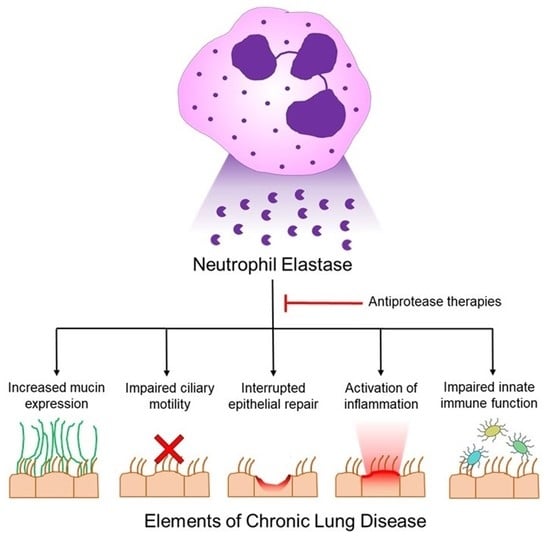
Neutrophil Elastase and Chronic Lung Disease
Abstract
:1. Introduction
2. Neutrophil Elastase Is Required for Microbial Clearance
3. NE-Dependent Mechanisms Inducing Airway Mucus Obstruction
4. NE Alters Cellular Differentiation and Cellular Fate
5. NE Activates Pro-Inflammatory Signaling
6. NE Impairs Innate Immunity
7. NE and Cystic Fibrosis Lung Disease
8. NE and Chronic Obstructive Pulmonary Disease
9. NE and Bronchiectasis
10. NE and Bronchopulmonary Dysplasia
11. NE Inhibitors and Mechanisms of Action
12. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belaaouaj, A.; Kim, K.S.; Shapiro, S.D. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science 2000, 289, 1185–1188. [Google Scholar] [CrossRef]
- Belaaouaj, A.; McCarthy, R.; Baumann, M.; Gao, Z.; Ley, T.J.; Abraham, S.N.; Shapiro, S.D. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 1998, 4, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.P.; Lu, H.; Jacobs, H.L.; Messina, C.G.; Bolsover, S.; Gabella, G.; Potma, E.O.; Warley, A.; Roes, J.; Segal, A.W. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 2002, 416, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [Green Version]
- AhYoung, A.P.; Lin, S.J.; Gerhardy, S.; van Lookeren Campagne, M.; Kirchhofer, D. An ancient mechanism of arginine-specific substrate cleavage: What’s ‘up’ with NSP4? Biochimie 2019, 166, 19–26. [Google Scholar] [CrossRef]
- Niemann, C.U.; Abrink, M.; Pejler, G.; Fischer, R.L.; Christensen, E.I.; Knight, S.D.; Borregaard, N. Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 2007, 109, 4478–4486. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.J.; Owen, C.A. The sulfate groups of chondroitin sulfate- and heparan sulfate-containing proteoglycans in neutrophil plasma membranes are novel binding sites for human leukocyte elastase and cathepsin G. J. Biol. Chem. 2007, 282, 14645–14654. [Google Scholar] [CrossRef] [Green Version]
- Liou, T.G.; Campbell, E.J. Quantum proteolysis resulting from release of single granules by human neutrophils: A novel, nonoxidative mechanism of extracellular proteolytic activity. J. Immunol. 1996, 157, 2624–2631. [Google Scholar]
- Kelly, E.; Greene, C.M.; McElvaney, N.G. Targeting neutrophil elastase in cystic fibrosis. Expert Opin. Ther. Targets 2008, 12, 145–157. [Google Scholar] [CrossRef]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Biol. 2008, 40, 1238–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, S.L.; Goth, C.K.; Eckhard, U.; Joshi, H.J.; Haue, A.D.; Vakhrushev, S.Y.; Schjoldager, K.T.; Overall, C.M.; Wandall, H.H. TAILS N-terminomics and proteomics reveal complex regulation of proteolytic cleavage by O-glycosylation. J. Biol. Chem. 2018, 293, 7629–7644. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Voynow, J.A.; Young, L.R.; Wang, Y.; Horger, T.; Rose, M.C.; Fischer, B.M. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am. J. Physiol. 1999, 276, 835–843. [Google Scholar]
- Fischer, B.M.; Cuellar, J.G.; Diehl, M.L.; deFreytas, A.M.; Zhang, J.; Carraway, K.L.; Voynow, J.A. Neutrophil elastase increases MUC4 expression in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, I.; Lillehoj, E.P.; Koga, T.; Isohama, Y.; Miyata, T.; Kim, K.C. The Signaling Pathway Involved in Neutrophil Elastase–Stimulated MUC1 Transcription. Am. J. Respir. Cell Mol. Biol. 2007, 37, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergin, D.A.; Greene, C.M.; Sterchi, E.E.; Kenna, C.; Geraghty, P.; Belaaouaj, A.; Taggart, C.C.; O’Neill, S.J.; McElvaney, N.G. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J. Biol. Chem. 2008, 283, 31736–31744. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-A.; He, F.; Martin, L.D.; Li, Y.; Chorley, B.N.; Adler, K.B. Human Neutrophil Elastase Induces Hypersecretion of Mucin from Well-Differentiated Human Bronchial Epithelial Cells in Vitro via a Protein Kinase Cδ-Mediated Mechanism. Am. J. