranes containing astragaloside IV-loaded liposomes and their potential use for wound healing. Evid.-Based Complement. Altern. 2022, 2022, 9716271. [Google Scholar] [CrossRef]
- Nam, S.; Lee, J.J.; Lee, S.Y.; Jeong, J.Y.; Kang, W.S.; Cho, H.J. Angelica gigas nakai extract-loaded fast-dissolving nanofiber based on poly(vinyl alcohol) and soluplus for oral cancer therapy. Int. J. Pharm. 2017, 526, 225–234. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, D.; Yang, X.; Zhang, L.; Chu, J.; Kai, G.; He, C.; Mo, X.; Wang, H. Cirsium Japonicum DC ingredients-loaded silk fibroin nanofibrous matrices with excellent hemostatic activity. Biomed. Phys. Eng. Express 2018, 4, 025035. [Google Scholar] [CrossRef]
- Dixit, V.; Tewari, J.; Obendorf, S.K. Fungal growth inhibition of regenerated cellulose nanofibrous membranes containing Quillaja Saponin. Arch. Environ. Contam. Toxicol. 2010, 59, 417–423. [Google Scholar] [CrossRef]
- Kaufmann, K.C.; Czakoski, A.; Barbin, D.F.; da Cunha, R.L. Incompatibility between sodium caseinate—Locust bean gum induced by NaCl and yerba mate extract. Int. J. Biol. Macromol. 2021, 183, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.K.; Chen, X.H.; Yu, D.G.; Yang, Y.Y.; Liu, P. Electrospun hydrophilic Janus nanocomposites for the rapid onset of therapeutic action of helicid. ACS Appl. Mater. Interf. 2018, 10, 2859–2867. [Google Scholar] [CrossRef]
- Bonifacio, B.V.; da Silva, P.B.; Ramos, M.A.D.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Yu, D.G. Preface—Bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Hou, X.X.; Yang, X.P.; Zhang, F.; Wu, S.Z.; Waclawik, E. Stretching-induced orientation to improve mechanical properties of electrospun PAN nanocomposites. Int. J. Mod. Phys. B 2008, 22, 5913–5918. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Fang, T.; Geng, J.; Freitag, D.; Arlt, W. Producing PVP nanofibers by electrospinning in N-2. Spring Int. Conf. Mater. Sci. Technol. 2012, 1, 701–708. [Google Scholar]
- Feng, L.; Li, S.H.; Zhai, J.; Song, Y.L.; Jiang, L.; Zhu, D.B. Template based synthesis of aligned polyacrylonitrile nanofibers using a novel extrusion method. Synthetic Met. 2003, 135–136, 817–818. [Google Scholar] [CrossRef]
- Liu, W.; Graham, M.; Evans, E.A.; Reneker, D.H. Poly(meta-phenylene isophthalamide) nanofibers: Coating and post processing. J. Mater. Res. 2002, 17, 3206–3212. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, B.; Wang, Y.; Cheng, H.; Zhang, X.; Xu, S.; Liu, H.; Liu, W.; Hu, C. Excellent electrochemical performances of intrinsic polyaniline nanofibers fabricated by electrochemical deposition. J. Wuhan Univ. Technol. 2019, 34, 216–222. [Google Scholar] [CrossRef]
- Shamsabadi, A.S.; Ranjbar, M.; Tavanai, H.; Farnood, A. Electrospinning of gold nanoparticles incorporated PAN nanofibers via in-situ laser ablation of gold in electrospinning solution. Mater. Res. Express 2019, 6, 055051. [Google Scholar] [CrossRef]
- Iqbal, H.; Mahar, F.K.; Razzaq, A.; Kamal, R.; Khan, N.U.; Ullah, K.; Iqbal, S. Green synthesis of cefadroxil loaded chitosan/PVA nanofibers by freeze drying. Mater. Res. Express 2019, 6, 125094. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Lee, M.W.; Sinha-Ray, S.; An, S.; Pourdeyhimi, B.; Yoon, S.S.; Yarin, A.L. Supersonic nanoblowing: A new ultra-stiff phase of nylon 6 in 20–50 nm confinement. J. Mater. Chem. C 2013, 1, 3491–3498. [Google Scholar] [CrossRef]
- Kajekar, A.J.; Dodamani, B.M.; Isloor, A.M.; Karim, Z.A.; Cheer, N.B.; Ismail, A.F.; Shilton, S.J. Preparation and characterization of novel PSf/PVP/PANI-nanofiber nanocomposite hollow fiber ultrafiltration membranes and their possible applications for hazardous dye rejection. Desalination 2015, 365, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Gomez, C.; Zambrano, S.; Ramirez, M.; de Hoyos, E.; Vasquez, H.; Lozano, K. Electrospinning to ForcespinningTM. Mater. Today 2010, 13, 12–14. [Google Scholar] [CrossRef]
- Li, H.; Wan, H.; Xia, T.; Chen, M.; Zhang, Y.; Luo, X.; Li, X. Therapeutic angiogenesis in ischemic muscles after local injection of fragmented fibers with loaded traditional Chinese medicine. Nanoscale 2015, 7, 13075–13087. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Kurečič, M.; Pivec, T.; Maver, U.; Gradišnik, L.; Gašparič, P.; Kaker, B.; Bratuša, A.; Hribernik, S.; Stana Kleinschek, K. Needleless electrospun carboxymethyl cellulose/polyethylene oxide mats with medicinal plant extracts for advanced wound care applications. Cellulose 2020, 27, 4487–4508. [Google Scholar] [CrossRef]
- Kumar, G.R.; Rajan, T.P. A review on electrospinning of natural bio herbs blended with polyvinyl alcohol nanofibres for biomedical applications. J. Nat. Fibers 2022, 19, 11984–12003. [Google Scholar]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research progress, models and simulation of electrospinning technology: A review. J. Mat. Sci. 2022, 57, 58–104. [Google Scholar] [CrossRef]
- Lamarra, J.; Calienni, M.N.; Rivero, S.; Pinotti, A. Electrospun nanofibers of poly(vinyl alcohol) and chitosan-based emulsions functionalized with cabreuva essential oil. Int. J. Biol. Macromol. 2020, 160, 307–318. [Google Scholar] [CrossRef]
- Saravanan, M. Development of Thespesia populnea doped PVA electrospun mat for biocompatibility studies. J. Nat. Fibers 2022, 19, 1951–1961. [Google Scholar]
- Vongsetskul, T.; Phurayar, P.; Chutimasakul, T.; Tuchinda, P.; Uamsiri, S.; Kumkate, S.; Pearngam, P.; Jitpibull, J.; Samphaongern, C.; Tangboriboonrat, P. Acanthus ebracteatus Vahl. extract-loaded cellulose acetate ultrafine fibers as a topical carrier for controlled-release applications. Polym. Bull. 2016, 73, 3319–3331. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional electrospun nanocomposites for efficient oxygen reduction reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Pang, Y.; Pan, J.Y.; Yang, J.H.; Zheng, S.Y.; Wang, C.S. Electrolyte/Electrode Interfaces in All-Solid-State Lithium Batteries: A Review. Electrochem. Energy Rev. 2021, 4, 169–193. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, M.; Wang, P.; Xu, X.; Liu, Y.; Yu, D.G. Ingenious Construction of Ni(DMG)2/TiO2-decorated porous nanofibers for the highly efficient photodegradation of pollutants in water. Colloids Surface A 2022, 650, 129561. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.G. Progresses on electrospun metal–organic frameworks nanofibers and their wastewater treatment applications. Mater. Today Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Yu, D.-G. Recent Progress in Electrospun Nanofibers and Their Applications in Heavy Metal Wastewater Treatment. Front. Chem. Sci. Eng. 2023, 18. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.G. Electrospun porous nanofifibers: Pore−forming mechanisms and applications for photocatalytic degradation of organic pollutants in wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef]
- Xie, D.; Zhou, X.; Xiao, B.; Duan, L.; Zhu, Z. Mucus-penetrating silk fibroin-based nanotherapeutics for efficient treatment of ulcerative colitis. Biomolecules 2022, 12, 1263. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.G.; Ge, R. Electrospun nanofibers-based glucose sensors for glucose detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.G. Processes of electrospun polyvinylidene fluoride-based nanofibers, their piezoelectric properties, and several fantastic applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-decorated BixTiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants Under Solar Radiation. J. Mater. Sci. Technol. 2023, in press. [CrossRef]
- Jiang, W.; Zhao, P.; Song, W.; Wang, M.; Yu, D.G. Electrospun zein/polyoxyethy lene core-sheath ultrathin fibers and their antibacterial food packaging applications. Biomolecules 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-Drug Delivery Systems: Pro and Con, Challenges, and Future Directions. Biomater. Sci. 2022, 24, 6853–7164. [Google Scholar] [CrossRef]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.G.; Liu, Y. Recent progress in electrospun polyacrylonitrile nanofiber-based wound dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.G. Electrospun nanofibers for periodontal treatment: A recent progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Yan, C.; Liu, H.; Yu, D.G. Advances in the application of electrospun drug-loaded nanofifibers in the treatment of oral ulcers. Biomolecules 2022, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, P.; Yang, Y.; Yu, D.G. Electrospun beads-on-the-string nanoproducts: Preparation and drug delivery application. Curr. Drug Deliv. 2022, 19. [Google Scholar] [CrossRef]
