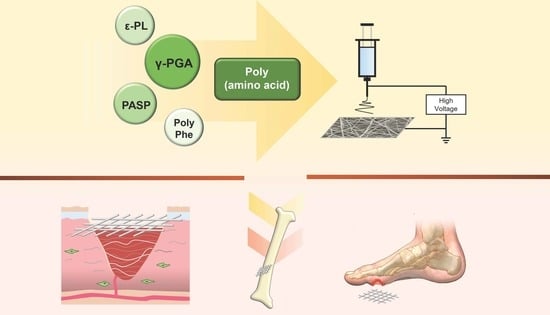
A Review on Electrospun Poly(amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing
Abstract
:1. Introduction
2. Electrospinning Technology
2.1. Introduction to Electrospinning Technology
2.2. Electrospinning Classification
2.2.1. Single Fluid Electrospinning
2.2.2. Double Fluid Electrospinning
2.2.3. Multi-Fluid Electrospinning

2.3. Electrospinning Influence Factors
3. Amino Acid
3.1. Introduction to Amino Acids
3.2. Polar Amino Acids
3.2.1. Basic Amino Acid
3.2.2. Acidic Amino Acid

3.2.3. Neutral Amino Acid
3.3. Nonpolar Amino Acids
4. Application
4.1. Application in Hemostasis and Wound Healing
4.2. Application in Special Trauma Repair

5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Liu, W.; Li, N.; Wang, M.; Liang, B.; Ullah, I.; Neve, A.L.; Feng, Y.; Chen, H.; Shi, C. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater. Sci. 2017, 5, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.M.; Mattheij, N.J.A.; Cosemans, J.M.E.M. Platelet-based coagulation: Different populations, different functions: Platelet-based coagulation. J. Thromb. Haemost. 2013, 11, 2–16. [Google Scholar] [CrossRef]
- Kubíčková, J.; Medek, T.; Husby, J.; Matonohová, J.; Vágnerová, H.; Marholdová, L.; Velebný, V.; Chmelař, J. Nonwoven textiles from hyaluronan for wound healing applications. Biomolecules 2022, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.B.; Conway, W.; Tschabrunn, C.M.; Mehta, M.; Perez-Cuevas, M.B.; Zhang, S.; Hammond, P.T. Clotting mimicry from robust hemostatic bandages based on self-assembling peptides. ACS Nano 2015, 9, 9394–9406. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in Hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. Platelets in atherothrombosis. Nat. Med. 2003, 8, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Maqsood, Z.; Iqubal, M.K.; Ali, J.; Baboota, S. Compendium of conventional and targeted drug delivery formulation used for the treatment and management of the wound healing. Curr. Drug Deliv. 2022, 19, 192–211. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Kumar, R.; Wong, C.C.; Reddy, D.N.K.; Huang, F.Y. Synthesis and characterization of curcumin incorporated multi component nano-scaffold with enhanced anti-bacterial and wound healing properties. Curr. Drug Deliv. 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, S.; Liu, P.; Yang, X.; Han, J.; Wang, A.; Zhang, J. Healing effects of curcumin nanoparticles in deep tissue injury mouse model. Curr. Drug Deliv. 2021, 18, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Tufail, S.; Siddique, M.I.; Sarfraz, M.; Sohail, M.F.; Shahid, M.N.; Omer, M.O.; Haliza, K.; Rasool, F. Simvastatin nanoparticles loaded polymeric film as a potential strategy for diabetic wound healing: In vitro and in vivo evaluation. Curr. Drug Deliv. 2022, 19, 534–546. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.; Mo, X.; Kenawy, E.R. Metronidazole topically immobilized electrospun nanofibrous scaffold: Novel secondary intention wound healing accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef]
- Gul, A.; Gallus, I.; Tegginamath, A.; Maryska, J.; Yalcinkaya, F. Electrospun antibacterial nanomaterials for wound dressings applications. Membranes 2021, 11, 908. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular endothelial growth factor-capturing aligned electrospun polycaprolactone/gelatin nanofibers promote patellar ligament regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Kant, V.; Kumari, P.; Jitendra, D.K.; Ahuja, M.; Kumar, V. Nanomaterials of natural bioactive compounds for wound healing: Novel drug delivery approach. Curr. Drug Deliv. 2021, 18, 1406–1425. [Google Scholar] [CrossRef]
- Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The development of hyaluronic acids used for skin tissue regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun asymmetric membranes as promising wound dressings: A review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Ficai, D.; Ficai, A.; Mihailescu, N.; Andronescu, E.; Turculet, C.F. Nanostructured fibers containing natural or synthetic bioactive compounds in wound dressing applications. Materials 2020, 13, 2407. [Google Scholar] [CrossRef] [PubMed]
