An Integrated Analysis of Mechanistic Insights into Biomolecular Interactions and Molecular Dynamics of Bio-Inspired Cu(II) and Zn(II) Complexes towards DNA/BSA/SARS-CoV-2 3CLpro by Molecular Docking-Based Virtual Screening and FRET Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Assessment of DNA/BSA Interacting Characteristics
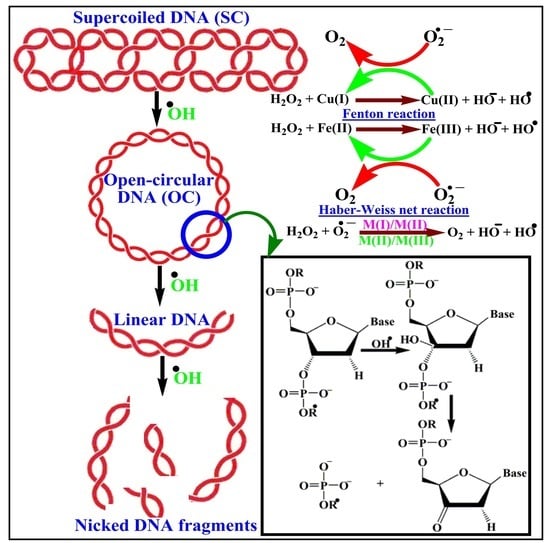
2.2.1. Assessment of DNA Nuclease Efficacy
2.2.2. Analysis of DNA-Interaction Characteristics
2.2.3. Assessment of Thermal Denaturation Characteristics
2.2.4. Assessment of DNA Affinity by Hydrodynamic Technique
2.2.5. Assessment of DNA/BSA Interacting Characteristics by Fluorometric Titration
2.2.6. Förster’s Theory-Based FRET Computation
2.2.7. Analysis of DNA Binding Characteristics Using the CV Method
2.2.8. Assessment of BSA Binding Characteristics by UV–Vis Absorption Titration
2.3. DFT and Molecular Modelling Properties
2.4. UV–Vis Absorption Titrations for In Vitro Antioxidant Assay
2.5. Assessment of In-Vitro Antimicrobial Properties
2.6. MTT Cell Viability Assay for Anticancer Characteristics
3. Results and Discussions
3.1. Synthetic Process and Properties
3.2. DNA/BSA-Binding Properties
3.2.1. Analysis of DNA Cleavage Characteristics
3.2.2. Assessment of DNA Binding Properties Using UV–Visible Absorption Titration
3.2.3. Assessment of Thermal Denaturation Characteristics
3.2.4. Assessment of DNA Binding Affinity Using Viscometric Techniques
3.2.5. Assessment of DNA/BSA Binding Characteristics Using Emission Titration
3.2.6. Förster’s Theory-Based FRET Computation
3.2.7. Analysis of DNA Binding Characteristics Using the CV Method
3.3. Evaluation of BSA Binding by UV–Visible Spectral Titration
3.4. DFT and Molecular Modelling Properties
3.5. Assessment of Antioxidant Properties Using UV–Visible Spectral Titration
3.5.1. Assessment of DPPH Radical Scavenging Property
3.5.2. Evaluation of Hydroxyl Radical Inhibition
3.5.3. Superoxide Scavenging Assay
3.5.4. Assessment of Nitric Oxide Inhibition
3.6. Evaluation of Antimicrobial Properties
3.7. Assessment of Cytotoxic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, G.R.; Tacke, M. N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Khan, T.A.; Patil, Y.P.; Pagariya, D.; Kishore, N.; Tapryal, S.; Naik, A.D.; Naik, S.G. Bio-affinity of copper(II) complexes with nitrogen and oxygen donor ligands: Synthesis, structural studies and in vitro DNA and HSA interaction of copper(II) complexes. J. Photochem. Photobiol. B. Biol. 2017, 174, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Can. J. Clinc. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sakthikumar, K.; Krause, R.W.M.; Isamura, B.K.; Raja, J.D.; Athimoolam, S. Spectro-electrochemical, fluorometric and biothermodynamic evaluation of pharmacologically active morpholine scaffold single crystal ligand and its metal(II) complexes: A comparative study on in-vitro and in-silico screening towards DNA/BSA/SARS-CoV-19. J. Inorg. Biochem. 2022, 236, 111953. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Application of Fragment-Based Drug Discovery to Versatile Targets. Front. Mol. Biosci. 2020, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- St. Denis, J.D.; Hall, R.J.; Murray, C.W.; Heightman, T.D.; Rees, D.C. Fragment-based drug discovery: Opportunities for organic synthesis. RSC Med. Chem. 2021, 12, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.; Atlabachew, M.; Liyew, M.; Ferede, E. Synthesis of organic salts from 1,10-phenanthroline for biological applications. Cogent Chem. 2018, 4, 1476077. [Google Scholar] [CrossRef]
- Kumaravel, G.; Ponya Utthra, P.; Raman, N. Exploiting the biological efficacy of benzimidazole based Schiff base complexes with l-Histidine as a co-ligand: Combined molecular docking, DNA interaction, antimicrobial and cytotoxic studies. Bioorg. Chem. 2018, 77, 269–279. [Google Scholar] [CrossRef]
- Migliore, R.; Biver, T.; Barone, G.; Sgarlata, C. Quantitative Analysis of the Interactions of Metal Complexes and Amphiphilic Systems: Calorimetric. Biomolecules 2022, 12, 408. [Google Scholar] [CrossRef]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A review: Pharmacological aspects of metal based 1,2,4-triazole derived Schiff bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Castro, J.; Dušek, M.; Tohidiyan, Z.; Eigner, V.; Khaleghi, M. Synthesis, spectral characterization, structural studies, molecular docking and antimicrobial evaluation of new dioxidouranium(VI) complexes incorporating tetradentate N2O2 Schiff base ligands. RSC Adv. 2015, 5, 95104–95117. [Google Scholar] [CrossRef]
- Mandegani, Z.; Asadi, Z.; Asadi, M.; Karbalaei-Heidari, H.R.; Rastegari, B. Synthesis, characterization, DNA binding, cleavage activity, cytotoxicity and molecular docking of new nano water-soluble [M(5-CH2PPh3-3,4-salpyr)](ClO4)2(M = Ni, Zn) complexes. Dalton Trans. 2016, 45, 6592–6611. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.L.; Franz, K.J. Application of metal coordination Chemistry to Explore and Manipulate. Cell Biol. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar]