- Gong, M.; Huang, C.; Huang, Y.; Li, G.; Chi, C.; Ye, J.; Xie, W.; Shi, R.; Zhang, L. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomed. Nanotechnol. 2019, 17, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of biodegradable polymeric stents for the treatment of cardiovascular diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Kant, V.; Kumari, P.; Jitendra, D.K.; Ahuja, M.; Kumar, V. Nanomaterials of natural bioactive compounds for wound healing: Novel drug delivery approach. Curr. Drug Deliv. 2021, 18, 1406–1425. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, Q.; Zhang, Y.; Zhuang, H.; Wang, E.; Xu, Q.; Ma, L.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a multifunctional biomaterial inspired by ancient Chinese medicine for hair regeneration in burned skin. ACS Appl. Mater. Interfaces 2020, 12, 12489–12499. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Barati, A.; Arjomandzadegan, M.; Vatankhah, E. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int. J. Biol. Macromol. 2020, 162, 762–773. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Suwantong, O.; Ruktanonchai, U.; Supaphol, P. In vitro biological evaluation of electrospun cellulose acetate fiber mats containing asiaticoside or curcumin. J. Biomed. Mater. Res. A 2010, 94A, 1216–1225. [Google Scholar]
- Verma, C.; Rohit, P.S.; Anjum, S.; Gupta, B. Novel approach for nanobiocomposites by nanoencapsulation of Lecithin-Clove oil within PVA nanofibrous web. Mater. Today Proc. 2019, 15, 183–187. [Google Scholar] [CrossRef]
- Ge, R.; Ji, Y.; Ding, Y.; Huang, C.; He, H.; Yu, D.-G. Electrospun self-emulsifying core-shell nanofibers for effective delivery of paclitaxel. Front. Bioeng. Biotechnol. 2023, 11, 1112338. [Google Scholar]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.G.; Wang, P.; Jiang, Q. Gold nanoparticles-loaded polyvinylpyrrolidone/ethylcellulose coaxial electrospun nanofibers with enhanced osteogenic capability for bone tissue regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Yu, D.G.; Liu, P. Elaborate design of shell component for manipulating the sustained release behavior from core—Shell nanofibres. J. Nanobiotechnol. 2022, 20, 244. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Song, W.L.; Yu, D.G.; Bligh, S.W.A. Electrospun Janus beads-on-a-string structures for different types of controlled release profiles of double drugs. Biomolecules 2021, 11, 635. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, Q.; Song, W.; Xu, L.; Zhang, K.; Zhou, T. Advanced technique-based combination of innovation education and safety education in higher education. J. Chem. Edu. 2023. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A review: Current status and emerging developments on natural polymer-based electrospun fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.-G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Swain, K.; Ramakrishna, S. Optimal delivery of poorly soluble drugs using electrospun nanofiber technology: Challenges, state of the art, and future directions. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1859. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Huang, Y.Y.; Zhang, H.D.; Sun, B.; Zhang, J.C.; Long, Y.Z. Needleless electrospinning for large scale production of ultrathin polymer fibres. Mater. Res. Innov. 2014, 18, S4-833–S4-837. [Google Scholar] [CrossRef]
- Dong, W.H.; Liu, J.X.; Mou, X.J.; Liu, G.S.; Huang, X.W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.Z. Performance of polyvinyl pyrrolidone-isatis root antibacterial wound dressings produced in situ by handheld electrospinner. Colloids Surface B 2020, 188, 110766. [Google Scholar] [CrossRef]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Akşit, N.N.; Gürdap, S.; İşoğlu, S.D.; İşoğlu, İ.A. Preparation of antibacterial electrospun poly(D, L-lactide-co-glycolide)/gelatin blend membranes containing Hypericum capitatum var. capitatum. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 797–809. [Google Scholar] [CrossRef]
- Luginina, M.; Schuhladen, K.; Orrú, R.; Cao, G.; Boccaccini, A.R.; Liverani, L. Electrospun PCL/PGS composite fibers incorporating bioactive glass particles for soft tissue engineering applications. Nanomaterials 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning—Green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Wang, X.f.; Huang, Z.m. Melt-electrospinning of PMMA. Chin. J. Polym. Sci. 2009, 28, 45. [Google Scholar] [CrossRef]
- Góra, A.; Sahay, R.; Thavasi, V.; Ramakrishna, S. Melt-Electrospun fibers for advances in biomedical engineering, clean energy, filtration, and separation. Polym. Rev. 2011, 51, 265–287. [Google Scholar] [CrossRef]
- Deng, R.; Liu, Y.; Ding, Y.; Xie, P.; Luo, L.; Yang, W. Melt electrospinning of low-density polyethylene having a low-melt flow index. J. Appl. Polym. Sci. 2009, 114, 166–175. [Google Scholar] [CrossRef]
- Coimbra, P.; Freitas, J.P.; Gonçalves, T.; Gil, M.H.; Figueiredo, M. Preparation of gentamicin sulfate eluting fiber mats by emulsion and by suspension electrospinning. Mater. Sci. Eng. C 2019, 94, 86–93. [Google Scholar] [CrossRef]
- Yan, S.; Xiaoqiang, L.; Shuiping, L.; Xiumei, M.; Ramakrishna, S. Controlled release of dual drugs from emulsion electrospun nanofibrous mats. Colloids Surfaces B 2009, 73, 376–381. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of proteins in poly(l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surfaces B 2010, 75, 418–424. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S. Encapsulation of phytoncide in nanofibers by emulsion electrospinning and their antimicrobial assessment. Fibers Polym. 2018, 19, 627–634. [Google Scholar] [CrossRef]
- Sun, J.; Bubel, K.; Chen, F.; Kissel, T.; Agarwal, S.; Greiner, A. Nanofibers by green electrospinning of aqueous suspensions of biodegradable block copolyesters for applications in medicine, pharmacy and agriculture. Macromol. Rapid Commun. 2010, 31, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surfaces A 2022, 636, 128163. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef]
- Afshar, S.K.; Abdorashidi, M.; Dorkoosh, F.A.; Javar, H.A. Electrospun fibers: Versatile approaches for controlled release applications. Int. J. Polym. Sci. 2022, 2022, 9116168. [Google Scholar]
- Li, C.; Yang, J.; He, W.; Xiong, M.; Niu, X.; Li, X.; Yu, D.G. A Review on Fabrication and Application of Tunable Hybrid Micro–Nano Array Surfaces. Adv. Mater. Interf. 2023. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Liu, P.; Zhang, Y.; Song, W.; Yu, D.G.; Lu, X. Electrospun healthcare nanofibers from medicinal liquor of Phellinus igniarius. Adv. Compos. Hybrid Mater. 2022, 5, 3045–3056. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1111–1125. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhao, P. The key elements for biomolecules to biomaterials and to bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric Teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun core–shell fibrous 2D scaffold with biocompatible poly(glycerol sebacate) and poly-l-lactic acid for wound healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.G.; Branford-White, C.; White, K.; Chatterton, N.P.; Zhu, L.M.; Huang, L.Y.; Wang, B. A modified coaxial electrospinning for preparing fibers from a high concentration polymer solution. Express Polym. Lett. 2011, 5, 732–741. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.G. Electrospun structural hybrids of acyclovir-polyacrylonitrile @ acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- Yu, D.G.; Yang, C.; Jin, M.; Williams, G.R.; Zou, H.; Wang, X.; Annie Bligh, S.W. Medicated Janus fibers fabricated using a teflon-coated side-by-side spinneret. Colloids Surf. B 2016, 138, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Huang, Y.; Hou, C.; Xin, B.; Li, T.; Jiang, Q. Electrospun poly(l-lactide-co-ε-caprolactone)/gelatin core–shell nanofibers encapsulated with doxorubicin hydrochloride as a drug delivery system. Polym. Int. 2022. early view. [Google Scholar] [CrossRef]
- Wang, M.; Yu, D.G.; Williams, G.R.; Bligh, S.W.A. Co-Loading of inorganic nanoparticles and natural oil in the electrospun Janus nanofibers for a synergetic antibacterial effect. Pharmaceutics 2022, 14, 1208. [Google Scholar] [CrossRef]
- Yu, D.G.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar]