- Köse, M.D.; Ungun, N.; Bayraktar, O. Eggshell membrane based turmeric extract loaded orally disintegrating films. Curr. Drug Deliv. 2022, 19, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Minhas, M.U.; Ahmad, S.; Khan, K.U.; Sohail, M.; Abdullah, O.; Khalid, I.; Malik, N.S. Synthesis and evaluation of polyethylene glycol-4000-co-poly (AMPS) based hydrogel membranes for controlled release of mupirocin for efficient wound healing. Curr. Drug Deliv. 2022, 19. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.; Yu, D.G. Electrospun hybrid films for fast and convenient delivery of active herb extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef]
- Dziemidowicz, K.; Sang, Q.; Wu, J.; Zhang, Z.; Zhou, F.; Lagaron, J.M.; Mo, X.; Parker, G.J.M.; Yu, D.G.; Zhu, L.M.; et al. Electrospinning for healthcare: Recent advancements. J. Mater. Chem. B 2021, 9, 939–951. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun core–shell fibrous 2D scaffold with biocompatible poly(glycerol sebacate) and poly-l-lactic acid for wound healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid films prepared from a combination of electrospinning and casting for offering a dual-phase drug release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
- Fusaro, L.; Gualandi, C.; Antonioli, D.; Soccio, M.; Liguori, A.; Laus, M.; Nadia, L.; Boccafoschi, F.; Focarete, M.L. Elastomeric electrospun scaffolds of a biodegradable aliphatic copolyester containing PEG-like sequences for dynamic culture of human endothelial cells. Biomolecules 2020, 10, 1620. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Wu, M.; Yu, D.G. Nanofibers-based food packaging. ES Food Agrofor. 2021, 7, 1–24. [Google Scholar] [CrossRef]
- Safonova, L.; Bobrova, M.; Efimov, A.; Davydova, L.; Tenchurin, T.; Bogush, V.; Agapova, O.; Agapov, I. Silk fibroin/spidroin electrospun scaffolds for full-thickness skin wound healing in rats. Pharmaceutics 2021, 13, 1704. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Li, D.; Xu, G.; Yin, D.; Xiao, Y.; Chen, X.; Zhu, X.; Shi, X. Negative isolation of circulating tumor cells using a microfluidic platform integrated with streptavidin-functionalized PLGA nanofibers. Adv. Fiber Mater. 2021, 3, 192–202. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, B.; Xu, L.; Dang, F.; Li, B.W. Enhanced microwave absorption performance in an ultralight porous single-atom Co–N–C absorber. Adv. Compos. Hybrid Mater. 2021, 4, 1292–1301. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Jiang, D.; Pei, Y.; Wang, Z.; Zhou, X.; Wu, J.; Mo, X.; Wang, H. Delivery of mRNA vaccines and anti-PDL1 siRNA through non-invasive transcutaneous route effectively inhibits tumor growth. Compos. B Eng. 2022, 233, 109648. [Google Scholar] [CrossRef]
- Jiang, S.; Schmalz, H.; Agarwal, S.; Greiner, A. Electrospinning of ABS nanofibers and their high filtration performance. Adv. Fiber Mater. 2020, 2, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z.; et al. Progress of fabrication and applications of electrospun hierarchically porous nanofibers. Adv. Fiber Mater. 2022, 4, 1–27. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Zhu, J.; Li, W.; Li, D.; Yang, X.; Zhao, W.; Ren, M.; Mo, X.; Fu, Q.; et al. Electrospun nanoyarn and exosomes of adipose-derived stem cells for urethral regeneration: Evaluations in vitro and in vivo. Colloids Surf. B Biointerfaces 2022, 209, 112218. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, M.; Zhou, Y.; Sun, M.; Xie, Y.; Yu, D.G. Comparisons of antibacterial performances between electrospun polymer@drug nanohybrids with drug-polymer nanocomposites. Adv. Compos. Hybrid Mater. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional electrospun nanocomposites for efficient oxygen reduction reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Yu, D.G. Preface—Bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Varshosaz, J.; Choopannejad, Z.; Minaiyan, M.; Kharazi, A.Z. Rapid hemostasis by nanofibers of polyhydroxyethyl methacrylate/polyglycerol sebacic acid: An in vitro/in vivo study. J. Appl. Polym. Sci. 2021, 138, 49785. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, M.; Li, X.; Liu, X.; Zhu, L.M.; Annie Bligh, S.W. Multifluid electrospinning for the generation of complex nanostructures. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1601. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X.; et al. Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun medicated nanofibers for wound healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Yu, D.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1772. [Google Scholar] [CrossRef]
