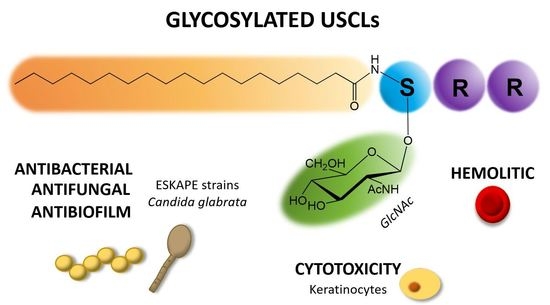
Glycosylated Lipopeptides—Synthesis and Evaluation of Antimicrobial Activity and Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of Fmoc-Ser(ß-d-GlcNAc(Ac)3)-OH
2.1.2. Synthesis of Lipopeptides
2.1.3. Removal of Acetyl-protecting Groups of Glucosamine Residues in Lipopeptides
2.1.4. Purification of Lipopeptides and Glycolipopeptides
2.1.5. Determination of Peptide Hydrophobicity with RP-HPLC
2.2. Antimicrobial Activity
2.2.1. Cultivation of Microorganisms
2.2.2. Activity against Planktonic Cultures
2.2.3. Activity against Biofilm
2.3. Hemolytic Activity
2.4. MTT Assay
3. Results and Discussion
3.1. Synthesis
3.2. Antimicrobial Activity against Planktonic Cultures
3.3. Antimicrobial Activity against Biofilm
3.4. Hemolysis of Human Erythrocytes
3.5. Cytotoxicity against HaCaT Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency Antimicrobial Resistance. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/antimicrobial-resistance (accessed on 21 November 2022).
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 21 November 2022).
- Greber, K.E.; Dawgul, M.; Kamysz, W.; Sawicki, W. Cationic net charge and counter ion type as antimicrobial activity determinant factors of short lipopeptides. Front. Microbiol. 2017, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findlay, B.; Szelemej, P.; Zhanel, G.G.; Schweizer, F. Guanidylation and Tail Effects in Cationic Antimicrobial Lipopeptoids. PLoS ONE 2012, 7, e41141. [Google Scholar] [CrossRef] [PubMed]
- Dawgul, M.A.; Greber, K.E.; Bartoszewska, S.; Baranska-Rybak, W.; Sawicki, W.; Kamysz, W. In Vitro Evaluation of Cytotoxicity and Permeation Study on Lysine- and Arginine-Based Lipopeptides with Proven Antimicrobial Activity. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makovitzki, A.; Baram, J.; Shai, Y. Antimicrobial Lipopolypeptides Composed of Palmitoyl Di- and Tricationic Peptides: In Vitro and in Vivo Activities, Self-Assembly to Nanostructures, and a Plausible Mode of Action. Biochemistry 2008, 47, 10630–10636. [Google Scholar] [CrossRef]
- Bulet, P.; Dimarcq, J.L.; Hetru, C.; Lagueux, M.; Charlet, M.; Hegy, G.; Van Dorsselaer, A.; Hoffmann, J.A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J. Biol. Chem. 1993, 268, 14893–14897. [Google Scholar] [CrossRef]
- Talat, S.; Thiruvikraman, M.; Kumari, S.; Kaur, K.J. Glycosylated analogs of formaecin I and drosocin exhibit differential pattern of antibacterial activity. Glycoconj. J. 2011, 28, 537–555. [Google Scholar] [CrossRef]
- Thomas, X.; Destoumieux-Garzón, D.; Peduzzi, J.; Afonso, C.; Blond, A.; Birlirakis, N.; Goulard, C.; Dubost, L.; Thai, R.; Tabet, J.-C.; et al. Siderophore Peptide, a New Type of Post-translationally Modified Antibacterial Peptide with Potent Activity. J. Biol. Chem. 2004, 279, 28233–28242. [Google Scholar] [CrossRef] [Green Version]
- Oman, T.J.; Boettcher, J.M.; Wang, H.; Okalibe, X.N.; van der Donk, W.A. Sublancin is not a lantibiotic but an S-linked glycopeptide. Nat. Chem. Biol. 2011, 7, 78–80. [Google Scholar] [CrossRef]