- Liu, Y.; Chen, X.; Gao, Y.; Liu, Y.; Yu, D.G.; Liu, P. Electrospun core-sheath nanofibers with variable shell thickness for modifying curcumin release to achieve a better antibacterial performance. Biomolecules 2022, 12, 1057. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Yu, D.G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable drug release from nanofibers coated with blank cellulose acetate layers fabricated using tri-axial electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lu, Z.H.; Zhao, P.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Zhang, M.; Williams, G.R. High-quality Janus nanofibers prepared using three-fluid electrospinning. Chem. Commun. 2017, 53, 4542–4545. [Google Scholar] [CrossRef] [Green Version]
- Ziyadi, H.; Baghali, M.; Bagherianfar, M.; Mehrali, F.; Faridi-Majidi, R. An investigation of factors affecting the electrospinning of poly (vinyl alcohol)/kefiran composite nanofibers. Adv. Compos. Hybrid Mater. 2021, 4, 768–779. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Zhou, Q.; Bao, M.; Yuan, H.; Zhao, S.; Dong, W.; Zhang, Y. Implication of stable jet length in electrospinning for collecting well-aligned ultrafine PLLA fibers. Polymer 2013, 54, 6867–6876. [Google Scholar] [CrossRef]
- Chowdhury, M.; Stylios, G.K. Analysis of the effect of experimental parameters on the morphology of electrospun polyethylene oxide nanofibres and on their thermal properties. J. Text. Ind. 2012, 103, 124–138. [Google Scholar] [CrossRef]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; Homem, N.C.; Felgueiras, H.P. Tunable spun fiber constructs in biomedicine: Influence of processing parameters in the fibers’ architecture. Pharmaceutics 2022, 14, 164. [Google Scholar] [CrossRef]
- Jacobs, V.; Anandjiwala, R.D.; Maaza, M. The influence of electrospinning parameters on the structural morphology and diameter of electrospun nanofibers. J. Appl. Polym. Sci. 2010, 115, 3130–3136. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloid 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Lai, C.; Zhong, G.; Yue, Z.; Chen, G.; Zhang, L.; Vakili, A.; Wang, Y.; Zhu, L.; Liu, J.; Fong, H. Investigation of post-spinning stretching process on morphological, structural, and mechanical properties of electrospun polyacrylonitrile copolymer nanofibers. Polymer 2011, 52, 519–528. [Google Scholar] [CrossRef]
- Leong, T.G.; Zarafshar, A.M.; Gracias, D.H. Three-dimensional fabrication at small size scales. Small 2010, 6, 792–806. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, S.; Wang, C.; Shao, H.; Wang, Z.; Cui, Y. A novel separation technique for aqueous nanoparticles based on a phase transfer approach. J. Mater. Chem. 2012, 22, 13469–13472. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef]
- Chang, L.; Wu, C.; Lan, P.; Bai, B.; Jiang, L.; Chen, S.; Jerrams, S.; Ma, J. Elastic melt-blown nonwoven fabrication of styrene-ethylene/butylene-styrene copolymer and polypropylene blends: A study of morphology and properties. Text. Res. J. 2022, 92, 1620–1630. [Google Scholar] [CrossRef]
- Yang, H.; Sugita, N.; Nakane, K. Factors influencing the PVA polymer-assisted freeze-drying synthesis of Al2O3 nanofibers. Ceram. Int. 2019, 45, 16731–16739. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, J.; Su, Y.; Wang, H.; Wang, X.X.; Huang, L.P.; Yu, M.; Ramakrishna, S.; Long, Y.Z. Recent progress and challenges in solution blow spinning. Mater. Horiz. 2021, 8, 426–446. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanizawa, K. Poly(ethylene terephthalate) nanofibers prepared by CO2 laser supersonic drawing. Polymer 2009, 50, 913–921. [Google Scholar] [CrossRef]
- Skrivanek, J.; Holec, P.; Batka, O.; Bilek, M.; Pokorny, P. Optimization of the spinneret rotation speed and airflow parameters for the nozzleless forcespinning of a polymer solution. Polymers 2022, 14, 1042. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, D.; Ji, P.; Sheng, N.; Zhang, M.; Wu, Z.; Chen, S.; Wang, H. High-strength superstretchable helical bacterial cellulose fibers with a “self-fiber-reinforced structure”. ACS Appl. Mater. Interf. 2021, 13, 1545–1554. [Google Scholar] [CrossRef]
- Wu, S.; Li, J.S.; Mai, J.; Chang, M.W. Three-dimensional electrohydrodynamic printing and spinning of flexible composite structures for oral multidrug forms. ACS Appl. Mater. Interf. 2018, 10, 24876–24885. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine algae polysaccharides as basis for wound dressings, drug delivery, and tissue engineering: A review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Zhang, L.; Yu, D. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biomater. Sci. Polym. E 2020, 31, 519–548. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, W.; Zhang, F.; Liu, Q.; Ye, J.; Dong, H.; Cao, X. Tannic acid-derived metal-phenolic networks facilitate PCL nanofiber mesh vascularization by promoting the adhesion and spreading of endothelial cells. J. Mater. Chem. B 2018, 6, 2734–2738. [Google Scholar] [CrossRef] [PubMed]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Shams Ardekani, M.R.; Sharifzadeh, M.; Khanavi, M. Preparation of polyurethane/pluronic F127 nanofibers containing peppermint extract loaded gelatin nanoparticles for diabetic wounds healing: Characterization, in Vitro, and in vivo Studies. Evid.-Based Complement. Altern. 2021, 2021, 6646702. [Google Scholar] [CrossRef]
- Agnes Mary, S.; Giri Dev, V.R. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Ind. 2015, 106, 886–895. [Google Scholar] [CrossRef]
- Shie Karizmeh, M.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Biomater. Adv. 2022, 135, 112667. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Poblete, J.; Gajardo, F.; Aguayo, C.; Fernández, K. Graphene oxide/polyethylene glycol aerogel reinforced with grape seed extracts as wound dressing. J. Mater. Sci. 2021, 56, 16082–16096. [Google Scholar] [CrossRef]
- Salami, M.S.; Bahrami, G.; Arkan, E.; Izadi, Z.; Miraghaee, S.; Samadian, H. Co-electrospun nanofibrous mats loaded with bitter gourd (momordica charantia) extract as the wound dressing materials: In vitro and in vivo study. BMC Complement. Med. 2021, 21, 111. [Google Scholar] [CrossRef]
- Parvathi, K.; Krishnan, A.G.; Anitha, A.; Jayakumar, R.; Nair, M.B. Poly(L-lactic acid) nanofibers containing Cissus quadrangularis induced osteogenic differentiation in vitro. Int. J. Biol. Macromol. 2018, 110, 514–521. [Google Scholar] [CrossRef]
- Wang, X.S.; Yang, J.M.; Ding, R.J.; Liu, X.Z.; Jiang, X.B.; Yang, Z.J.; Ling, Z.M.; Hu, T.X.; Wei, F.X. Fabrication of a polylactide-glycolide/poly-ε-caprolactone/dextran/plastrum testudinis extract composite anti-inflammation nanofiber membrane via electrospinning for annulus fibrosus regeneration. J. Biomed. Nanotechnol. 2021, 17, 873–888. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, J.W.; Lee, J.S.; Lee, J.H.; Yoon, T.R.; Kuroyanagi, Y.; Park, M.H.; Kim, H.J. Hyaluronic acid and silver sulfadiazine-impregnated polyurethane foams for wound dressing application. J. Mater. Sci. Mater. M 2002, 13, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chou, P.Y.; Chen, Z.Y.; Chuang, D.C.C.; Hsieh, S.T.; Lin, F.H. An electrospun nerve wrap comprising Bletilla striata polysaccharide with dual function for nerve regeneration and scar prevention. Carbohydr. Polym. 2020, 250, 116981. [Google Scholar] [CrossRef] [PubMed]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf 2021, 27, 100613. [Google Scholar] [CrossRef]
- Nourmohammadi, J.; Hadidi, M.; Nazarpak, M.H.; Mansouri, M.; Hasannasab, M. Physicochemical and antibacterial characterization of nanofibrous wound dressing from silk fibroin-polyvinyl alcohol-Elaeagnus angustifolia extract. Fibers Polym. 2020, 21, 456–464. [Google Scholar] [CrossRef]
- Chan, W.P.; Huang, K.C.; Bai, M.Y. Silk fibroin protein-based nonwoven mats incorporating baicalein Chinese herbal extract: Preparation, characterizations, and in vivo evaluation. J. Biomed. Mater. Res. B 2017, 105, 420–430. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Xu, J.; Wang, J.; Gu, X.; Li, P.; Fan, Y. Three-dimensional magnetic fibrous scaffold with icariin expanded by supercritical CO2 for bone tissue engineering under static magnetic field. Compos. B Eng. 2021, 226, 109304. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Agnes Mary, S.; Ramakrishna, S.; Lakshmi, B.S.; Giri Dev, V.R. Aloe vera incorporated biomimetic nanofibrous scaffold: A regenerative approach for skin tissue engineering. Iran Polym. J. 2014, 23, 237–248. [Google Scholar] [CrossRef]
- Mathiazhagan, S.; Periasamy, V.; Vadivel, A. Ecofriendly antimicrobial Acalypha indica leaf extract immobilized polycaprolactone nanofibrous mat for food package applications. J. Food Process. Preserv. 2021, 45, e15302. [Google Scholar] [CrossRef]