- Song, Y.; Huang, H.; He, D.; Yang, M.; Wang, H.; Zhang, H.; Li, J.; Li, Y.; Wang, C. Gallic Acid/2-Hydroxypropyl-β-cyclodextrin inclusion complexes electrospun nanofibrous webs: Fast dissolution, improved aqueous solubility and antioxidant property of gallic acid. Chem. Res. Chin. Univ. 2021, 37, 450–455. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-based nanofiber–nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Electrospun functional nanofiber membrane for antibiotic removal in water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Nasser, S.; Ibrahim, M.; Atassi, Y. Hemostatic wound dressings based on drug loaded electrospun PLLA nanofibrous mats. Mater. Chem. Phys. 2021, 267, 124686. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Parın, F.N.; Ullah, A.; Yeşilyurt, A.; Parın, U.; Haider, M.K.; Kharaghani, D. Development of PVA–psyllium husk meshes via emulsion electrospinning: Preparation, characterization, and antibacterial activity. Polymers 2022, 14, 1490. [Google Scholar] [CrossRef]
- Hosseini, A.; Ramezani, S.; Tabibiazar, M.; Mohammadi, M.; Golchinfar, Z.; Mahmoudzadeh, M.; Jahanban-Esfahlan, A. Immobilization of α-amylase in ethylcellulose electrospun fibers using emulsion-electrospinning method. Carbohydr. Polym. 2022, 278, 118919. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Kang, S.; Wang, K.; Yang, Y.; Yu, D.G.; Wan, F.; Williams, G.R.; Bligh, S.W.A. Combination of structure-performance and shape-performance relationships for better biphasic release in electrospun Janus fibers. Int. J. Pharm. 2021, 596, 120203. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-core/PHBV-shell fibers to eliminate tailing off for an improved sustained release of curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf. A 2022, 636, 128163. [Google Scholar] [CrossRef]
- Yu, H.; Liu, W.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. Targeting delivery system for lactobacillus plantarum based on functionalized electrospun nanofibers. Polymers 2020, 12, 1565. [Google Scholar] [CrossRef]
- Li, J.J.; Yang, Y.Y.; Yu, D.G.; Du, Q.; Yang, X.L. Fast dissolving drug delivery membrane based on the ultra-thin shell of electrospun core-shell nanofibers. Eur. J. Pharm. Sci. 2018, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lu, Z.H.; Zhao, P.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Yu, D.G.; Liu, P. Elaborate design of shell component for manipulating the sustained release behavior from core–shell nanofibres. J. Nanobiotechnol. 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Yu, D.G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable drug release from nanofibers coated with blank cellulose acetate layers fabricated using tri-axial electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X.; Li, S.; Song, W.L.; Yu, D.G.; Annie Bligh, S.W. The effect of drug heterogeneous distributions within core-sheath nanostructures on Its sustained release profiles. Biomolecules 2021, 11, 1330. [Google Scholar] [CrossRef]
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.G.; Shen, H. Sheath-separate-core nanocomposites fabricated using a trifluid electrospinning. Mater. Des. 2020, 192, 108782. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos. Hybrid Mater. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the blending ratio on the design of keratin/poly(butylene succinate) nanofibers for drug delivery applications. Biomolecules 2021, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Ziyadi, H.; Baghali, M.; Bagherianfar, M.; Mehrali, F.; Faridi-Majidi, R. An investigation of factors affecting the electrospinning of poly (vinyl alcohol)/kefiran composite nanofibers. Adv. Compos. Hybrid Mater. 2021, 4, 768–779. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Zhan, L.; Deng, J.; Ke, Q.; Li, X.; Ouyang, Y.; Huang, C.; Liu, X.; Qian, Y. Grooved fibers: Preparation principles through electrospinning and potential applications. Adv. Fiber Mater. 2022, 4, 203–213. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Bakar, S.S.S.; Fong, K.C.; Eleyas, A.; Nazeri, M.F.M. Effect of voltage and flow rate electrospinning parameters on polyacrylonitrile electrospun fibers. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 318, p. 012076. [Google Scholar]