- Mackintosh, J.A.; Veal, D.A.; Beattie, A.J.; Gooley, A.A. Isolation from an Ant Myrmecia gulosa of Two Inducible O-Glycosylated Proline-rich Antibacterial Peptides. J. Biol. Chem. 1998, 273, 6139–6143. [Google Scholar] [CrossRef] [Green Version]
- Cudic, M.; Bulet, P.; Hoffmann, R.; Craik, D.J.; Otvos, L. Chemical synthesis, antibacterial activity and conformation of diptericin, an 82-mer peptide originally isolated from insects. Eur. J. Biochem. 1999, 266, 549–558. [Google Scholar] [CrossRef]
- Stepper, J.; Shastri, S.; Loo, T.S.; Preston, J.C.; Novak, P.; Man, P.; Moore, C.H.; Havlíček, V.; Patchett, M.L.; Norris, G.E. Cysteine S-glycosylation, a new post-translational modification found in glycopeptide bacteriocins. FEBS Lett. 2011, 585, 645–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarska, N.G.; Wren, B.W.; Willcocks, S.J. The importance of the glycosylation of antimicrobial peptides: Natural and synthetic approaches. Drug Discov. Today 2017, 22, 919–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beuth, J.; Ko, H.L.; Uhlenbruck, G.; Pulverer, G. Lectin-Mediated bacterial adhesion to human tissue. Eur. J. Clin. Microbiol. 1987, 6, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Scheuerl, S.L.; Kartha, K.P.R.; Field, R.A. Practical synthesis of the 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucosides of Fmoc-serine and Fmoc-threonine and their benzyl esters. Carbohydr. Res. 2003, 338, 1039–1043. [Google Scholar] [CrossRef]
- Horton, D. 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-glucopyranosyl Chloride. In General Carbohydrate Method; Elsevier: Amsterdam, The Netherlands, 1972; Volume 5, pp. 282–285. [Google Scholar]
- Jensen, K.J.; Hansen, P.R.; Venugopal, D.; Barany, G. Synthesis of 2-Acetamido-2-deoxy-β-D-glucopyranose-O-Glycopeptides from N-Dithiasuccinoyl-Protected Derivatives. J. Am. Chem. Soc. 1996, 118, 3148–3155. [Google Scholar] [CrossRef]
- Fothergill, A.W. Antifungal Susceptibility Testing: Clinical Laboratory and Standards Institute (CLSI) Methods. In Interactions of Yeasts, Moulds, and Antifungal Agents; Hall, G.S., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 65–74. ISBN 978-1-58829-847-8. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria That Grow Aerobically; Approved Standard—Ninth edition, 9th ed.; CLSI: Wayne, PA, USA, 2012; Volume 32, ISBN 1562387839. [Google Scholar]
- Neubauer, D.; Jaśkiewicz, M.; Sikorska, E.; Bartoszewska, S.; Bauer, M.; Kapusta, M.; Narajczyk, M.; Kamysz, W. Effect of Disulfide Cyclization of Ultrashort Cationic Lipopeptides on Antimicrobial Activity and Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 7208. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Gołacki, K.; Kamysz, W. Ultrashort Cationic Lipopeptides–Effect of N-Terminal Amino Acid and Fatty Acid Type on Antimicrobial Activity and Hemolysis. Molecules 2020, 25, 257. [Google Scholar] [CrossRef] [Green Version]
- Avrahami, D.; Shai, Y. A New Group of Antifungal and Antibacterial Lipopeptides Derived from Non-membrane Active Peptides Conjugated to Palmitic Acid. J. Biol. Chem. 2004, 279, 12277–12285. [Google Scholar] [CrossRef] [Green Version]
- Kishk, S.M.; McLean, K.J.; Sood, S.; Smith, D.; Evans, J.W.D.; Helal, M.A.; Gomaa, M.S.; Salama, I.; Mostafa, S.M.; de Carvalho, L.P.S.; et al. Design and Synthesis of Imidazole and Triazole Pyrazoles as Mycobacterium Tuberculosis CYP121A1 Inhibitors. ChemistryOpen 2019, 8, 995–1011. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Olejniczak-Kęder, A.; Sikorska, E.; Sikora, K.; Kamysz, W. Biological and Physico-Chemical Characteristics of Arginine-Rich Peptide Gemini Surfactants with Lysine and Cystine Spacers. Int. J. Mol. Sci. 2021, 22, 3299. [Google Scholar] [CrossRef]
- Maciejewska, M.; Bauer, M.; Neubauer, D.; Kamysz, W.; Dawgul, M. Influence of Amphibian Antimicrobial Peptides and Short Lipopeptides on Bacterial Biofilms Formed on Contact Lenses. Materials 2016, 9, 873. [Google Scholar] [CrossRef] [Green Version]
- Kamysz, E.; Sikorska, E.; Jaśkiewicz, M.; Bauer, M.; Neubauer, D.; Bartoszewska, S.; Barańska-Rybak, W.; Kamysz, W. Lipidated Analogs of the LL-37-Derived Peptide Fragment KR12—Structural Analysis, Surface-Active Properties and Antimicrobial Activity. Int. J. Mol. Sci. 2020, 21, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondejewski, L.H.; Jelokhani-Niaraki, M.; Farmer, S.W.; Lix, B.; Kay, C.M.; Sykes, B.D.; Hancock, R.E.W.; Hodges, R.S. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J. Biol. Chem. 1999, 274, 13181–13192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of Peptide Hydrophobicity in the Mechanism of Action of α-Helical Antimicrobial Peptides. Antimicrob. Agents Chemother. 2007, 51, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavano, L.; Infante, M.R.; Riya, M.A.; Pinazo, A.; Vinardell, M.P.; Mitjans, M.; Manresa, M.A.; Perez, L. Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter 2012, 9, 306–319. [Google Scholar] [CrossRef]
- Grimsey, E.; Collis, D.W.P.; Mikut, R.; Hilpert, K. The effect of lipidation and glycosylation on short cationic antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183195. [Google Scholar] [CrossRef]





| Peptide | Sequence | MS Analysis | Adjusted Retention Time (min) | ||
|---|---|---|---|---|---|
| z a | m/z b | m/z c | |||
| 1 | C14-SRR-NH2 | 1 2 | 627.47 314.24 | 627.64 314.50 | 16.55 |
| 2 | C14-RSR-NH2 | 1 2 | 627.47 314.24 | 627.68 314.57 | 15.96 |
| 3 | C14-RRS-NH2 | 1 2 | 627.47 314.24 | 627.64 314.55 | 15.91 |
| 4 | C16-SRR-NH2 | 1 2 | 655.50 328.25 | 655.68 328.50 | 20.55 |
| 5 | C16-RSR-NH2 | 1 2 | 655.50 328.25 | 655.71 328.65 | 19.93 |
| 6 | C16-RRS-NH2 | 1 2 | 655.50 328.25 | 655.7 328.59 | 19.89 |
| 7 | C18-SRR-NH2 | 1 2 | 683.52 342.27 | 683.73 342.66 | 24.78 |
| 8 | C18-RSR-NH2 | 1 2 | 683.52 342.27 | 683.75 342.61 | 24.13 |
| 9 | C18-RRS-NH2 | 1 2 | 683.52 342.27 | 683.72 342.6 | 24.04 |
| 10 | C14-S(GlcNAc)RR-NH2 | 1 2 | 830.55 415.78 | 830.72 416.17 | 14.47 |
| 11 | C14-RS(GlcNAc)R-NH2 | 1 2 | 830.55 415.78 | 830.79 416.22 | 14.82 |
| 12 | C14-RRS(GlcNAc)-NH2 | 1 2 | 830.55 415.78 | 830.80 416.25 | 15.01 |
| 13 | C16-S(GlcNAc)RR-NH2 | 1 2 | 858.58 429.79 | 858.80 430.06 | 18.26 |
| 14 | C16-RS(GlcNAc)R-NH2 | 1 2 | 858.58 429.79 | 858.78 430.28 | 18.63 |
| 15 | C16-RRS(GlcNAc)-NH2 | 1 2 | 858.58 429.79 | 858.82 430.34 | 18.80 |
| 16 | C18-S(GlcNAc)RR-NH2 | 1 2 | 886.61 443.80 | 886.73 443.23 | 22.12 |
| 17 | C18-RS(GlcNAc)R-NH2 | 1 2 | 886.61 443.80 | 886.81 444.27 | 22.50 |
| 18 | C18-RRS(GlcNAc)-NH2 | 1 2 | 886.61 443.80 | 886.81 444.09 | 22.67 |
| Peptide | Fatty Acid | E. faecium ATCC 700221 | S. aureus ATCC 33591 | K. pneumoniae ATCC 700603 | A. baumannii ATCC BAA 1605 | P. aeruginosa ATCC 9027 | K. aerogenes ATCC 13048 | C. glabrata ATCC 15126 |
|---|---|---|---|---|---|---|---|---|
| Unmodified | ||||||||
| 1 | C14 | 16 * | 16 * | 128 * | 32 * | 32 * | 128 * | 32 |
| 2 | 8 | 8 | 512 | 64 | 64 | 512 | 32 | |
| 3 | 8 | 16 | 512 | 64 | 64 | 512 | 32 | |
| 4 | C16 | 8 | 8 | 512 | 32 | 16 | 16 | 4 |
| 5 | 8 | 8 | 64 | 32 | 64 | 32 | 8 | |
| 6 | 8 | 8 | 32 | 32 | 16 | 64 | 8 | |
| 7 | C18 | 8 | 16 | 16 | 32 | 64 | 16 | 4 |
| 8 | 8 | 16 | 32 | 64 | 64 | 32 | 4 | |
| 9 | 8 | 8 | 16 | 32 | 64 | 32 | 4 | |
| Glycosylated | ||||||||
| 10 | C14 | 32 | 64 | >512 | 128 | 256 | >512 | 256 |
| 11 | 32 | 64 | >512 | 64 | 128 | >512 | 128 | |
| 12 | 16 | 32 | >512 | 64 | 256 | >512 | 128 | |
| 13 | C16 | 16 | 16 | 256 | 32 | 32 | 512 | 32 |
| 14 | 16 | 16 | 128 | 32 | 32 | 64 | 16 | |
| 15 | 8 | 16 | 256 | 32 | 64 | 256 | 16 | |
| 16 | C18 | 16 | 16 | 64 | 16 | 32 | 32 | 4 |
| 17 | 8 | 16 | 16 | 16 | 32 | 32 | 4 | |
| 18 | 8 | 16 | 32 | 32 | 32 | 32 | 4 | |
| Peptide | Acid | S. aureus | K. pneumoniae | A. baumannii | P. aeruginosa | C. glabrata |
|---|---|---|---|---|---|---|
| Unmodified | ||||||
| 7 | C18 | 512 | 512 | 512 | 512 | 128 |
| 8 | 256 | 512 | 512 | >512 | 128 | |
| 9 | 256 | 512 | 512 | >512 | 128 | |
| Glycosylated | ||||||
| 16 | C18 | 256 | 256 | 512 | 512 | 64 |
| 17 | 128 | 256 | 512 | >512 | 64 | |
| 18 | 128 | 128 | 256 | 512 | 128 | |
| Peptide | Acid | HC50 | Selectivity Index (SI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E. faecium | S. aureus | K. pneumoniae | A. baumannii | P. aeruginosa | K. aerogenes | C. glabrata | |||
| Unmodified | |||||||||
| 1 | C14 | 41.54 ± 1.29 * | 2.60 | 2.60 | 0.32 | 1.30 | 1.30 | 0.32 | 1.30 |
| 2 | 186.64 ± 2.14 | 23.33 | 23.33 | 0.36 | 2.92 | 2.92 | 0.36 | 5.83 | |
| 3 | 227.01 ± 5.69 | 28.38 | 14.19 | 0.44 | 3.55 | 3.55 | 0.44 | 7.09 | |
| 4 | C16 | 75.46 ± 0.73 | 9.43 | 9.43 | 0.15 | 2.36 | 4.72 | 4.72 | 18.87 |
| 5 | 82.21 ± 2.07 | 10.28 | 10.28 | 1.28 | 2.57 | 1.28 | 2.57 | 10.28 | |
| 6 | 75.95 ± 1.57 | 9.49 | 9.49 | 2.37 | 2.37 | 4.75 | 1.19 | 9.49 | |
| 7 | C18 | 82.31 ± 1.36 | 10.29 | 5.14 | 5.14 | 2.57 | 1.29 | 5.14 | 20.58 |
| 8 | 335.12 ± 73.39 | 41.89 | 20.95 | 10.47 | 5.24 | 5.24 | 10.47 | 83.78 | |
| 9 | 94.31 ± 2.64 | 11.79 | 11.79 | 5.89 | 2.95 | 1.47 | 2.95 | 23.58 | |
| Glycosylated | |||||||||
| 10 | C14 | >512 | >16.00 | >8.00 | - | 4.00 | >2.00 | - | >2.00 |
| 11 | 364.17 ± 105.10 | 11.38 | 5.69 | - | 5.69 | 2.85 | - | 2.85 | |
| 12 | 345.72 ± 51.94 | 21.61 | 10.80 | - | 5.40 | 1.35 | - | 2.70 | |
| 13 | C16 | 95.39 ± 2.40 | 5.96 | 5.96 | 0.37 | 2.98 | 2.98 | 0.19 | 2.98 |
| 14 | 73.34 ± 1.63 | 4.58 | 4.58 | 0.57 | 2.29 | 2.29 | 1.15 | 4.58 | |
| 15 | 111.45 ± 11.65 | 13.93 | 6.97 | 0.44 | 3.48 | 1.74 | 0.44 | 6.97 | |
| 16 | C18 | 77.45 ± 1.13 | 4.84 | 4.84 | 1.21 | 4.84 | 2.42 | 2.42 | 19.36 |
| 17 | 45.25 ± 0.59 | 5.66 | 2.83 | 2.83 | 2.83 | 1.41 | 1.41 | 11.31 | |
| 18 | 48.04 ± 2.45 | 6.01 | 3.00 | 1.50 | 1.50 | 1.50 | 1.50 | 12.01 | |
| Peptide | Acid | IC50 | Selectivity Index (SI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E. faecium | S. aureus | K. pneumoniae | A. baumannii | P. aeruginosa | K. aerogenes | C. glabrata | |||
| Unmodified | |||||||||
| 1 | C14 | 1.41 ± 0.29 | 0.09 | 0.09 | 0.01 | 0.04 | 0.04 | 0.01 | 0.04 |
| 2 | 14.98 ± 2.05 | 1.87 | 1.87 | 0.03 | 0.23 | 0.23 | 0.03 | 0.47 | |
| 3 | 14.27 ± 1.96 | 1.78 | 0.89 | 0.03 | 0.22 | 0.22 | 0.03 | 0.45 | |
| 4 | C16 | 4.02 ± 1.17 | 0.50 | 0.50 | 0.01 | 0.13 | 0.25 | 0.25 | 1.01 |
| 5 | 5.72± 2.27 | 0.72 | 0.72 | 0.09 | 0.18 | 0.09 | 0.18 | 0.72 | |
| 6 | 7.84 ± 1.44 | 0.98 | 0.98 | 0.25 | 0.25 | 0.49 | 0.12 | 0.98 | |
| 7 | C18 | 8.28 ± 1.18 | 1.04 | 0.52 | 0.52 | 0.26 | 0.13 | 0.52 | 2.07 |
| 8 | 7.97 ± 1.51 | 1.00 | 0.50 | 0.25 | 0.12 | 0.12 | 0.25 | 1.99 | |
| 9 | 10.68 ± 1.61 | 1.34 | 1.34 | 0.67 | 0.33 | 0.17 | 0.33 | 2.67 | |
| Glycosylated | |||||||||
| 10 | C14 | 32.12 ± 6.26 | 1.00 | 0.50 | - | 0.25 | 0.13 | - | 0.13 |
| 11 | 25.16 ± 3.47 | 0.79 | 0.39 | - | 0.39 | 0.20 | - | 0.20 | |
| 12 | 20.46 ± 3.05 | 1.28 | 0.64 | - | 0.32 | 0.08 | - | 0.16 | |
| 13 | C16 | 10.61 ± 2.04 | 0.66 | 0.66 | 0.04 | 0.33 | 0.33 | 0.02 | 0.33 |
| 14 | 14.08 ± 3.48 | 0.88 | 0.88 | 0.11 | 0.44 | 0.44 | 0.22 | 0.88 | |
| 15 | 5.16 ± 1.32 | 0.65 | 0.32 | 0.02 | 0.16 | 0.08 | 0.02 | 0.32 | |
| 16 | C18 | 10.12 ± 0.66 | 0.63 | 0.63 | 0.16 | 0.63 | 0.32 | 0.32 | 2.53 |
| 17 | 9.45 ± 0.89 | 1.18 | 0.59 | 0.59 | 0.59 | 0.30 | 0.30 | 2.36 | |
| 18 | 13.85 ± 1.71 | 1.73 | 0.87 | 0.43 | 0.43 | 0.43 | 0.43 | 3.46 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, K.; Bauer, M.; Bartoszewska, S.; Neubauer, D.; Kamysz, W. Glycosylated Lipopeptides—Synthesis and Evaluation of Antimicrobial Activity and Cytotoxicity. Biomolecules 2023, 13, 172. https://doi.org/10.3390/biom13010172
Sikora K, Bauer M, Bartoszewska S, Neubauer D, Kamysz W. Glycosylated Lipopeptides—Synthesis and Evaluation of Antimicrobial Activity and Cytotoxicity. Biomolecules. 2023; 13(1):172. https://doi.org/10.3390/biom13010172
Chicago/Turabian StyleSikora, Karol, Marta Bauer, Sylwia Bartoszewska, Damian Neubauer, and Wojciech Kamysz. 2023. "Glycosylated Lipopeptides—Synthesis and Evaluation of Antimicrobial Activity and Cytotoxicity" Biomolecules 13, no. 1: 172. https://doi.org/10.3390/biom13010172