- Szymańska, M.; Pospieszna-Markiewicz, I.; Mańka, M.; Insińska-Rak, M.; Dutkiewicz, G.; Patroniak, V.; Fik-Jaskółka, M.A. Synthesis and Spectroscopic Investigations of Schiff Base Ligand and Its Bimetallic Ag(I) Complex as DNA and BSA Binders. Biomolecules 2021, 11, 1449. [Google Scholar] [CrossRef]
- Peng, B.; Gao, Z.; Li, X.; Li, T.; Chen, G.; Zhou, M.; Zhang, J. DNA binding, DNA cleavage and HSA interaction of several metal complexes containing N-(2-hydroxyethyl)-N′-benzoylthiourea and 1,10-phenanthroline ligands. J. Biolog. Inorg. Chem. 2016, 21, 903–916. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D. Recent advances on non-steroidal anti-inflammatory drugs, NSAIDs: Organotin complexes of NSAIDs. J. Organomet. Chem. 2006, 691, 1767–1774. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Biagini, S.; Bianchi, A.; Faggi, E.; Giorgi, C.; Gratteri, P.; Pina, F.; Valtancoli, B. Thermodynamic and fluorescence emission properties of the Zn(II), Cd(II) and Pb(II) complexes with a fluorescent chelator bearing phenanthroline and naphthalene subunits. Inorg. Chim. Acta 2012, 381, 229–235. [Google Scholar] [CrossRef]
- Sakthikumar, K.; Solomon, R.V.; Raja, J.D. Spectro-electrochemical assessments of DNA/BSA interactions, cytotoxicity, radical scavenging and pharmacological implications of biosensitive and biologically active morpholine-based metal(II) complexes: A combined experimental and computational investigation. RSC Adv. 2019, 9, 14220–14241. [Google Scholar]
- Sakthikumar, K.; Dhaveethu Raja, J.; Vijay Solomon, R.; Sankarganesh, M. Density functional theory molecular modelling, DNA interactions, antioxidant, antimicrobial, anticancer and biothermodynamic studies of bioactive water soluble mixed ligand complexes. J. Biomol. Struct. 2018, 37, 2498–2514. [Google Scholar] [CrossRef]
- Song, Y.M.; Wu, Q.; Yang, P.J.; Luan, N.N.; Wang, L.F.; Liu, Y.M. DNA Binding and cleavage activity of Ni (II) complex with all-trans retinoic acid. J. Inorg. Biochem. 2006, 100, 1685–1691. [Google Scholar] [CrossRef]
- Mizyed, S. Synthesis of new azacrown ether Schiff-bases and their complexes with C60. Jordan J. Chem. 2013, 8, 71–78. [Google Scholar] [CrossRef]
- Sakthikumar, K.; Raja, J.D.; Sankarganesh, M.; Rajesh, J. Antimicrobial, Antioxidant and DNA Interaction Studies of Water-soluble Complexes of Schiff Base Bearing Morpholine Moiety. Indian J. Pharm. Sci. 2018, 80, 723–738. [Google Scholar] [CrossRef]
- Uma Maheswari, P.; Palaniandavar, M. DNA binding and cleavage properties of certain tetrammine ruthenium(II) complexes of modified 1,10-phenanthrolines—Effect of hydrogen-bonding on DNA-binding affinity. J. Inorg. Biochem. 2004, 98, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Rajendiran, V.; Uma Maheswari, P.; Balamurugan, R.; Kilner, C.A.; Halcrow, M.A.; Palaniandavar, M. Copper(II) complexes of tridentate pyridylmethylethylenediamines: Role of ligand steric hindrance on DNA binding and cleavage. J. Inorg. Biochem. 2005, 99, 1717–1732. [Google Scholar] [CrossRef]
- Nagaraj, K.; Murugan, K.S.; Thangamuniyandi, P.; Sakthinathan, S. Nucleic acid binding study of surfactant copper(II) complex containing dipyrido[3,2-a:2′-3′-c]phenazine ligand as an intercalator: In vitro antitumor activity of complex in human liver carcinoma (HepG2) cancer cells. RSC Adv. 2014, 4, 56084–56094. [Google Scholar] [CrossRef]
- Shahabadi, N.; Kashanian, S.; Darabi, F. DNA binding and DNA cleavage studies of a water soluble cobalt(II) complex containing dinitrogen Schiff base ligand: The effect of metal on the mode of binding. Eur. J. Med. Chem. 2010, 45, 4239–4245. [Google Scholar] [CrossRef]
- Ramana, M.M.V.; Betkar, R.; Nimkar, A.; Ranade, P.; Mundhe, B.; Pardeshi, S. Synthesis of a novel 4H-pyran analog as minor groove binder to DNA using ethidium bromide as fluorescence probe. Spectrochim. Acta A Mol. Biomol. 2016, 152, 165–171. [Google Scholar] [CrossRef]
- Feizi-Dehnayebi, M.; Dehghanian, E.; Mansouri-Torshizi, H. Probing the biomolecular (DNA/BSA) interaction by new Pd(II) complex via in-depth experimental and computational perspectives: Synthesis, characterization, cytotoxicity. J. Iran. Chem. Soc. 2022, 19, 3155–3175. [Google Scholar] [CrossRef]
- Meti, M.D.; Nandibewoor, S.T.; Joshi, S.D.; More, U.A.; Chimatadar, S.A. Multi-spectroscopic investigation of the binding interaction of fosfomycin with bovine serum albumin. J. Pharm. Anal. 2005, 5, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.J.; Ou-Yang, Y.; Zhang, Y.; Liu, Y. Affinity and Specificity of Ciprofloxacin-Bovine Serum Albumin Interactions: Spectroscopic Approach. Protein J. 2010, 29, 234–241. [Google Scholar] [CrossRef]