- Yang, G.Z.; Li, H.P.; Yang, J.H.; Wan, J.; Yu, D.G. Influence of working temperature on the formation of electrospun polymer nanofibers. Nanoscale. Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.S.; Mishra, V.; Mukherjee, M.D.; Mukherjee, M.D.; Saini, P.; Ranjan, K.R. Amino acid derived biopolymers: Recent advances and biomedical applications. Int. J. Biol. Macromol. 2021, 188, 542–567. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Muthupandian, S.; Farah, S.; Kumar, N.; Domb, A.J. Biodegradable polymers derived from amino acids. Macromol. Biosci. 2011, 12, 1625–1636. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Qi, Y.; Ma, X.; Wang, Y.; Song, H.; Zhao, Y.; Li, W.; Qin, J. Poly (aspartic acid) based self-healing hydrogel with blood coagulation characteristic for rapid hemostasis and wound healing applications. Colloids Surf. B 2022, 214, 112430. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Novel electrospun gelatin-glycerin-ε-Poly-lysine nanofibers for controlling Listeria monocytogenes on beef. Food Packag. Shelf Life 2018, 18, 21–30. [Google Scholar] [CrossRef]
- Han, Y.; Shi, C.; Cui, F.; Chen, Q.; Tao, Y.; Li, Y. Solution properties and electrospinning of polyacrylamide and ε-polylysine complexes. Polymer 2020, 204, 122806. [Google Scholar] [CrossRef]
- Mekhail, M.; Jahan, K.; Tabrizian, M. Genipin-crosslinked chitosan/poly-l-lysine gels promote fibroblast adhesion and proliferation. Carbohydr. Polym. 2014, 108, 91–98. [Google Scholar] [CrossRef]
- Amariei, G.; Kokol, V.; Vivod, V.; Boltes, K.; Letón, P.; Rosal, R. Biocompatible antimicrobial electrospun nanofibers functionalized with ε-poly-l-lysine. Int. J. Pharm. 2018, 553, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.G.R.; Zucolotto, V.; De Queiroz, A.A.A. Electrospinning of hyperbranched poly-L-Lysine/polyaniline nanofibers for application in cardiac tissue engineering. J. Macromol. Sci. A 2010, 47, 1203–1207. [Google Scholar] [CrossRef]
- Allur Subramaniyan, S.; Sheet, S.; Balasubramaniam, S.; Berwin Singh, S.V.; Rampa, D.R.; Shanmugam, S.; Kang, D.R.; Choe, H.S.; Shim, K.S. Fabrication of nanofiber coated with l-arginine via electrospinning technique: A novel nanomatrix to. Cell Commun. Adhes. 2018, 24, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef]
- Park, S.B.; Sung, M.H.; Uyama, H.; Han, D.K. Poly(glutamic acid): Production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021, 113, 101341. [Google Scholar] [CrossRef]
- Ma, X.; Xu, T.; Chen, W.; Qin, H.; Chi, B.; Ye, Z. Injectable hydrogels based on the hyaluronic acid and poly (γ-glutamic acid) for controlled protein delivery. Carbohydr. Polym. 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Ko, Y.G.; Yoon, K.H.; Park, C.; Sung, M.H.; Kwon, O.K.; Ahn, C.H.; Kim, Y.J.; Kwon, O.H. Preparation and Evaluation of Poly(γ-glutamic acid)-Based Anti-Adhesion Membranes. Key Eng. Mater. 2007, 342, 225–228. [Google Scholar] [CrossRef]
- Wang, S.; Cao, X.; Shen, M.; Guo, R.; Bányai, I.; Shi, X. Fabrication and morphology control of electrospun poly(γ-glutamic acid) nanofibers for biomedical applications. Colloids Surf. B 2012, 89, 254–264. [Google Scholar] [CrossRef]
- Tajima, T.; Ueno, S.; Yabu, N.; Sukigara, S.; Ko, F. Fabrication and characterization of poly-γ-glutamic acid nanofiber. J. Appl. Polym. Sci. 2011, 122, 150–158. [Google Scholar] [CrossRef]
- Murakami, S.; Aoki, N.; Matsumura, S. Bio-based biodegradable hydrogels prepared by crosslinking of microbial poly(γ-glutamic acid) with L-lysine in aqueous solution. Polym. J. 2011, 43, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.H.; Uyama, H.; Kwon, O.H.; Sung, M.H. Fabrication of ultrafine fibers of poly(γ-glutamic acid) and its derivative by electrospinning. Polym. Bull. 2009, 63, 735–742. [Google Scholar] [CrossRef]
- Xu, T.; Yang, R.; Ma, X.; Chen, W.; Liu, S.; Liu, X.; Cai, X.; Xu, H.; Chi, B. Bionic poly(γ-glutamic acid) electrospun fibrous scaffolds for preventing hypertrophic scars. Adv. Healthc. Mater. 2019, 8, 1900123. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Yu, Q.; Jia, L. Functional polyaspartic acid fibers hydrogel membrane with enhanced mechanical performance prepared by coaxial electrospinning. Mater. Res. Express 2021, 8, 036407. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Z.; Chen, D.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Poly(aspartic acid) based self-healing hydrogels with antibacterial and light-emitting properties for wound repair. Colloids Surf. B 2021, 200, 111568. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Q.; Pan, W.; Li, R.Q.; Yu, B.; Liu, W.; Yang, M.; Xu, F.J. Rodlike supramolecular nanoassemblies of degradable poly(aspartic acid) derivatives and hydroxyl-rich polycations for effective delivery of versatile tumor-suppressive ncRNAs. Small 2018, 14, 1703152. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Chen, N.; Dash, B.C.; Hsia, H.C.; Berthiaume, F. Self-assembled nanomaterials for chronic skin wound Healing. Adv. Wound Care 2021, 10, 221–233. [Google Scholar] [CrossRef]
- Adelnia, H.; Tran, H.D.; Little, P.J.; Blakey, I.; Ta, H.T. Poly(aspartic acid) in biomedical applications: From polymerization, modification, properties, degradation, and biocompatibility to applications. ACS Biomater. Sci. Eng. 2021, 7, 2083–2105. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Yu, Q.; Jia, L.; Wan, L.Y. Poly(aspartic acid) electrospun nanofiber hydrogel membrane-based reusable colorimetric sensor for Cu(II) and Fe(III) detection. ACS Omega 2019, 4, 14633–14639. [Google Scholar] [CrossRef] [Green Version]
- Molnar, K.; Juriga, D.; Nagy, P.M.; Sinko, K.; Jedlovszky-Hajdu, A.; Zrinyi, M. Electrospun poly(aspartic acid) gel scaffolds for artificial extracellular matrix: Electrospun poly(aspartic acid) gel scaffolds. Polym. Int. 2014, 63, 1608–1615. [Google Scholar] [CrossRef]
- Molnar, K.; Jedlovszky-Hajdu, A.; Zrinyi, M.; Jiang, S.; Agarwal, S. Poly(amino acid)-based gel fibers with pH responsivity by coaxial reactive electrospinning. Macromol. Rapid Commun. 2017, 38, 1700147. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Alazzawi, M.; Kadim Abid Alsahib, N.; Turkoglu Sasmazel, H. Core/Shell glycine-polyvinyl alcohol/polycaprolactone nanofibrous membrane intended for guided bone regeneration: Development and characterization. Coatings 2021, 11, 1130. [Google Scholar] [CrossRef]
- Finn, N.; Carlinet, C.; Maurdev, G. Electrospun poly(acrylic acid)/lysine fibers and the interactive effects of moisture, heat, and cross-link density on their behavior. J Appl. Polym. Sci. 2015, 132, 41252. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Xing, Y.; Yu, Q. Lysine-doped polypyrrole/spider silk protein/poly(l -lactic) acid containing nerve growth factor composite fibers for neural application. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 56, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Yang, J.H.; Tsou, S.C.; Ding, C.H.; Hsu, C.C.; Yang, K.C.; Yang, C.C.; Chen, K.S.; Chen, S.W.; Wang, J.S. Cell proliferation on PVA/sodium alginate and PVA/poly(γ-glutamic acid) electrospun fiber. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 66, 170–177. [Google Scholar] [CrossRef]
- Zhan, H.; Liu, S.; Hu, X.; Jiang, D. Performance and drug release studies of poly (ε-caprolactone)/γ-poly (glutamic acid) fibrous membranes. Text. Res. J. 2019, 89, 1642–1657. [Google Scholar] [CrossRef]
- Wang, B.; Dong, J.; Niu, L.; Chen, W.; Chen, D.; Shen, C.; Zhu, J.; Zhang, X. In vitro and in vivo degradation of potential anti-adhesion materials: Electrospun membranes of poly(ester-amide) based on L-phenylalanine and p-(dioxanone): In Vitro and In Vivo Degradation of PDPA Membranes. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.K.; del Valle, L.J.; Kobauri, S.; Katsarava, R.; Puiggali, J. Electrospun fibrous mats from a l-phenylalanine based poly(ester amide): Drug delivery and accelerated degradation by loading enzymes. Polym. Degrad. Stab. 2015, 119, 275–287. [Google Scholar] [CrossRef]
- Li, M.; Qiu, W.; Wang, Q.; Li, N.; Wang, X.; Yu, J.; Li, X.; Li, F.; Wu, D. Nitric oxide-releasing L-Tryptophan and L-Phenylalanine based Poly(ester urea)s electrospun composite mats as antibacterial and antibiofilm dressing for wound healing. Compos. Part. B Eng. 2022, 229, 109484. [Google Scholar] [CrossRef]
- Sun, B.; Hou, L.; Sun, B.; Han, Y.; Zou, Y.; Huang, J.; Zhang, Y.; Feng, C.; Dou, X.; Xu, F. Use of Electrospun phenylalanine/poly-ε-caprolactone chiral hybrid saffolds to promote endothelial remodeling. Front. Bioeng. Biotechnol. 2021, 9, 773635. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanagisawa, K. Creation of superhydrophobic poly(L-phenylalanine) nonwovens by electrospinning. Polymers 2018, 10, 1212. [Google Scholar] [CrossRef] [Green Version]
- Srinath, D.; Lin, S.; Knight, D.K.; Rizkalla, A.S.; Mequanint, K. Fibrous biodegradable l-alanine-based scaffolds for vascular tissue engineering: Fibrous biodegradable l-alanine-based scaffolds for vascular TE. J. Tissue Eng. Regen. Med. 2014, 8, 578–588. [Google Scholar] [CrossRef]
- Çatıker, E.; Konuk, E.; Gültan, T.; Gümüşderelioğlu, M. Enhancement of scaffolding properties for poly(3-hydroxybutyrate): Blending with poly-β-alanine and wet electrospinning. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 338–349. [Google Scholar] [CrossRef]
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The therapeutic potential of targeting tryptophan catabolism in cancer. Br. J. Cancer 2020, 122, 30–44. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, nutrition, and health benefits of tryptophan. Int. J. Tryptophan Res. 2018, 11, 117864691880228. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhao, P.; Yang, Y.Y.; Yu, D.G. Electrospun Beads-on-the-string Nanoproducts: Preparation and Drug Delivery Application. Curr. Drug Deliv. 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Lv, H.; Yang, Y.; Yu, D.G. Electrospun polyacrylonitrile-based lace nanostructures and their Cu(II) adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-term effect against methicillin-resistant staphylococcus aureus of emodin released from coaxial electrospinning nanofiber membranes with a biphasic profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef] [Green Version]
- Pavlou, E.G.; Georgatzakou, H.T.; Fortis, S.P.; Tsante, K.A.; Tsantes, A.G.; Nomikou, E.G.; Kapota, A.I.; Petras, D.I.; Venetikou, M.S.; Papageorgiou, E.G.; et al. Coagulation abnormalities in renal pathology of chronic kidney disease: The interplay between blood cells and soluble factors. Biomolecules 2021, 11, 1309. [Google Scholar] [CrossRef]
- Da Silva, J.; Leal, E.C.; Carvalho, E. Bioactive antimicrobial peptides as therapeutic agents for infected diabetic foot ulcers. Biomolecules 2021, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hao, J. Identifying new pathways and targets for wound healing and therapeutics from natural sources. Curr. Drug Deliv. 2021, 18, 1064–1084. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent Advances in poly(α-L-glutamic acid)-based nanomaterials for drug delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.G. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv. Fiber Mater. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroğlu, D.Ç.; Uysal, B. Comparing antibiotic pastes with electrospun nanofibers as modern drug delivery systems for regenerative endodontics. Curr. Drug Deliv. 2022, 19, 1567–2018. [Google Scholar] [CrossRef]
- Sun, L.; Song, L.; Zhang, X.; Zhou, R.; Yin, J.; Luan, S. Poly(γ-glutamic acid)-based electrospun nanofibrous mats with photodynamic therapy for effectively combating wound infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110936. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, F.; Liu, W.; Cuim, L.; Kang, L. A novel preparation for a PVA/L-histidine/AgNPs membrane and its antibacterial property. RSC Adv. 2015, 5, 54182–54187. [Google Scholar] [CrossRef]
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Development of a poly(vinyl alcohol)/lysine electrospun membrane-based drug delivery system for improved skin regeneration. Int. J. Pharm. 2019, 570, 118640. [Google Scholar] [CrossRef] [PubMed]