- Han, J.; Zhang, H.T.; Zhu, L.M.; Branford-White, C. Electrospun biodegradable nanofiber mats for controlled release of herbal medicine. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; Volume 1, pp. 1–4. [Google Scholar]
- Kashte, S.; Sharma, R.; Kadam, S. Layer-by-layer decorated herbal cell compatible scaffolds for bone tissue engineering: A synergistic effect of graphene oxide and Cissus quadrangularis. J. Bioact. Compat. Pol. 2020, 35, 57–73. [Google Scholar] [CrossRef]
- Patil, N.A.; Gore, P.M.; Jaya Prakash, N.; Govindaraj, P.; Yadav, R.; Verma, V.; Shanmugarajan, D.; Patil, S.; Kore, A.; Kandasubramanian, B. Needleless electrospun phytochemicals encapsulated nanofibre based 3-ply biodegradable mask for combating COVID-19 pandemic. Chem. Eng. J. 2021, 416, 129152. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Aras, C.; Tümay Özer, E.; Göktalay, G.; Saat, G.; Karaca, E. Evaluation of Nigella sativa oil loaded electrospun polyurethane nanofibrous mat as wound dressing. J. Biomater. Sci. Polym. E 2021, 32, 1718–1735. [Google Scholar] [CrossRef]
- Shahid, M.A.; Ali, A.; Uddin, M.N.; Miah, S.; Islam, S.M.; Mohebbullah, M.; Jamal, M.S.I. Antibacterial wound dressing electrospun nanofibrous material from polyvinyl alcohol, honey and Curcumin longa extract. J. Ind. Text. 2021, 51, 455–469. [Google Scholar] [CrossRef]
- Ali, A.; Shahid, M.A. Polyvinyl alcohol (PVA)–Azadirachta indica (neem) nanofibrous mat for biomedical application: Formation and characterization. J. Polym. Environ. 2019, 27, 2933–2942. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.K.; Ramamoorthy, R.; Muthalagu, M.; Sampath, D.; Sivagnanam, K.; Arumugam, G. Synthesis, characterization, and antimicrobial properties of novel dual drug loaded electrospun mat for wound dressing applications. J. Bioact. Compat. Polym. 2021, 36, 431–443. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, S. Electrospun poly(vinyl alcohol) nanofibrous membranes containing Coptidis Rhizoma extracts for potential biomedical applications. Text. Res. J. 2019, 89, 3506–3518. [Google Scholar] [CrossRef]
- Aghamohamadi, N.; Sanjani, N.S.; Majidi, R.F.; Nasrollahi, S.A. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater. Sci. Eng. C 2019, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos. Hybrid Mater. 2022, 5, 1017–1029. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L.; Ahmed, A. Batch preparation and characterization of electrospun porous polylactic acid-based nanofiber membranes for antibacterial wound dressing. Adv. Fiber Mater. 2022, 4, 832–844. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L.; Ahmed, A. Fabrication and characterization of core-shell electrospun fibrous mats containing medicinal herbs for wound healing and skin tissue engineering. Mar. Drugs 2019, 17, 27. [Google Scholar]
- Mohamed, A.E.; Shetta, A.; Kegere, J.; Mamdouh, W. Antibacterial and antioxidant properties of Cichorium intybus extract embedded in chitosan nanocomposite nanofibers. Int. J. Biol. Macromol. 2022, 215, 387–397. [Google Scholar] [CrossRef]
- Hameed, M.; Rasul, A.; Waqas, M.K.; Saadullah, M.; Aslam, N.; Abbas, G.; Latif, S.; Afzal, H.; Inam, S.; Akhtar Shah, P. Formulation and evaluation of a clove oil-encapsulated nanofiber formulation for effective wound-healing. Molecules 2021, 26, 2491. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Bahrami, S.H.; Nazarpak, M.H.; Solouk, A. Multilayer nanofibrous patch comprising chamomile loaded carboxyethyl chitosan/poly(vinyl alcohol) and polycaprolactone as a potential wound dressing. Int. J. Biol. Macromol. 2020, 147, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Electrospinning of double-layer chitosan-flaxseed mucilage nanofibers for sustained release of Ziziphora clinopodioides essential oil and sesame oil. LWT Food Sci. Technol. 2021, 140, 110812. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Snetkov, P.P.; Sitnikova, V.E.; Uspenskaya, M.V.; Morozkina, S.N.; Olekhnovich, R.O. Hyaluronic acid-curcumin electrospun fibers. Russ. Chem. B 2020, 69, 596–600. [Google Scholar] [CrossRef]
- [Snetkov, P.; Morozkina, S.; Olekhnovich, R.; Vu, T.H.N.; Tyanutova, M.; Uspenskaya, M. Curcumin/usnic acid-loaded electrospun nanofibers based on hyaluronic acid. Materials 2020, 13, 3476. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Fahim, H.; Mahboubi, M. Fabrication and characterization of composite film based on gelatin and electrospun cellulose acetate fibers incorporating essential oil. J. Food Meas. Charact. 2021, 15, 2108–2118. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Luan, P.; Zhao, X.; Copenhaver, K.; Ozcan, S.; Zhu, H. Turning natural herbaceous fibers into advanced materials for sustainability. Adv. Fiber Mater. 2022, 4, 736–757. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/zein/gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef]
- Mirza, S.; Zia, I.; Jolly, R.; Kazmi, S.; Owais, M.; Shakir, M. Synergistic combination of natural bioadhesive bael fruit gum and chitosan/nano-hydroxyapatite: A ternary bioactive nanohybrid for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 215–224. [Google Scholar] [CrossRef]
- Singh, P.; Verma, C.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Preparation of thyme oil loaded κ-carrageenan-polyethylene glycol hydrogel membranes as wound care system. Int. J. Pharm. 2022, 618, 121661. [Google Scholar] [CrossRef] [PubMed]
- Kalachaveedu, M.; Jenifer, P.; Pandian, R.; Arumugam, G. Fabrication and characterization of herbal drug enriched guar galactomannan based nanofibrous mats seeded with GMSC’s for wound healing applications. Int. J. Biol. Macromol. 2020, 148, 737–749. [Google Scholar] [CrossRef]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Asadian, F.; Zareshahrabadi, Z.; Saharkhiz, M.J.; Ahadian, S.; Zomorodian, K. Antimicrobial core-shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 2021, 603, 120698. [Google Scholar] [CrossRef]
- Salazar, D.; Arancibia, M.; Casado, S.; Viteri, A.; López-Caballero, M.E.; Montero, M.P. Green banana (Musa acuminata AAA) wastes to develop an edible film for food applications. Polymers 2021, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, A.; Kikionis, S.; Tagka, A.; Koliarakis, N.; Evangelatou, A.; Papagiannis, P.; Stratigos, A.; Karalis, V.; Dallas, P.; Vitsos, A.; et al. Management of acute radiodermatitis in non-melanoma skin cancer patients using electrospun nanofibrous patches loaded with Pinus halepensis bark extract. Cancers 2021, 13, 2596. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A.C.; Chronakis, I.S. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, X.Y.; Xu, R.; Dong, S.; Wu, M.Y.; Zheng, X.; Lu, W.M.; Li, B. A handy skin wound dressing prepared by alginate and cationic nanofibrillated cellulose derived from solid residues of herbs. Bioresources 2021, 16, 5926–5946. [Google Scholar] [CrossRef]
- Luo, S.; Saadi, A.; Fu, K.; Taxipalati, M.; Deng, L. Fabrication and characterization of dextran/zein hybrid electrospun fibers with tailored properties for controlled release of curcumin. J. Sci. Food Agric. 2021, 101, 6355–6367. [Google Scholar] [CrossRef]
- Ramanathan, G.; Muthukumar, T.; Tirichurapalli Sivagnanam, U. In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur. J. Pharmacol. 2017, 814, 45–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Li, J.; Cui, X.; Jiang, M.; Zhang, M.; Wang, X.; Zhang, W.; Liu, Z. Bilayer membrane composed of mineralized collagen and chitosan cast film coated with berberine-loaded PCL/PVP electrospun nanofiber promotes bone regeneration. Front. Bioeng. Biotech. 2021, 9, 684335. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ezhilarasu, H.; Prasannan, P.; Ong, S.T.; Subramanian, S.; Kamruddin, M.; Lakshminarayanan, R.; Ramakrishna, S.; et al. Poly-ε-caprolactone/gelatin hybrid electrospun composite nanofibrous mats containing ultrasound assisted herbal extract: Antimicrobial and cell proliferation study. Nanomaterials 2019, 9, 462. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.Y.; Hu, C.H.; Huang, W.C.; Ho, C.Y.; Yao, C.H.; Huang, C.H. Effects of bilayer nanofibrous scaffolds containing curcumin/lithospermi radix extract on wound healing in streptozotocin-induced diabetic rats. Polymers 2019, 11, 1745. [Google Scholar] [CrossRef]
- Yao, C.H.; Chen, K.Y.; Chen, Y.S.; Li, S.J.; Huang, C.H. Lithospermi radix extract-containing bilayer nanofiber scaffold for promoting wound healing in a rat model. Mater. Sci. Eng. C 2019, 96, 850–858. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Z.; Guo, C. Chemical modification of silk proteins: Current status and future prospects. Adv. Fiber Mater. 2022, 4, 705–719. [Google Scholar] [CrossRef]