- Na, N.; Zhao, D.Q.; Li, H.; Jiang, N.; Wen, J.Y.; Liu, H.Y. DNA Binding, Photonuclease Activity and Human Serum Albumin Interaction of a Water-Soluble Freebase Carboxyl Corrole. Molecules 2015, 21, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidyanathan, V.G.; Nair, B.U. Synthesis, characterization and electrochemical studies of mixed ligand complexes of ruthenium(II) with DNA. Dalton Trans. 2005, 17, 2842–2848. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.M.; Rehmann, J.P.; Meshoyrer, R.; Kumar, C.V.; Turro, N.J.; Barton, J.K. Mixed-ligand complexes of ruthenium(II): Factors governing binding to DNA. J. Am. Chem. Soc. 1989, 111, 3051–3058. [Google Scholar] [CrossRef]
- Farooq, S.; Siebert, J.P. Gaussian projection, siggraph’09. In Proceedings of the Posters on—Siggraph’09, New Orleans, LA, USA, 4–6 August 2009; p. 1. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. Theochem. 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Improta, R.; Barone, V. PCM/TD-DFT study of the two lowest excited states of uracil derivatives in solution: The effect of the functional and of the cavity model. J. Mol. Struct. Theochem. 2009, 914, 87–93. [Google Scholar] [CrossRef]
- Sandeep, G.; Nagasree, K.P.; Hanisha, M.; Kumar, M.M.K. AUDocker LE: A GUI for virtual screening with AUTODOCK Vina. BMC Res. Notes 2011, 4, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Matondo, A.; Dendera, W.; Isamura, B.K.; Ngbolua, K.; Mambo, H.V.S.; Muzomwe, M.; Mudogo, V. In silico Drug Repurposing of Anticancer Drug 5-FU and Analogues Against SARS-CoV-2 Main Protease: Molecular Docking, Molecular Dynamics Simulation, Pharmacokinetics and Chemical Reactivity Studies. Adv. Appl. Bioinform. Chem. 2022, 15, 59–77. [Google Scholar] [CrossRef]
- Alfaro, M.; Alfaro, I.; Angel, C. Identification of potential inhibitors of SARS-CoV-2 papain-like protease from tropane alkaloids from Schizanthus porrigens: A molecular docking study. Chem. Phy. Lett. 2020, 761, 138068. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 2014, 34, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J. Food Proc. 2014, 2014, 918018. [Google Scholar] [CrossRef] [Green Version]
- Sakthikumar, K.; Sankarganesh, M.; Raja, J.D.; Mitu, L. Water Soluble Mixed Ligand Complexes Spectral, antioxidant, antimicrobial and DNA interaction studies. Rev. Chim. 2018, 69, 3169–3177. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, I.; Wani, W.A.; Saleem, K.; Hsieh, M.F. Anticancer metallo drugs of glutamic acid sulphonamides: In silico, DNA binding, hemolysis and anticancer studies. RSC Adv. 2014, 4, 29629–29641. [Google Scholar] [CrossRef]
- Sweeney, E.E.; McDaniel, R.E.; Maximov, P.Y.; Fan, P.; Jordan, V.C. Models and mechanisms of acquired antihormone resistance in breast cancer: Significant clinical progress despite limitations. Horm. Mol. Biol. Clin. Investig. 2012, 9, 143–163. [Google Scholar] [CrossRef] [Green Version]
- Ulukaya, E.; Colakogullari, M.; Wood, E.J. Interference by Anti-Cancer Chemotherapeutic Agents in the MTT-Tumor Chemosensitivity Assay. Chemotherapy 2004, 5, 43–50. [Google Scholar] [CrossRef]
- Seng, H.-L.; Ong, H.-K.A.; Rahman, R.N.Z.R.; Yamin, B.M.; Tiekink, E.R.T.; Tan, K.W.; Maah, M.J.; Caracelli, I.; Ng, C.H. Factors affecting nucleolytic efficiency of some ternary metal complexes with DNA binding and recognition domains. Crystal and molecular structure of Zn(phen)(edda). J. Inorg. Biochem. 2008, 102, 1997–2011. [Google Scholar] [CrossRef]
- Sathyaraj, G.; Weyhermüller, T.; Nair, B.U. Synthesis, characterization and DNA binding studies of new ruthenium(II)bisterpyridine complexes. Eur. J. Med. Chem. 2010, 45, 284–291. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, R.; Wang, S.; Li, G.; Sheng, Y.; Rui, H.; Zhang, J.; Xu, J.; Jiang, D. New cofactors and inhibitors for a DNA-cleaving DNAzyme: Superoxide anion and hydrogen peroxide mediated an oxidative cleavage process. Sci. Rep. 2017, 7, 378. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Detmer, C.A.; Pamatong, F.V.; Bocarsly, J.R. Nonrandom Double Strand Cleavage of DNA by a Monofunctional Metal Complex: Mechanistic Studies. Inorg. Chem. 1996, 35, 6292–6298. [Google Scholar] [CrossRef]
- Sudhamani, C.N.; Bhojya Naik, H.S.; Gowda, K.R.S.; Giridhar, M.D.; Girija, D.; Kumar, P.N.P. Novel iron phenanthroline-based photosensitizers for antimicrobial PDT: Synthesis, DNA binding and photo-induced DNA cleavage activity. Med. Chem. Res. 2017, 26, 1160–1169. [Google Scholar] [CrossRef]
- Wang, W.; Jun Lee, G.; Joo Jang, K.; Sub Cho, T.; Kim, S.K. Real-time detection of Fe·EDTA/H2O2-induced DNA cleavage by linear dichroism. Nucleic Acids Res. 2008, 36, e85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.N.; Gandhi, D.S.; Parmar, P.A.; Joshi, H.N. DNA-binding and cleavage activity of polypyridyl ruthenium(II) complexes. J. Coord. Chem. 2012, 65, 1926–1936. [Google Scholar] [CrossRef]
- Barcelo, F.; Barcelo, I.; Gavilanes, F.; Ferragut, J.A.; Yanovich, S.; Gonzales-Ros, J.M. Interaction of anthracyclines with nucleotides and related compounds studied by spectroscopy. Biochim. Biophys. Acta Gen. Subj. 1986, 884, 172–181. [Google Scholar] [CrossRef]
- Manikandamathavan, V.M.; Parameswari, R.P.; Weyhermüller, T.; Vasanthi, H.R.; Nair, B.U. Cytotoxic copper (II) mixed ligand complexes: Crystal structure and DNA cleavage activity. Eur. J. Med. Chem. 2011, 46, 4537–4547. [Google Scholar] [CrossRef]