- Németh, C.; Gyarmati, B.; Gacs, J.; Salakhieva, D.V.; Molnár, K.; Abdullin, T.; László, K.; Szilágyi, A. Fast dissolving nanofibrous matrices prepared by electrospinning of polyaspartamides. Eur. Polym. J. 2020, 130, 109624. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Sridhar, R.; Ramakrishna, S. Composite poly-l-lactic acid/poly-(α,β)-dl-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1443–1451. [Google Scholar] [CrossRef]
- Yerra, A.; Dadala, M.M. Silk fibroin electrospun nanofiber blends with antibiotics and polyvinyl alcohol for burn wound healing. J. Appl. Polym. Sci. 2022, 139, 51930. [Google Scholar] [CrossRef]
- Talukder, M.E.; Hasan, K.M.; Wang, J.; Yao, J.; Li, C.; Song, H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J. Mater. Sci. 2021, 56, 12814–12834. [Google Scholar] [CrossRef]
- Hermosilla, J.; Pastene-Navarrete, E.; Acevedo, F. Electrospun fibers loaded with natural bioactive compounds as a biomedical system for skin burn treatment. A Review. Pharmaceutics 2021, 13, 2054. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.S.; Saadatjo, Z.; Mahmoudi, R.; Delaviz, H.; Bardania, H.; Rajabi, S.S.; Rafati, A.; Zarshenas, M.M.; Barmak, M.J. Preparation and evaluation of polycaprolactone/chitosan/Jaft biocompatible nanofibers as a burn wound dressing. Burns 2021, S0305417921003624. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Mishra, S.C.; Chhatbar, K.C.; Kashikar, A.; Mehndiratta, A. Diabetic foot. BMJ 2017, 359, j5064. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Zhao, J.; Mo, X. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Sandoval-Herrera, I.; Romero-García, J.; Ledezma-Pérez, A.; Alvarado-Canché, C.; Torres-Lubian, R.; De-León, A. Controlled release of chlorogenic acid from polyvinyl alcohol/poly(γ-glutamic acid) blended electrospun nanofiber mats with potential applications in diabetic foot treatment. Polymers 2021, 13, 2943. [Google Scholar] [CrossRef]
- Kim, Y.K.; Ku, J.K. Guided bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for guided bone regeneration: A road from bench to bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shao, Q.; Han, Y.; Liu, Q.; He, L.; Sun, Q.; Ruan, S. Fibers by electrospinning and their emerging applications in bone tissue engineering. Appl. Sci. 2021, 11, 9082. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Xiong, J.; Li, J.; Miao, X.; Lan, X.; Liu, X.; Wang, W.; Cai, N.; Tang, Y. Fabrication and in vitro evaluation of PCL/gelatin hierarchical scaffolds based on melt electrospinning writing and solution electrospinning for bone regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 128, 112287. [Google Scholar] [CrossRef] [PubMed]
- Dejob, L.; Toury, B.; Tadier, S.; Grémillard, L.; Gaillard, C.; Salles, V. Electrospinning of in situ synthesized silica-based and calcium phosphate bioceramics for applications in bone tissue engineering: A review. Acta Biomater. 2021, 123, 123–153. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Y.L.; Qin, F.; Cao, C.; Yu, X.L.; Wu, Y.H.; Wang, T.L.; Xu, R.G.; Zhao, L.; Wu, F.; et al. Electrospun Poly (aspartic acid)-modified zein nanofibers for promoting bone regeneration. Int. J. Nanomed. 2019, 14, 9497–9512. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.H.; Yang, S.P.; Chen, Y.S.; Chen, K.Y. Electrospun poly(γ–glutamic acid)/β–tricalcium phosphate composite fibrous mats for bone regeneration. Polymers 2019, 11, 227. [Google Scholar] [CrossRef] [Green Version]






| Spinning solution properties | Polymer molecular weight | The fiber diameter increases with the increase in polymer molecular weight |
| Solvent evaporation rate | The faster the solvent evaporates, the greater the fiber diameter; the faster the solvent evaporates, the greater the fiber porosity and specific surface area; the slower the solvent evaporates, the less easy to remove the solvent residue | |
| Spinning solution viscosity | The higher the viscosity of the spinning solution, the easier it is to block the spinneret; the lower the viscosity, the smaller the fiber diameter, but too low will produce electrospray | |
| Conductivity of spinning solution | The larger the dielectric constant, the smaller the fiber diameter; the smaller the dielectric constant, the easier it is to produce beads of fiber | |
| Spinning parameters | Spinning voltage | The higher the spinning voltage, the smaller the fiber size; too much voltage will lead to unstable spinning; too little voltage fiber diameter will be coarse, or even produce droplets |