- Yin, L.; Wang, K.; Lv, X.; Sun, R.; Yang, S.; Yang, Y.; Liu, Y.; Liu, J.; Zhou, J.; Yu, Z. The fabrication of an ICA-SF/PLCL nanofibrous membrane by coaxial electrospinning and its effect on bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 8616. [Google Scholar] [CrossRef] [Green Version]
- Göksen, G.; Fabra, M.J.; Ekiz, H.I.; López-Rubio, A. Phytochemical-loaded electrospun nanofibers as novel active edible films: Characterization and antibacterial efficiency in cheese slices. Food Control 2020, 112, 107133. [Google Scholar] [CrossRef]
- Rodriguez, C.; Padilla, V.; Lozano, K.; McDonald, A.; Materon, L.; Chapa, A.; Ahmad, F.; De Leo, C.T.; Gilkerson, R. Fabrication of forcespinning® nanofibers incorporating nopal extract. Polym. Int. 2021, 70, 679–686. [Google Scholar] [CrossRef]
- Saadat, S.; Emam-Djomeh, Z.; Askari, G. Antibacterial and antioxidant gelatin nanofiber scaffold containing ethanol extract of pomegranate peel: Design, characterization and in vitro assay. Food Bioprocess Technol. 2021, 14, 935–944. [Google Scholar] [CrossRef]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J. Tissue Eng. Regen. M 2017, 11, 905–915. [Google Scholar] [CrossRef]
- Tufail, S.; Siddique, M.I.; Sarfraz, M.; Sohail, M.F.; Shahid, M.N.; Omer, M.O.; Haliza, K.; Rasool, F. Simvastatin nanoparticles loaded polymeric film as a potential strategy for diabetic wound healing: In vitro and in vivo evaluation. Curr. Drug Deliv. 2022, 19, 534–546. [Google Scholar]
- Lin, M.; Dai, Y.; Xia, F.; Zhang, X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater. Sci. Eng. C 2021, 119, 111626. [Google Scholar] [CrossRef]
- Saji, V.S. Supramolecular organic nanotubes for drug delivery. Mater. Today Adv. 2022, 14, 100239. [Google Scholar] [CrossRef]
- Dave, K.; Venuganti, V.V.K. Dendritic polymers for dermal drug delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Giram, P.S.; Shitole, A.; Nande, S.S.; Sharma, N.; Garnaik, B. Fast dissolving moxifloxacin hydrochloride antibiotic drug from electrospun Eudragit L-100 nonwoven nanofibrous mats. Mater. Sci. Eng. C 2018, 92, 526–539. [Google Scholar] [CrossRef]
- Eskitoros-Togay, Ş.M.; Bulbul, Y.E.; Tort, S.; Demirtaş Korkmaz, F.; Acartürk, F.; Dilsiz, N. Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int. J. Pharm. 2019, 565, 83–94. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid films prepared from a combination of electrospinning and casting for offering a dual-phase drug release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Köse, D.M.; Ungun, N.; Bayraktar, O. Eggshell membrane based turmeric extract loaded orally disintegrating films. Curr. Drug Deliv. 2022, 19, 547–559. [Google Scholar] [PubMed]
- Amjad, S.; Jafri, A.; Sharma, A.K.; Serajuddin, M. A novel strategy of nanotized herbal drugs and their delivery in the treatment of diabetes: Present status and future prospects. J. Herb. Med. 2019, 17–18, 100279. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Jenifer, P.; Kalachaveedu, M.; Pandian, R. In vivo evaluation of Acalypha indica extract incorporated electrospun guar/PVA nanofibrous mats for use as active wound dressing material. Pharmacogn. Mag. 2021, 17, 882–885. [Google Scholar]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Avci, H.; Gergeroglu, H. Synergistic effects of plant extracts and polymers on structural and antibacterial properties for wound healing. Polym. Bull. 2019, 76, 3709–3731. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Wang, X.; Zhang, M.; Wang, X.; Li, Y.; Cui, Z.; Chen, X.; Han, Y.; Zhao, W. Electrospun multifunctional nanofibrous mats loaded with bioactive anemoside B4 for accelerated wound healing in diabetic mice. Drug Deliv. 2022, 29, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Guleken, Z.; Depciuch, J.; Ege, H.; İlbay, G.; Kalkandelen, C.; Ozbeyli, D.; Bulut, H.; Sener, G.; Tarhan, N.; Erdem Kuruca, S. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochim. Acta A 2021, 254, 119639. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.u.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular endothelial growth factor-capturing aligned electrospun polycaprolactone/gelatin nanofibers promote patellar ligament regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Zhu, J.; Li, W.; Li, D.; Yang, X.; Zhao, W.; Ren, M.; Ren, J.; Mo, X.; et al. Electrospun nanoyarn and exosomes of adipose-derived stem cells for urethral regeneration: Evaluations in vitro and in vivo. Colloids Surfaces B 2022, 209, 112218. [Google Scholar] [CrossRef]
- Sedghi, R.; Sayyari, N.; Shaabani, A.; Niknejad, H.; Tayebi, T. Novel biocompatible zinc-curcumin loaded coaxial nanofibers for bone tissue engineering application. Polymer 2018, 142, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Y.; Zhang, Y.G.; Watts, D.C.; Almassri, H.N.S.; Shao, H.Y.; Yang, X.; Ma, Y.H.; Zhang, D.; Wu, X.H. Electrospun naringin-loaded beaded nanofiber with controlled release property for bone tissue engineering applications. Sci. Adv. Mater. 2019, 11, 1433–1442. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, J.; Liu, L.; Shi, J.; Muhammad, A.; Zhai, X.; Zou, X.; Xiao, J.; Li, Z.; et al. Development of nanofiber indicator with high sensitivity for pork preservation and freshness monitoring. Food Chem. 2022, 381, 132224. [Google Scholar] [CrossRef]
- Pereira, N.R.L.; Lopes, B.; Fagundes, I.V.; de Moraes, F.M.; Morisso, F.D.P.; Parma, G.O.C.; Zepon, K.M.; Magnago, R.F. Bio-packaging based on cellulose acetate from banana pseudostem and containing Butia catarinensis extracts. Int. J. Biol. Macromol. 2022, 194, 32–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, S.; Liu, P.; Yang, X.; Han, J.; Wang, A.; Zhang, J. Healing effects of curcumin nanoparticles in deep tissue injury mouse model. Curr. Drug Deliv. 2021, 18, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Huesca-Urióstegui, K.; García-Valderrama, E.J.; Gutierrez-Uribe, J.A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Nanofiber systems as herbal bioactive compounds carriers: Current applications in healthcare. Pharmaceutics 2022, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An innovative tool to release natural extracts with antimicrobial properties. Pharmaceutics 2021, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Rehman, T.U.; Rehan, Z.A.; Noreen, R.; Iqbal, S.; Batool, S.; Qayyum, M.A.; Ahmed, T.; Farooq, T. Development of polymeric nanofibers blended with extract of neem (Azadirachta indica), for potential biomedical applications. Front. Mater. 2022, 9, 1042304. [Google Scholar] [CrossRef]
- Feng, X.; Hao, J. Identifying new pathways and targets for wound healing and therapeutics from natural sources. Curr. Drug Deliv. 2021, 18, 1064–1084. [Google Scholar] [CrossRef]
- Wang, X.; Wu, B.; Zhang, Y.; Dou, X.; Zhao, C.; Feng, C. Polydopamine-Doped Supramolecular Chiral Hydrogels for Postoperative Tumor Recurrence Inhibition and Simultaneously Enhanced Wound Repair. Acta Biomater. 2022, 153, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xing, C.; Dou, X.; Zhao, Y.; Peng, Y.; Feng, C.; Fang, Y. Chiral Hydrogel Accelerates Re-Epithelization in Chronic Wounds via Mechanoregulation. Adv. Healthc. Mater. 2022, 11, 2201032. [Google Scholar] [CrossRef]
- Wang, X.; Feng, C. Chiral Fiber Supramolecular Hydrogels for Tissue Engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 15, e1847. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, Y.; Qin, S.; Zhang, Y.; Liu, J.; Zhang, J.; Feng, C.; Zhao, W. Fluorine Substitution Tunes the Nanofiber Chirality of Supramolecular Hydrogels to Promote Cell Adhesion and Proliferation. Adv. Fiber Mater. 2023, 5, 1–11. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.G.; Kim, I. Smart L-Borneol-Loaded Hierarchical Hollow Polymer Nanospheres with Antipollution and Antibacterial Capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Deng, C.; Wang, R.; Zhang, H. Protocol for Atmospheric Water Harvesting Using in situ Polymerization Honeycomb Hygroscopic Polymers. STAR Protoc. 2022, 3, 101780. [Google Scholar] [CrossRef]
- Wu, Y.L.; Li, Y.L.; Lv, G.L.; Bu, W.B. Redox Dyshomeostasis Strategy for Tumor Therapy Based on Nanomaterials Chemistry. Chem. Sci. 2022, 13, 2202–2217. [Google Scholar] [CrossRef]
- Chen, L.J.; Jiang, X.W.; Lv, M.; Wang, X.L.; Zhao, P.R.; Zhang, M.; Lv, G.L.; Wu, J.Y.; Liu, Y.Y.; Yang, Y.; et al. Reductive-Damage-Induced Intracellular Maladaptation for Cancer Electronic Interference Therapy. Chem 2022, 8, 866–879. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Chen, Y.; Yuan, K.; He, W.; Yang, S.; Tang, T.; Yu, D.-G. Electrospraying Micro-Nano Structures on Chitosan Composite Coatings for Enhanced Antibacterial Effect. Prog. Org. Coat. 2023, 174, 107310. [Google Scholar] [CrossRef]
- Tang, Z.M.; Wu, S.M.; Zhao, P.R.; Wang, H.; Ni, D.L.; Li, H.Y.; Jiang, X.W.; Wu, Y.L.; Meng, Y.; Yao, Z.W.; et al. Chemical Factory-Guaranteed Enhanced Chemodynamic Therapy for Orthotopic Liver Cancer. Adv. Sci. 2022, 9, 2201232. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Chen, L.; Chen, Y.; Shi, J.; Zhang, Z.; Wang, Y.; Wu, F.; Jiang, X.; Yang, W.; Zhang, L.; et al. Reactive Metal Boride Nanoparticles Trap Lipopolysaccharide and Peptidoglycan for Bacteria-infected Wound Healing. Nat. Commun. 2022, 13, 7353. [Google Scholar] [CrossRef]












| Traditional Herbal Medicine | Component | Effect | Undesirable Properties | Ref. |
|---|---|---|---|---|
| Nigella sativa (seed) | Thymoquinone (Essential Oil), carbohydrates, proteins, alkaloids, vitamins, minerals | Analgesic, anti-inflammatory, anticancer, antibacterial, antifungal, antioxidant, wound healing | Easy to degrade, volatile | [9] |
| Satureja mutica (seed) | Thymol, carvacrol | Antibacterial, antioxidant | Easy to degrade, volatile | [10] |
| Salvia hispanica L. (seed) | Xylose, glucose, methyl glucuronic acid | Nutritional benefits | / | [11] |
| Flax (seed) | Polysaccharides, cyanogenic glycosides, cyclic peptides, linolenic acid, cyclic peptides, alkaloids | Antioxidant, free radical scavenger, antibacterial, anti-inflammatory, chronic disease treatment | Poor physical-mechanical properties, profound brittleness, rapid dissolution rate | [12] |
| Mustard (seed) | Allyl isothiocyanate | Antibacterial, anti-mildew, anti-yeast, food preservation | Strong volatility, heavy smell | [13] |
| Turmeric (root) | Polyphenols (curcumin) | Anti-inflammatory, anti-oxidant, anti-cancer, analgesic, lowering blood pressure, relieving anxiety, angiogenesis, nerve healing | Poor bioavailability, low water solubility, photodegradation, in vivo instability | [14] |
| Rehmannia (root) | Catalpol (iridoid glycoside) | Neuroprotection | / | [15] |
| B. Vulgaris (root) | Polysaccharide (pectin) | Accelerate wound healing | / | [16] |
| Lithospermum erythrorhizon (root) | Naphthoquinone pigments (shikonin, isobutyl-shikonin, β-hydroxyl-isovaleryl-shikonin, α-methyl-n-butyl-shikonin), quinones | Antibacterial, antioxidant, wound healing, anti-inflammatory | / | [17] |
| Blumea balsamifera (stem) | Flavonoids, L-borneol, caryophyllene, Iedol,D-camphor | Antioxidant, antifungal, antibacterial, anti-tumor | Easy to degrade, volatile | [18] |
| Cissus quadrangularis (stem) | Phytogenic isolated steroid | Anti-osteoporosis, anti-microbial, anti-inflammatory | / | [19] |
| Pineapple (stem) | Bromelain | Analgesic, anti-inflammatory | / | [20] |
| Ziziphus jujuba (stem) | Terpenoids, tannins, zizipho, tannic acid, flavonoids | Anti-inflammatory, antibacterial, antioxidant, oral treatment | / | [21] |
| Zingiber cassumunar Roxb (rhizome) | Phenylbutanoids, cyclohexene derivatives, naphthoquinones, vanillin, vanillic acid, veratric acid, terpenoids, β-sitosterol, curcuminoids | Anti-bacterial, anti-inflammatory, relieve musculoskeletal pain | Easy to degrade, volatile | [22] |
| Dioscorea (rhizome) | Mucilage, polysaccharide, starch | Neuroprotection, adipocyte aquaglyceroporin modulation, antioxidant, fungicide, amnesia amelioration activities | / | [2] |
| Agrimonia eupatoria L. (aerial parts) | Tannins, flavonoids, phenolic acids, triterpenoids | anti-inflammatory, antioxidant, antimicrobial, antibiofilm properties | / | [23] |
| Scutellariae (radix) | Flavonoid glycoside baicalin, wogonoside | Anti-inflammatory, antioxidant | Poor water solubility | [24] |
| Aloe vera (leave) | Soluble sugars, anthraquinones, polysaccharides, sterols, amino acids, salicylic acid, vitamins, proteins, minerals | Antibacterial, antiviral, antifungal, anti-inflammatory, antioxidant, immune regulation, hypoglycemic, wound healing | Limited by external factors, lack of electrical sensitivity and mechanical properties | [25] |
| Thyme (leave) | Thymol, carvacrol | Anti-inflammatory, antibacterial, antioxidant, insecticidal, low toxicity | Easy to degrade, volatile | [26] |
| Azadirachta indica (leave) | Azadirachtin | Anti-ulcer, antibacterial, anti-inflammatory, antimalarial, antifungal, antiviral, antioxidant, antimutagenic and anticancer | / | [27] |
| Mikania micrantha (leave) | Deoxymikanolide, scandenolide, dihydroscandenolide, mikanolide, dihydromikanolide, m—methoxy benzoic acid | Antitumor, Antifungal, Cytotoxic, Analgesic, Antibacterial, Antioxidant, Antiviral | / | [28] |
| Falcaria vulgaris (leave) | Carvacrol | Antibacterial, antioxidant | / | [29] |
| Moringa oleifera Lam. (leave) | Quercetin, kampeferol, ascorbic acid (polyphenolic compounds) | Antioxidant properties, retard oxidative stress, and their degenerative effect | Light and oxygen sensitive | [30] |
| Henna (leave) | Lawsone (2-hydroxy-1,4- naphthoquinone) | Antioxidant, analgesic, anti-inflammatory, antibacterial, antifungal, anticancer activities, healing burns | / | [31] |
| Plantago australis Lam. (leave) | Verbascoside | Antiviral, antimicrobial, anti-inflammatory, antiulcer, antidiarrheal | / | [32] |
| Zataria multiflora (leave) | Phenolic compounds (carvacrol, thymol) | Antispasmodic, Anti-Nociceptive, Antioxidant, Anti-Inflammatory, Antifungal, Antibacterial | / | [33] |
| Morinda citrifolia (leave) | Phenols, alkaloids, terpenes, flavonoids, tannins, caprylic acid, L-asperuloside, caproic acid | Anti-bacterial, anti-helminthic, anti-viral, analgesic, anti-fungal, anti-inflammatory, hypotensive, immune-enhancing | / | [34] |
| Clove (bud) | Eugenol, eugenol acetate, β-caryophyllene, β-cartilene | Fungi, antioxidant, analgesic, anesthetic, insecticide | Easy to degrade, volatile | [35] |
| Calendula officinalis (flower) | Phenolic compounds, triterpenoids, steroids, terpenoids, carotenes, fatty acids, essential oils, carbohydrates, quinones, minerals, tocopherols, saponins | Anti-inflammatory, antioxidant, antibacterial, antifungal, antiviral properties, blood clotting activity, immunomodulatory | / | [36] |
| Althea Officinalis (petal) | Pectin, starch, flavonoids, sucrose, phytosterol, tannin, amino acids | Antibacterial, anti-inflammatory | / | [37] |
| Atropa belladonna (fruit) | Tropane alkaloids, atropine, hyoscyamine, scopolamine, anisodamine | Antioxidant, anticancer properties | / | [38] |
| C. carandas (fruit) | Phenolics, flavonoids, vitamin C | Anti-inflammatory, antioxidant, antibacterial | / | [39] |
| Citrullus colocynthis (fruit) | Glycosides, flavonoids, alkaloids, curcurbitacins, colocynth oxides, fatty acids, essential oils | Antioxidant, cytotoxic, antidiabetic, antilipidemic, insecticide, antimicrobial, anti-inflammatory | / | [40] |
| Ganoderma lucidum (spore) | Ganoderma lucidum triterpenoids | Anti-cancer, anti-inflammatory, anti-oxidation, anti-proliferation | Easy to be oxidized | [41] |
| Poria cocos (mushroom) | Poricoic acid A | Prebiotic functions, diuretic, anti-osteoporosis, anti-inflammatory, anti-tumor | / | [42] |
| Cinnamon (bark) | Phenol compound | Antibacterial, anti-tumor, antioxidant | Easy to degrade, volatile | [43] |
| Pinus halepensis (bark) | Anthocyanin, phenolic acid | Antioxidant, antiviral, analgesic, cytotoxic, anti-inflammatory, antibacterial | / | [44] |
| Magnoliae (cortex) | Magnolol | Anti-tumor, anti-inflammatory, antibacterial, antioxidant, antiplatelet, antiarrhythmic | / | [45] |
| Pomegranate (peel) | Tannins, alkaloids, flavonoids, organic acids | Antioxidant, antifungal, antimicrobial | Easy to oxidize, difficult to absorb | [46] |
| Oregano (herb) | Rosmarinic acid (phenolic compounds) | Antioxidant, antibacterial, antifungal, sweating, antispasmodic, analgesic | Easy to degrade, volatile | [47] |
| Oliveria decumbens (plant) | Thymol, carvacrol | Antibacterial, antioxidant | Easy to degrade, volatile | [10] |
| Frankincense (tree) | Monoterpenes, diterpenes, lipophilic pentacyclic triterpene acids, polysaccharides, volatile oil | Anti-inflammatory, treatment of inflammatory diseases | Poor oral bioavailability | [48] |
| Black pepper | Essential oil, limonene | Antibiosis | Easy to degrade, volatile | [49] |
| Wolfberry | Lycium barbarum polysaccharides (polysaccharides), betaine, zeaxanthin, β-carotene, vitamins, amino acids, trace minerals | Anti-aging, anti-tumor, antioxidant and immune regulation, neuroprotection | / | [50] |
| Spinacia oleracea | Multivitamins, minerals, bioflavonoids, chlorophyll, quercetin | Anti-proliferation, anti-oxidation, anti-inflammatory, central nervous system inhibition, liver protection, anti-allergy, anti-γ radiation, anti-osteoporosis | / | [19] |
| Cayenne pepper | Capsaicin (8-Methyl-N-vanillyl-6-nonenamide) | anti-microbial, anti-virulence | / | [4] |
| Honey | Methylglyoxal | Antibacterial, anti-inflammatory, antioxidant | / | [51] |
| Elaeagnus Aangustifolia | β-carboline alkaloids, cardiac glycosides, esters, flavonoids, phenols, phenolic acids, ketones, phenyl ethers, pyrimidines, steroids, terpenes, vitamins | Analgesic, antibacterial, anti-inflammatory, antioxidant, therapeutic for rheumatoid arthritis and osteoarthritis | / | [52] |
| Herba epimedii | Icariin (flavonoids) | Angiogenesis, anti-osteoporosis, anti-inflammation | / | [53] |
| Tripterygium wilfordii Hook F. | Celastrol (quinone methide triterpene) | Antioxidant, anti-inflammatory | / | [54] |
| Salvia miltiorrhiza | Tanshinone, Danshen acid | Antioxidant, anti-inflammatory, anti-hypoxia, anti-arteriosclerosis, anti-apoptosis | Poor water solubility, low bioavailability, instability | [55] |
| Amla oil | Vitamin(A/C), fiber, mineral (potassium, magnesium, calcium) | Antioxidation, enhance bone mineralization | Easy to degrade, volatile | [56] |
| Centella asiatica | Asiatic acid, asiaticoside, madecassic acid, madecassoside(triterpenoid) | Heal wounds, burns, ulcerous abnormalities of the skin, stomach and duodenal ulcers, leprosy, lupus, scleroderma, diseases of the veins | / | [57] |
| Astragalus membranaceus | Polysaccharides, saponins | Anti-inflammatory, immunoregulatory, stimulating cell metabolism, lowering blood sugar, promoting cell proliferation | Poor water solubility | [58] |
| Angelica gigas Nakai | Decursin, decursinol angelate, decursinol | Anti-cancer | Poor water solubility | [59] |
| Ferula gum | resin, essence, gum, free ferulic acid, vanillin | Antibiosis | / | [20] |
| Cirsium Japonicum DC | Total flavonoid, essential oil, lipid, saccharides | Hemostasis, anti-inflammatory, antitumor | / | [60] |
| Quilaja saponaria | Saponin | Growth Promotion, Antimicrobial, Insecticidal | / | [61] |
| Yerba mate | Polyphenol (xanthines, saponin, caffeoyl derivatives) | Antioxidant, cholesterol-lowering, liver, diuretic, regulate blood sugar, stimulate the central nervous system | Easy thermal degradation | [62] |
| Helicid nilgrinica Bedd | Helicid | Treatment of insomnia and headache | Poor water solubility | [63] |
| Technology | Driving Force | Merit | Defect | Ref. |
|---|---|---|---|---|
| Electrospinning Technique | Electrostatic force | Top-down integrated process with small and uniform fiber diameter, high porosity and large surface area | Narrow range of materials, high voltage safety issues, relatively low productivity | [140] |
| Stretch | Stretching force | Simple process, low cost, thin fiber diameter, high crystallinity, good alignment and enhanced tensile strength | Complex equipment, fixed structure, cannot change with the process and material changes | [162] |
| Self-assembly | Evaporation/Van der Waals force | Various functional and active building blocks can be integrated to obtain complex 3D structures with high fault tolerance | For biomolecules, high cost, polydispersity and purification limitations, lack of stability in nonaqueous solutions and at high temperatures | [163] |
| Phase separation | Centrifugal force | Good crystallinity and tensile properties | Centrifugal dispersion operation easily affects morphology | [164] |
| Template synthesis | / | Wide range of materials available, nanomaterials with clearly defined size, shape and structure | Fiber damage during template removal | [165] |
| Melt spraying | High temperature extrusion | Short process flow, high production efficiency, fine and uniform fibers | High energy consumption, poor fiber orientation, low web strength | [166] |
| Freeze drying | Lyophilization | Maintains original chemical composition and physical properties to improve substrate instability | Cost high | [167] |
| Solvent casting | Evaporation drying | Able to prepare ultra-thin films with high optical transparency and rapidly degradable porous films | Organic solvents cause health and environmental problems | [168] |
| Laser ablation | Laser high energy | Green simple process, wide selection | Low yield | [169] |
| Chemical vapor deposition | / | High yield, fiber diameter, crystallinity and orientation can be tailored by precisely controlling the synthesis conditions | Discontinuous short fibers | [170] |
| Blown spinning | Air compression | Can be applied to any surface or substrate to overcome the limitations of electronic spinning on in-situ synthesis and conductive targets | Small surface area and poor morphology of fiber | [171] |
| Carbon Dioxide (CO2) Laser Supersonic Drawing | Supersonic stretching | Uniform diameter, continuous nanofibers, solvent-free, high efficiency | For polymers with good thermoplasticity only | [172] |
| Force spinning technique | Centrifugal force | Increased productivity, increased material selection and reduced fiber costs | Complex structure and heavy load of production equipment | [173] |
| Dry-wet spinning | / | Spinning solution with high viscosity, low solvent recovery and consumption, fast forming speed, uniform fiber structure, high strength and elasticity | After the spinning solution is broken, it is easy to flow along the spinneret | [174] |
| Electrohydrodynamic (EHD) printing | Electrostatic force | Pattern fiber materials by digitally controlled material deposition (usually layer by layer) to create ordered freeform geometries | / | [175] |
| Synthetic Polymer | Doped Polymer | Solvent | Herbal Extract | Electrospinning Technique | Characteristic | Ref. |
|---|---|---|---|---|---|---|
| PCL | / | DCM/DMF | Icariin | Blend | Cell penetration, collagen deposition and angiogenesis with bone regeneration potential | [191] |
| / | Chloroform/Methanol | Aloe vera | Blend | Can support tissue and has a controlled surface morphology and structure to treat different skin conditions or injuries | [192] | |
| / | / | Eugenol | Blend | Promote the production of collagen | [35] | |
| / | DCM/DMF | Acalypha indica leaf extract | Blend/Post-treatment | Microbial inhibition to keep food fresh | [193] | |
| / | DCM/DMF | Shikonin | Blend | Loading and controlling the release of bioactive components has great potential in the treatment of wound healing or atopic dermatitis | [194] | |
| / | THF/Methanol | Cissus quadrangularis extract | Blend/Post-treatment | Enhanced roughness, mechanical properties and wettability of the scaffold to provide osteoinductive properties in vivo | [195] | |
| PLA | / | DCM | Azadirachta indicaing extract/Eucalyptus Citriodora extract | Blend | Biodegradable masks to inhibit bacterial spread for antimicrobial use | [196] |
| PLLA | / | Chloroform/Methanol | Cissus quadrangularis extract | Blend | Enhancing protein absorption and cell viability to induce osteogenic differentiation of MSCs | [184] |
| PLGA | / | HFIP | Aloe vera | Blend | Improved wound closure and re-healing, topical application of high-concentration aloe PLGA-AV nanofibers provides strategy for the treatment of chronic wounds | [197] |
| / | HFIP | Aloe vera | Blend | Promising Strategies for Treating Chronic Wounds | [107] | |
| PU | / | DMF | Ayurveda oil | Blend | Improved surface roughness and anticoagulant properties with excellent physicochemical and blood compatibility | [56] |
| / | DMF | Nigella sativa oil | Blend | No cytotoxicity, increased cell viability, synergistically accelerates wound healing | [198] | |
| PVA | / | Deionized water | Honey/Curcumin | Blend | Shows enhanced water management properties and antimicrobial properties for potential wound dressings and tissue engineering | [199] |
| / | Distilled water | Mikania micrantha extract | Blend | Shows enhanced water management, excellent antibacterial efficiency and biocompatibility | [28] | |
| / | Ethanol/Pure water | Astragalus polysaccharide/Astragaloside IV | Blend | Synergistic effect, better inhibit inflammation, promote collagen deposition and wound re-epithelialization, conducive to diabetic wounds. | [58] | |
| / | distilled water/EtOH | Angelica gigas Nakai | Blend | Improved water solubility and rapid dissolution, higher antiproliferative activity, provide a promising method for the treatment of oral cancer | [59] | |