- Mei, W.J.; Liu, J.; Zheng, K.C.; Lin, L.J.; Chao, H.; Li, A.X.; Yun, F.C.; Ji, L.N. Experimental and theoretical study on DNA-binding and photocleavage properties of chiral complexes Δ- and Λ-[Ru(bpy)2L] (L = o-hpip, m-hpip and p-hpip)Electronic supplementary information (ESI) available: Electronic spectra and photo cleavage diagrams. Dalton Trans. 2003, 7, 1352–1359. [Google Scholar] [CrossRef]
- Khan, N.H.; Pandya, N.; Maity, N.C.; Kumar, M.; Patel, R.M.; Kureshy, R.I.; Abdi, S.H.R.; Mishra, S.; Das, S.; Bajaj, H.C. Influence of chirality of V(V) Schiff base complexes on DNA, BSA binding and cleavage activity. Eur. J. Med. Chem. 2011, 46, 5074–5085. [Google Scholar] [CrossRef]
- Gubendran, A.; Kumar, G.G.V.; Kesavan, M.P.; Rajagopal, G.; Athappan, P.; Rajesh, J. New anthracene based Schiff base ligands appended Cu(II) complexes: Theoretical study, DNA binding and cleavage activities. Appl. Organomet. Chem. 2017, 32, e4128. [Google Scholar] [CrossRef]
- Tanzadehpanah, H.; Mahaki, H.; Moghadam, N.H.; Salehzadeh, S.; Rajabi, O.; Najafi, R.; Amini, R.; Saidijam, M. Binding site identification of anticancer drug gefitinib to HSA and DNA in the presence of five different probes. J. Biomol. Struct. Dyn. 2018, 37, 823–836. [Google Scholar] [CrossRef]
- Aslanoglu, M.; Ayne, G. Voltammetric studies of the interaction of quinacrine with DNA. Anal. Bioanal. Chem. 2004, 380, 658–663. [Google Scholar] [CrossRef]
- Patra, A.K.; Nethaji, M.; Chakravarty, A.R. Synthesis, crystal structure, DNA binding and photo-induced DNA cleavage activity of (S-methyl-l-cysteine)copper(II) complexes of heterocyclic bases. J. Inorg. Biochem. 2007, 101, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Mergny, J.L.; Lacroix, L. Analysis of Thermal Melting Curves. Oligonucleotides 2003, 13, 515–537. [Google Scholar] [CrossRef]
- Reddy, P.R.; Shilpa, A.N.; Raju., N.; Raghavaiah, P. Synthesis, structure, DNA binding and cleavage properties of ternary amino acid Schiff base-phen/bipy Cu(II) complexes. J. Inorg. Biochem. 2011, 105, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, E.; Tjahjono, T.D.H.; Yoshioka, N.; Inoue., H. Spectroscopic Studies on the Thermodynamic and Thermal Denaturation of the Ct-DNA Binding of Methylene Blue. Spectrochim. Acta A Mol. Biomol. 2010, 77, 528–534. [Google Scholar]
- Wijaya, K.; Tjahjono, D.H.; Yoshioka, N.; Inoue, H. DNA-Binding Properties of Iron(II) Mixed-Ligand Complexes Containing 1,10-Phenanthroline and Dipyrido[3,2-a:2′,3′-c]phenazine. Z. Nat. B. 2004, 59, 310–318. [Google Scholar]
- Cory, M.; McKee, D.D.; Kagan, J.; Henry, D.W.; Miller, J.A. Design, synthesis, and DNA binding properties of bifunctional intercalators. Comparison of polymethylene and diphenyl ether chains connecting phenanthridine. J. Am. Chem. Soc. 1985, 107, 2528–2536. [Google Scholar] [CrossRef]
- Selvakumar, B.; Rajendiran, V.; Uma Maheswari, P.; Stoeckli-Evans, H.; Palaniandavar, M. Structures, spectra, and DNA-binding properties of mixed ligand copper(II) complexes of iminodiacetic acid: The novel role of diimine co-ligands on DNA conformation and hydrolytic and oxidative double strand DNA cleavage. J. Inorg. Biochem. 2006, 100, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Gellert, M.; Smith, C.E.; Neville, D.; Felsenfeld, G. Actinomycin binding to DNA: Mechanism and specificity. J. Mol. Biol. 1965, 11, 445–457. [Google Scholar] [CrossRef]
- Shahabadi, N.; Fatahi, N.; Mahdavi, M.; Nejad, Z.K.; Pourfoulad, M. Multispectroscopic studies of the interaction of calf thymus DNA with the anti-viral drug, valacyclovir. Spectrochim. Acta A Mol. Biomol. 2011, 83, 420–424. [Google Scholar] [CrossRef]
- Chaires, J.B. Energetics of Drug–DNA Interactions. Biopolymers 1997, 44, 201–215. [Google Scholar] [CrossRef]
- Kelly, J.M.; Tossi, A.B.; McConnell, D.J.; OhUigin, C. A study of the interactions of some polypyridylruthenium(II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res. 1985, 13, 6017–6034. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, S.; Dabrowiak, J.C.; Chaires, J.B. Neither.DELTA.-nor.LAMBDA.-tris(phenanthroline)ruthenium(II) binds to DNA by classical intercalation. Biochemistry 1992, 31, 9319–9324. [Google Scholar] [CrossRef]
- Parveen, S.; Arjmand, F. De novo design, synthesis and spectroscopic characterization of chiral benzimidazole-derived amino acid Zn(II) complexes: Development of tryptophan-derived specific hydrolytic DNA artificial nuclease agent. Spectrochim. Acta A Mol. Biomol. 2012, 85, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.J.; Wang, X.T.; Xie, C.Z.; Tian, H.; Song, X.Q.; Pan, H.T.; Qiao, X.; Xu, J.Y. Mixed-ligand copper (II) Schiff base complexes: The role of the co-ligand in DNA binding, DNA cleavage, protein binding and cytotoxicity. Dalton Trans. 2016, 45, 9073–9087. [Google Scholar] [CrossRef]
- Dhar, S.; Nethaji, M.; Chakravarty, A.R. Effect of charge transfer bands on the photo-induced DNA cleavage activity of [1-(2-thiazolylazo)-2-naphtholato]copper(II) complexes. J. Inorg. Biochem. 2005, 99, 805–812. [Google Scholar] [CrossRef]
- Bhat, S.S.; Kumbhar, A.S.; Lönnecke, P.; Hey-Hawkins, E. Self-Association of Ruthenium(II) Polypyridyl Complexes and Their Interactions with Calf Thymus DNA. Inorg. Chem. 2010, 49, 4843–4853. [Google Scholar] [CrossRef]
- Li, W.-Y.; Xu, J.-G.; Guo, X.-Q.; Zhu, Q.-Z.; Zhao, Y.-B. Study on the interaction between rivanol and DNA and its application to DNA assay. Spectrochim. Acta A Mol. Biomol. 1997, 53, 781–787. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef]
- Deepa, S.; Mishra, A.K. Fluorescence spectroscopic study of serum albumin–bromadiolone interaction: Fluorimetric determination of bromadiolone. J. Pharm. Biomed. Anal. 2005, 38, 556–563. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Hosseini, S.; Naseri, A.; Safarnejad, A.; Rasoulzadeh, F. Interactions of cephalexin with bovine serum albumin: Displacement reaction and molecular docking. Bioimpacts 2016, 6, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Kumar Mishra, A. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Arif, R.; Nayab, P.S.; Ansari, I.A.; Shahid, M.; Irfan, M.; Alam, S.; Abid, M. Synthesis, molecular docking and DNA binding studies of phthalimide-based copper(II) complex: In vitro antibacterial, hemolytic and antioxidant assessment. J. Mol. Struct. 2018, 1160, 142–153. [Google Scholar] [CrossRef]
- Ray, A.; Koley Seth, B.; Pal, U.; Basu, S. Nickel(II)-Schiff base complex recognizing domain II of bovine and human serum albumin: Spectroscopic and docking studies. Spectrochim. Acta A Mol. Biomol. 2012, 92, 164–174. [Google Scholar] [CrossRef]
- Feng, Q.; Li, N.Q.; Jiang, Y.Y. Electrochemical studies of porphyrin interacting with DNA and determination of DNA. Anal. Chim Acta 1997, 344, 97–104. [Google Scholar] [CrossRef]
- Roy, M.; Biswal, D.; Sarkar, O.; Pramanik, N.R.; Paul, S.; Manna, C.K.; Mondal, T.K.; Chakrabarti, D.S. Synthesis, characterization, DFT calculations, protein binding and molecular docking studies of mononuclear dioxomolybdenum(VI) complexes with ONS donor ligand. J. Mol. Struct. 2021, 1234, 130192. [Google Scholar] [CrossRef]
- Jain, A.; Blum, C.; Subramaniam, V. Fluorescence Lifetime Spectroscopy and Imaging of Visible Fluorescent Proteins. Adv. Biomed. Eng. 2009, 147–176. [Google Scholar]
- Bhuin, S.S.; Halder, S.; Saha, S.K.; Chakravarty, M. Binding interactions and FRET between bovine serum albumin and various phenothiazine-/anthracene-based dyes: A structure–property relationship. RSC Adv. 2021, 11, 1679–1693. [Google Scholar] [CrossRef]
- Leone, A.M.; Tibodeau, J.D.; Bull, S.H.; Feldberg, S.W.; Thorp, H.H.; Murray, R.W. Ion Atmosphere Relaxation and Percolative Electron Transfer in Co Bipyridine DNA Molten Salts. J. Am. Chem. Soc. 2003, 125, 6784–6790. [Google Scholar] [CrossRef]
- Muhammad, N.; Shah, A.; Shuja, S.; Ali, S.; Qureshi, R.; Meetsma, A.; Tahir, M.N. Organotin(IV) 4-nitrophenylethanoates: Synthesis, structural characteristics and intercalative mode of interaction with DNA. J. Organomet. Chem. 2009, 694, 3431–3437. [Google Scholar] [CrossRef]
- Raman, N.; Sobha, S. Exploring the DNA binding mode of transition metal based biologically active compounds. Spectrochim. Acta A Mol. Biomol. 2012, 85, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.T.; Rodriguez, M.; Bard , A.J. Voltammetric studies of the interaction of metal chelates with DNA. 2. Tris-chelated complexes of cobalt(III) and iron(II) with 1,10-phenanthroline and 2,2′-bipyridine. J. Bard. J. Am. Chem. Soc. 1989, 111, 8901–8911. [Google Scholar] [CrossRef]
- Motati, D.R.; Uredi, D.; Watkins, E.B. A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines. Chem. Sci. 2018, 9, 1782–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, N.; Yunus, U.; Razzque, S.; Khan, M.; Saleem, S.; Mirza, B.; Rashid, N. Electrochemical and spectroscopic investigations of isoniazide and its analogs with ds.DNA at physiological pH: Evaluation of biological activities. Eur. J. Med. Chem. 2012, 47, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Akbar, K. Comparative DNA binding and antioxidant studies of acetyl and benzoyl substituted ferrocene incorporated seleno ureas. Russ. J. Electrochem. 2015, 51, 198–208. [Google Scholar] [CrossRef]
- Poorniammal, R.; Balachandar, D.; Gunasekaran, S. Evaluation of antioxidant property of some fungal pigments by DNA protection assay. Ann. Phytomed. Int. J. 2018, 7, 106–111. [Google Scholar] [CrossRef]
- Sethupathi, M.; Thulasinathan, B.; Sengottuvelan, N.; Ponnuchamy, K.; Perdih, F.; Alagarsamy, A.; Karthikeyan, M. Macrocyclic ‘tet a’-Derived Cobalt(III) Complex with a N,N′-Disubstituted Hexadentate Ligand: Crystal Structure, Photonuclease Activity and as a Photosensitizer. ACS Omega 2021, 7, 669–682. [Google Scholar] [CrossRef]
- Aslanoglu, M. Electrochemical and Spectroscopic Studies of the Interaction of Proflavine with DNA. Anal. Sci. 2006, 22, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Bai, G.; Dong, C. Studies on interaction of an intramolecular charge transfer fluorescence probe: 4′-Dimethylamino-2,5-dihydroxychalcone with DNA. Bioorg. Med. Chem. 2005, 13, 5694–5699. [Google Scholar] [CrossRef]
- Arshad, N.; Mir, M.I.; Perveen, F.; Javed, A.; Javaid, M.; Saeed, A.; Channar, P.A.; Farooqi, S.I.; Alkahtani, S.; Anwar, J. Investigations on Anticancer Potentials by DNA Binding and Cytotoxicity Studies for Newly Synthesized and Characterized Imidazolidine and Thiazolidine-Based Isatin Derivatives. Molecules 2022, 27, 354. [Google Scholar] [CrossRef]
- Daskalakis, M.; Nguyen, T.T.; Nguyen, C.; Guldberg, P.; Köhler, G.; Wijermans, P.; Jones, P.A.; Lübbert, M. Demethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-Aza-2′-deoxycytidine (decitabine) treatment. Blood 2002, 100, 2957–2964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.J.; Baird, E.E.; Dervan, P.B. Binding site size limit of the 2:1 pyrrole-imidazole polyamide-DNA motif. Proc. Natl. Acad. Sci. USA 1996, 93, 6981–6985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.Y.; Gerena, L.; Swaminathan, S.; Bolton, P.H. Determination of the number and location of the manganese binding sites of DNA quadruplexes in solution by EPR and NMR. Nucleic Acids Res. 1995, 23, 844–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezhkovskiy, L.M. On the Calculation of the Concentration Dependence of Drug Binding to Plasma Proteins with Multiple Binding Sites of Different Affinities: Determination of the Possible Variation of the Unbound Drug Fraction and Calculation of the Number of Binding Sites of the Protein. J. Pharm. Sci. 2007, 96, 249–257. [Google Scholar]
- Pravin, N.; Raman, N. Investigation of in vitro anticancer and DNA strap interactions in live cells using carboplatin type Cu(II) and Zn(II) metalloinsertors. Eur. J. Med. Chem. 2014, 85, 675–687. [Google Scholar] [CrossRef]
- Maruyama, K.; Mishima, Y.; Minagawa, K.; Motonaka, J. Electrochemical and DNA-binding properties of dipyridophenazine complexes of osmium(II). J. Electroanal. Chem. 2001, 510, 96–102. [Google Scholar] [CrossRef]
- Iqbal, M.; Ali, S.; Tahir, M.N. Octahedral copper(II) carboxylate complex: Synthesis, structural description, DNA-binding and anti-bacterial studies. J. Coord. Chem. 2018, 71, 991–1002. [Google Scholar] [CrossRef]
- Somasundaram, I.; Palaniandavar, M. Factors influencing the stability of ATP in ternary complexes: Spectroscopic investigation of the interaction of certain biomimetic copper(II) complexe with ATP and AMP. J. Inorg. Biochem. 1994, 53, 95–107. [Google Scholar] [CrossRef]
- Shakeel, M.; Butt, T.M.; Zubair, M.; Siddiqi, H.M.; Janjua, N.K.; Akhter, Z.; Yaqub, A.; Mahmood, S. Electrochemical investigations of DNA-Intercalation potency of bisnitrophenoxy compounds with different alkyl chain lengths. Heliyon 2020, 6, e04124. [Google Scholar] [CrossRef]
- Janjua, N.K.; Akhter, Z.; Jabeen, F.; Iftikhar, B. Cyclic Voltammetric Investigation of Interactions between Bisnitroaromatic Compounds and ds.DNA. J. Korean Chem. Soc. 2014, 58, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Peng, T.; Yang, C.F. Electrochemical determination of interaction parameters for DNA and mitoxantrone in an irreversible redox process. Biophys. Chem. 2003, 104, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Mugesh, S.; Murugan, M.; Ahamed, F.; Annaraj, J. Mixed-ligand copper(ii)-phenolate complexes: Structure and studies on DNA/protein binding profiles, DNA cleavage, molecular docking and cytotoxicity. RSC Adv. 2016, 6, 1810–1825. [Google Scholar] [CrossRef]
- Idowu, M.; Lamprecht, E.; Nyokong, T. Interaction of water-soluble thiol capped CdTe quantum dots and bovine serum albumin. J. Photochem. Photobiol. A 2008, 198, 7–12. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, Y.Y.; Wang, J.W.; Zhu, Y.M. Cytotoxic Activities and DNA Binding Properties of 1-Methyl-7H-indeno[1,2-b]Quinolinium-7-(4-dimethylamino) Benzylidene Triflate. DNA Cell Biol. 2012, 31, 1046–1053. [Google Scholar] [CrossRef]
- Sang, P.; Tian, S.H.; Meng, Z.H.; Yang, L.Q. Anti-HIV drug repurposing against SARS-CoV-2. RSC Adv. 2020, 10, 15775–15783. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Chen, W.; Xiao, D.; Wang, C. Computational molecular docking and virtual screening revealed promising SARS-CoV-2 drugs. Precis. Clin. Med. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, M. Synthesis and characterization of novel copper(II) complexes as potential drug candidates against SARS-CoV-2 main protease. New J. Chem. 2022, 46, 4911–4926. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved a-ketoamide inhibitors. Science 2020, 80, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. J. Food. Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Dhaveethu Raja, J.; Sakthikumar, K. Synthesis of water soluble transition metal(II) complexes from Morpholine condensed tridentate schiff base: Structural elucidation, antimicrobial, antioxidant and DNA interaction studies. J. Chem. Pharm. Res. 2015, 7, 23–34. [Google Scholar]
- Dhaveethu Raja, J.; Sukkur Saleem, S.; Sakthikumar, K.; Sankarganesh, M.; Vadivel, M. Synthesis of transition metal(II) complexes from piperonal condensed Schiff base: Structural elucidation, antimicrobial, antioxidant, DNA-binding and oxidative cleavage studies. J. Chem. Pharm. Res. 2015, 7, 67–76. [Google Scholar]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Meadows, K.A.; McMillin, D.R. DNA-binding studies of Cu(bcp)2+ and Cu(dmp)2+: DNA elongation without intercalation of Cu(bcp)2+. J. Am. Chem. Soc. 1993, 115, 6699–6704. [Google Scholar] [CrossRef]
- Nitha, L.P.; Aswathy, R.; Mathews, N.E.; Sindhu Kumari, B.; Mohanan, K. Synthesis, spectroscopic characterisation, DNA cleavage, superoxidase dismutase activity and antibacterial properties of some transition metal complexes of a novel bidentate Schiff base derived from isatin and 2-aminopyrimidine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 154–161. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Kaseem, M. Synthesis and antioxidant activities of Schiff bases and their complexes: A review. Appl. Organomet. Chem. 2016, 30, 810–817. [Google Scholar] [CrossRef]
- Patil, S.A.; Unki, S.N.; Kulkarni, A.D.; Naik, V.H.; Badami, P.S. Synthesis, characterization, in vitro antimicrobial and DNA cleavage studies of Co(II), Ni(II) and Cu(II) complexes with ONOO donor coumarin Schiff bases. J. Mol. Struct. 2011, 985, 330–338. [Google Scholar] [CrossRef]
- Thimmaiah, K.N.; Lloyd, W.D.; Chandrappa, G.T. Stereochemistry and fungitoxicity of complexes of p-anisaldehydethiosemicarbazone with Mn(II), Fe(II), Co(II) and Ni(II). Inorg. Chim. Acta 1985, 106, 81–83. [Google Scholar] [CrossRef]
- Mohanan, K.; Nirmala Devi, S.; Murukan, B. Complexes of Copper(II) with 2-(N-Salicylideneamino)-3-carboxyethyl-4,5,6,7-tetrahydrobenzo[b]thiophene Containing Different Counter Anions. Syn. React. Inorg. Met.-Org. Nano-Met. Chem. 2006, 36, 441–449. [Google Scholar] [CrossRef]
- Paulpandiyan, R.; Arunadevi, A.; Raman, N. Role of Knoevenagel condensate pyrazolone derivative Schiff base ligated transition metal complexes in biological assay and cytotoxic efficacy. Appl. Organomet. Chem. 2017, 31, e3792. [Google Scholar] [CrossRef]
- Griffon, G.; Merlin, J.-L.; Marchal, C. Comparison of sulforhodamine B, tetrazolium and clonogenic assays for in vitro radiosensitivity testing in human ovarian cell lines. Anti-Can. Drugs 1995, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiPiro, J.T.; Spruill, W.J.; Wade, W.E.; Blouin, R.A.; Pruemer, J.M.; Ducharme, M.P. Concepts in Clinical Pharmacokinetics, 4th Edition. Ann. Pharmacother. 2006, 40, 1479. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Liu, X.; Tian, J.; Yan, S. Synthesis, crystal structures, DNA/bovine serum albumin binding, DNA cleavage and cytotoxicity of five mononuclear zinc(II) complexes. Appl. Organomet. Chem. 2017, 31, e3802. [Google Scholar] [CrossRef]
- Petrović, Z.D.; Đorović, J.; Simijonović, D.; Trifunović, S.; Petrović, V.P. In vitro study of iron coordination properties, anti-inflammatory potential and cytotoxic effects of N-salicylidene and N-vanillidene anil Schiff bases. Chem. Pap. 2018, 72, 2171–2180. [Google Scholar] [CrossRef]
- Kumaravel, G.; Ponnukalai, P.U.; Mahendiran, D.; Raman, N. Exploring the DNA interactions, FGF growth receptor interaction and biological screening of metal(II) complexes of NNN donor ligand derived from 2-(aminomethyl)benzimidazole. Int. J. Biol. Macromol. 2019, 126, 1303–1317. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Zimenkovsky, B.; Radko, L.; Stypula-Trebas, S.; Roman, O.; Gzella, R.; Lesyk, A.K. Synthesis and cytotoxicity of new thiazolo[4,5-b]pyridine-2(3H)-one derivatives based on α,β-unsaturated ketones and α-ketoacids. Chem. Pap. 2017, 72, 669–681. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Zhang, G.; Liao, Y. Intercalation of the daphnetin–Cu(II) complex with calf thymus DNA. RSC Adv. 2016, 6, 5408–5418. [Google Scholar] [CrossRef]















| Compounds | λmax (nm) | Δλ nm (% H) | Kb × 104 M−1 | (kJmol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Free (Bound) | WS–I (WS–II) | BH–I (BH–II) | SK–I (SK–II) | WS–I (WS–II) | BH–I (BH–II) | SK–I (SK–II) | ||
| (HL) | 336 (340) | 4 (46.88) | 1.5169 (1.5480) | 1.0545 (1.0545) | 0.8775 (1.5618) | −23.85 (−23.90) | −22.95 (−22.95) | −22.50 (−23.92) |
| (1b) | 335 (342) | 7 (56.88) | 2.0610 (2.1357) | 2.5359 (2.5251) | 2.0314 (2.1747) | −24.61 (−24.70) | −25.12 (−25.11) | −24.58 (−24.74) |
| (2b) | 334 (339) | 5 (55.75) | 1.9930 (2.0811) | 2.0131 (2.0035) | 1.9471 (2.0897) | −24.53 (−24.64) | −24.55 (−24.54) | −24.47 (−24.65) |
| Compounds | Tm °C (K) | ΔTm °C | Binding Constant Kr (or) K1 @ 298 K (M−1) | Binding Constant Km (or) K2 @ Tm K (M−1) | ΔH° (kcal mol−1) | ΔS° (cal mol−1) | ΔG° (kcal mol−1) |
|---|---|---|---|---|---|---|---|
| (HL) | 74 (347.0) | 6 | 1.5169 × 104 | 1.4625 × 103 | −9.8083 | −13.7851 | −5.0249 |
| (1b) | 80.5 (353.5) | 12.5 | 2.0610 × 104 | 2.3722 × 103 | −8.1538 | −7.6237 | −5.4588 |