| Liquid feeding speed | The larger the flow rate, the larger the fiber diameter, too large will produce droplets; low feed rate spinning process is easy to interrupt | |
| Collector | Influence the three-dimensional structure and arrangement of the product | |
| Distance between spinning head and collecting plate | Spacing is too small solvent cannot be fully evaporated; spacing is too large to affect the electric field strength, but also make the fiber is not easy to deposit and fly into the air | |
| Environmental parameters | Spinning environment temperature | Increasing the temperature increases the rate of solvent volatilization, and hollow nanofibers can be obtained by increasing the temperature |
| Spinning environment humidity | Elevated humidity reduces the rate of solvent evaporation, and nanocrystalline films can be obtained by increasing humidity |
| Amino Acids | Additional Polymer | Solvent | Electrospun Technique | Characteristic | Ref. |
|---|---|---|---|---|---|
| Lysine | Gelatin/glycerin | Acetic acid | Blend | Excellent antibacterial ability against Listeria monocytogenes, a promising food packaging material | [92] |
| PAA/PVA | Distilled water | Blend | Long-lasting antibacterial activity with good biocompatibility | [95] | |
| PAN | DCM/DMF | Blend | High biocompatibility and potential for culturing heart cells | [96] | |
| PAA | Distilled water | Blend | The addition of polylysine enhances the mechanical strength and stability of PAA | [117] | |
| PLLA/PPY | HFIP | Coaxial | Stable electrical properties, good biocompatibility, high cell adhesion rate | [118] | |
| Glutamic acid | PLGA | TFA | Blend | Promotes wound healing and prevents tissue adhesions | [101] |
| Cystamine (aftertreatment) | TFA | Blend | Good water stability | [102] | |
| OXA | Ethanol/water/ hydrochloric | Blend | Good mechanical properties and similar to skin, with certain moisture absorption properties | [103] | |
| PEG | Distilled water | Blend | Uniform nanofiber diameter | [105] | |
| PEO | Distilled water | Blend | Promotes cell adhesion and proliferation and inhibits proliferative scarring | [106] | |
| PVA | Distilled water | Blend | Promotes cell adhesion and can be used as a tissue engineering scaffold | [119] | |
| PCL | HFIP | Blend | Improves the solubility of florfenicol (FF) and promotes the in vitro release of the drug | [120] | |
| Aspartic acid | PSI | — | Blend | Strong adsorption of metal ions and reduced water solubility after cross-linking, can be used as a colorimetric sensor for aqueous solutions | [112] |
| PSI | DMF | Blend | A biocompatible fiber scaffold | [113] | |
| PSI/PEO/THD | DMF | Coaxial | pH sensitive for smart drug release applications | [114] | |
| Arginine | PVA/HA | Distilled water | Blend | Accelerates wound healing and tissue regeneration | [98] |
| Glycine | PVA | Distilled water | Blend | High specific surface area for bio-scaffold and drug transport applications | [116] |
| Amino Acids | Additional Polymer | Solvent | Electrospun Technique | Characteristic | Ref. |
|---|---|---|---|---|---|
| Phenylalanine | PCL | HFIP | Blend | Cell adhesion is good and can be applied to vascular endothelial remodeling | [124] |
| / | TFA/CHCl3 | Blend | Super hydrophobic material to ensure stable adhesion of droplets | [125] | |
| Alanine | P3HB | HFIP | Blend | Good biocompatibility and mechanical properties, conducive to cell adhesion and proliferation | [127] |
| Tryptophan | L-phenylalanine | HFIP | Blend | Treat wound infection and promote wound healing | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie Bligh, S.W. A Review on Electrospun Poly(amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12, 794. https://doi.org/10.3390/biom12060794
Ji Y, Song W, Xu L, Yu D-G, Annie Bligh SW. A Review on Electrospun Poly(amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules. 2022; 12(6):794. https://doi.org/10.3390/biom12060794
Chicago/Turabian StyleJi, Yuexin, Wenliang Song, Lin Xu, Deng-Guang Yu, and Sim Wan Annie Bligh. 2022. "A Review on Electrospun Poly(amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing" Biomolecules 12, no. 6: 794. https://doi.org/10.3390/biom12060794