| / | Water | Azadirachta indica extract | Blend | Enhanced water management and thermal performance for bacterial inhibition | [200] | |
| / | Ethanol/distilled water | Plantago australis Hydroethanolic extract | Blend | Promote cell proliferation and accelerate wound closure | [32] | |
| / | DMSO | Thespesia populnea extract | Blend | Better drug release, extended activity, excellent antibacterial activity without hindering cell growth | [81] | |
| / | / | Cissus quadrangularis extract/Galinsoga parviflora Cav extract | Blend | Sufficient tensile properties and excellent antibacterial activity, cell viability, blood compatibility | [201] | |
| / | double-distilled water | Morinda citrifolia extract | Blend | Nanostructured morphology that interacts effectively with protein GSK3β binding sites to enhance cell proliferation and adhesion | [34] | |
| / | / | Coptidis Rhizoma extract | Blend | High drug loading efficiency and sustained release with high antibacterial activity | [202] | |
| PVP | / | Ethanol | Isatis root extract | Blend | Good air permeability, wettability, antibacterial properties and faster wound healing | [120] |
| / | Ethanol/Deionized water/DMF | Aloe vera/Aloe vera acetate | Blend | Good compatibility, thermal stability, hydrophilicity, tensile strength, this hydrated material is conducive to application in tissue engineering scaffolds | [203] | |
| / | Ethanol/DMAc | Helicide | Tri-fluid side-by-side | Fast, efficient and easy delivery of actives | [204] | |
| PEO | PCL | Ethanol/Glacial acetic acid | Ganoderma lucidum triterpenoids/Methotrexate | Coaxial | Chinese and Western medicine successfully encapsulated and uniformly dispersed in the polymer matrix, with biphasic release characteristics, synergistic inhibition of HeLa cells | [41] |
| EE100 | / | Ethanol | Oregano ethanolic extract | Blend | High stability and sustained release | [47] |
| Nylon66 | / | Formic acid | B.vulgaris extract | Blend | Promote the adhesion, proliferation and differentiation of H-keratin and MSCs into epithelial lineage | [16] |
| Carbomer | PAN | DMF | Ziziphus jujuba extract | Blend | Controlled release, improved anti-inflammatory function, for periodontal disease, improve patient compliance | [21] |
| PHA | / | DCM | Plai oil | Blend | Enhanced mechanical strength, non-toxic and cell-attached, available with the development and design of pain-relieving transdermal patch products | [22] |
| Natural Polymer | Doped Polymer | Solvent | Herbal Extract | Electrospinning Technique | Characteristic | Ref. |
|---|---|---|---|---|---|---|
| Chitosan | / | Glacial acetic acid/Double-distilled water | Honey/Capsaicin extract | Blend | High levels of cell viability and proliferation and better wound closure | [4] |
| Hyaluronic acid | / | Distilled water/DMSO | curcumin/usnic acid | Blend | Preparation of Biocompatible Nanofibers Without Other Carrier Polymers and Catalysts | [213] |
| Cellulose acetate | / | Acetone/DMAc | Asiaticoside | Blend | Greater water retention and low toxicity, with potential for topical/transdermal or wound dressing patches | [57] |
| / | Acetone/DMF | Blumea balsamifera oil | Blend | Biphasic release, good wound permeability, sufficient biocompatibility and high efficacy inhibit bacterial growth | [18] | |
| / | Acetone/DMAc | Asiaticoside/Curcumin | Blend | Antioxidant activity to support fibroblast adhesion and proliferation | [108] | |
| / | Acetone | Acanthus ebracteatus Vahl. extract | Blend | Antioxidant activity, reduced extract cytotoxicity, potentially for local delivery of bioactive components | [82] | |
| / | Acetone/DMF/Water | Quillaja saponin | Blend | Good antifungal activity | [61] | |
| Arabinose | Gelatin/PVA/PVP | Deionized water/Ethanol | Aloe vera/Trachyspermum ammi essential oil | Coaxial | Enhancing wound closure and eliminating bacterial infection at the wound site | [221] |
| Guar gum | PVA | Distilled water | Acalypha indica/Aristolochia bracteolata/Lawsonia inermis/Thespesia populnea | Blend | Enhances wound contraction, fully forms the dermis and epidermis, and has smaller scars by expressing stem cells and wound healing-related genes | [220] |
| Pullulan | Chitosan | Deionized water | Opuntia cochenillifera extract/Cactaceae extract | Blend | Demonstrated water absorption, enhanced thermal stability and good bacterial inhibition and biocompatibility | [236] |
| Sodium alginate | CA/PEO | Acetone/Water | Pinus halepensis bark extract | Blend | Eliminates skin inflammation, reduces patient discomfort and can be used for radiation dermatitis treatment | [224] |
| Dextran | Zein | Acetic acid/Water | Curcumin | Blend | Effective free radical scavenging activity and iron reducing ability, and controlled release behavior required for curcumin delivery | [227] |
| Xanthan gum | Chitosan | Formic acid | Curcumin | Blend | High entrapment efficiency, physical stability in aqueous media and long-term pH-stimulated release | [225] |
| Collagen | Poly(3-hydroxybutyric acid/Gelatin | HFP | Coccinia grandis extract | Blend/Post-treatment | Improved tensile strength and increased wound epithelial regeneration | [228] |
| Gelatin | / | Ethanol/Acetic acid | Crude C. carandas fruits ethanol extract | Blend | It has higher thermal stability and high antioxidant activity, and has application potential in cosmetic masks. | [39] |
| / | Acetic acid/Double-distilled water | Ethanolic pomegranate peel extract | Blend | High encapsulation efficiency and increased bioavailability of bioactive compounds for pharmaceutical or food applications | [237] | |
| / | / | Centella asiatica extract | Blend | Biodegradable, promotes fibroblast proliferation and collagen synthesis, and exhibits antibacterial activity | [238] | |
| Silk fibroin | PEO | Glacial acetic acid | Cirsium Japonicum DC | Blend | Chinese herbal medicine ingredients significantly improve cell compatibility and hemostatic performance of nanofiber matrix | [60] |
| Zein | / | Glacial acetic acid/Ethanol | Laurus nobilis essential oil/Rosmarinus officinalis essential oil | Blend | Prominent antibacterial activity, more sustained release, suitable for edible active food packaging | [235] |
| Keratin | PCL/PEO/CS | Formic acid/Acetic acid | Aloe vera extracts | Coaxial | Increased tensile strength, showing biocompatibility and improved adhesion | [206] |
| Drug Plant | Families Genera | Extraction Method | Use Ingredients | Inhibiting Flora | Ref. |
|---|---|---|---|---|---|
| Aloe vera | Liliaceae | / | Aloe gel | Staphylococcus aureus/Staphylococcus epidermidis | [206] |
| Curcuma longa L. | Turmericaceae | Acid hydrolysis | Curcumin | Bacillus cereus/Escherichia coli/Salmonella typhimurium/Staphylococcus aureus | [14] |
| Azadirachta indica | Meliaceae | Methanol, Acetyl and Water | Azadirachtin | Staphylococcus aureus | [200] |
| Calendula officinalis | Composite family | Ethyl alcohol | / | Staphylococcus aureus/Escherichia coli | [36] |
| Centella asiatica | Umbelliferae | / | Asiaticoside | / | [57] |
| Lawsonia inermis (Henna) | Lythraceae | Water/ethanol | Lawsone | Staphylococcus aureus/Escherichia coli | [223] |
| Zataria multiflora | Lamiaceae | / | / | Candida albicans/Pseudomonas aeruginosa/Staphylococcus aureus | [33] |
| Halepensis | Coniferous species | Water | / | / | [44] |
| Honeyor propolis | / | / | / | Staphylococcus aureus/Escherichia coli/Pseudomonas aeruginosa/Candida albicans | [51] |
| Lithospermum erythrorhizon (Lithospermi radix) | Borage family | Methanol | Shikonin | Staphylococcus aureus/Escherichia coli | [231] |
| Nigella sativa | Ranunculaceae | Cold pressing | / | Staphylococcus aureus/Escherichia coli | [9] |
| Clove | Olive family | / | / | Escherichia coli/Staphylococcus aureus, and Pseudomonas aeruginosa | [208] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Bai, Y.; Huang, C.; Wang, Y.; Ji, Y.; Du, Y.; Xu, L.; Yu, D.-G.; Bligh, S.W.A. Recent Progress of Electrospun Herbal Medicine Nanofibers. Biomolecules 2023, 13, 184. https://doi.org/10.3390/biom13010184
Liu H, Bai Y, Huang C, Wang Y, Ji Y, Du Y, Xu L, Yu D-G, Bligh SWA. Recent Progress of Electrospun Herbal Medicine Nanofibers. Biomolecules. 2023; 13(1):184. https://doi.org/10.3390/biom13010184
Chicago/Turabian StyleLiu, Hang, Yubin Bai, Chang Huang, Ying Wang, Yuexin Ji, Yutong Du, Lin Xu, Deng-Guang Yu, and Sim Wan Annie Bligh. 2023. "Recent Progress of Electrospun Herbal Medicine Nanofibers" Biomolecules 13, no. 1: 184. https://doi.org/10.3390/biom13010184