| (2b) | 78.5 (351.5) | 10.5 | 1.9930 × 104 | 2.1862 × 103 | −8.5979 | −9.1806 | −5.3709 |
| Compounds | Binding Ratio (R) = [Complex]/[DNA] | ||||||
|---|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | |||
| Relative Specific Viscosity (η/η0)1/3 | Slope | R2 | |||||
| EB (Control) | 1.01 | 1.35 | 1.63 | 1.82 | 1.99 | 1.2859 | 0.97379 |
| (HL) | 0.61 | 0.67 | 0.75 | 0.85 | 1.01 | 0.4616 | 0.95002 |
| (1b) | 0.82 | 1.10 | 1.25 | 1.45 | 1.71 | 1.0805 | 0.98652 |
| (2b) | 0.80 | 0.91 | 1.16 | 1.21 | 1.53 | 0.8400 | 0.94373 |
| Compounds | Binding Constants for DNA/BSA with Test Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stern–Volmer Methods for DNA Binding Properties (Stern–Volmer Methods for BSA Binding Properties) | LWB Method KLB × 104 M−1 | Scatchard Analysis | Kapp × 107 M−1 | |||||||
| Method–I | Method–II | |||||||||
| Kq × 1012 M−1s−1 | KSV × 104 M−1 | Kass × 104 M−1 | n | (kJM−1) | P | KSA × 104 M−1 | n | |||
| (HL) | 1.1636 (2.639) | 1.1636 (2.639) | 0.9882 (1.524) | 0.976 (0.958) | –22.790 (–23.86) | 0.090 (0.225) | 0.6985 | 2.3194 | 1.096 | 0.4200 |
| (1b) | 4.0303 (9.564) | 4.0303 (9.564) | 3.1817 (7.199) | 1.088 (1.021) | –26.942 (–27.71) | 0.128 (0.322) | 2.1654 | 3.3302 | 1.008 | 1.0000 |
| (2b) | 2.0669 (7.935) | 2.0669 (7.935) | 1.9688 (5.984) | 1.083 (1.009) | –24.873 (–27.25) | 0.124 (0.285) | 1.8218 | 3.2941 | 0.909 | 0.4804 |
| Compounds | J × 10−14 (LM−1cm3) | R0 (nm) | E | r (nm) | kET (J/s) | B (M−1cm−1) |
|---|---|---|---|---|---|---|
| (HL) | 0.8215 | 2.4400 | 0.3462 | 2.6685 | 5.8443 | 5339.79 |
| (1b) | 0.6852 | 2.3673 | 0.2769 | 2.7780 | 3.8294 | 4999.08 |
| (2b) | 0.9460 | 2.4980 | 0.3231 | 2.8257 | 4.7730 | 5650.81 |
| Compounds | ΔEP (V) | E° (or) E1/2 (V) | Found (I) (Calcd) | Free (Bound) | Do × 10−5 cm2 s−1 | Kb × 104 M−1 (Methods) | S (bp) | ||
|---|---|---|---|---|---|---|---|---|---|
| Free (Bound) | Free (Bound) | Free (Bound) | I Red (Oxi) | II | III | ||||
| (HL) | 0.7420 (0.8890) | 0.3490 (0.3680) | 1.1125 (2.0964) | 1.4295 (1.3424) | 2.8570 (2.5809) | 0.5874 (0.528) | 0.2477 | 0.4837 | 0.446 |
| (1b) | 0.2366 (0.2031) | 0.7015 (0.7317) | 1.2053 (3.2434) | 0.8494 (0.7920) | 4.4035 (4.0688) | 2.0878 (1.732) | 3.5514 | 2.1695 | 0.267 |
| (2b) | 0.4261 (0.4350) | 0.5372 (0.5712) | 1.1168 (3.7609) | 0.6072 (0.6464) | 4.0597 (3.6573) | 1.6317 (1.461) | 3.2242 | 1.8300 | 0.248 |
| Compounds | λmax (nm) | Δλ (nm) | Chromism (% H) | Binding Constant Kapp × 104 M−1 by BH Method | (kJmol−1) | |
|---|---|---|---|---|---|---|
| Free | Bound | |||||
| (HL) | 278 | 276 | 2 | 47.79 | 0.8237 | −22.3387 |
| (1b) | 278 | 270 | 8 | 64.70 | 3.5140 | −25.9330 |
| (2b) | 278 | 270 | 8 | 62.16 | 2.1878 | −24.7590 |
| Compounds | Antibacterial Activity | Antifungal Activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| Ligand (HL) | 9 (33) | 9 (33) | 11 (45) | 8 (25) | 14 (57) | 9 (33) | 9 (33) | 10 (40) | 10 (40) | 11 (45) |
| Complex (1b) | 9 (33) | 10 (40) | 11 (45) | 9 (33) | 17 (65) | 11 (45) | 10 (40) | 10 (40) | 11 (45) | 10 (40) |
| Complex (2b) | 10 (40) | 10 (40) | 11 (45) | 08 (25) | 14 (57) | 11 (45) | 11 (45) | 11 (45) | 10 (40) | 10 (40) |
| Amikacin | 22 (73) | 22 (73) | 24 (75) | 20 (70) | 20 (70) | 20 (70) | 20 (70) | -- | -- | -- |
| Streptomycin | 24 (75) | 26 (77) | 24 (75) | 21 (71) | 25 (76) | 21 (71) | 21 (71) | -- | -- | -- |
| Ketoconazole | -- | -- | -- | -- | -- | -- | -- | 16 (63) | 18 (67) | 18 (67) |
| Amphotericin B | -- | -- | -- | -- | -- | -- | -- | 15 (60) | 17 (65) | 17 (65) |
| Compounds | IC50 (µM) | |||
|---|---|---|---|---|
| A549 | HepG2 | MCF-7 | NHDF | |
| Cisplatin | 31.9 ± 1.6 | 22.9 ± 1.1 | 20.2 ± 1.0 | 26.9 ± 1.3 |
| (HL) | 126.4 ± 6.3 | 108.4 ± 5.4 | 105.2 ± 5.3 | 208.6 ± 10.4 |
| (1b) | 29.7 ± 1.2 | 30.9 ± 1.2 | 31.7 ± 1.3 | 72.6 ± 2.9 |
| (2b) | 32.1 ± 1.3 | 33.4 ± 1.4 | 32.5 ± 1.3 | 74.5 ± 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakthikumar, K.; Isamura, B.K.; Krause, R.W.M. An Integrated Analysis of Mechanistic Insights into Biomolecular Interactions and Molecular Dynamics of Bio-Inspired Cu(II) and Zn(II) Complexes towards DNA/BSA/SARS-CoV-2 3CLpro by Molecular Docking-Based Virtual Screening and FRET Detection. Biomolecules 2022, 12, 1883. https://doi.org/10.3390/biom12121883
Sakthikumar K, Isamura BK, Krause RWM. An Integrated Analysis of Mechanistic Insights into Biomolecular Interactions and Molecular Dynamics of Bio-Inspired Cu(II) and Zn(II) Complexes towards DNA/BSA/SARS-CoV-2 3CLpro by Molecular Docking-Based Virtual Screening and FRET Detection. Biomolecules. 2022; 12(12):1883. https://doi.org/10.3390/biom12121883
Chicago/Turabian StyleSakthikumar, Karunganathan, Bienfait Kabuyaya Isamura, and Rui Werner Maçedo Krause. 2022. "An Integrated Analysis of Mechanistic Insights into Biomolecular Interactions and Molecular Dynamics of Bio-Inspired Cu(II) and Zn(II) Complexes towards DNA/BSA/SARS-CoV-2 3CLpro by Molecular Docking-Based Virtual Screening and FRET Detection" Biomolecules 12, no. 12: 1883. https://doi.org/10.3390/biom12121883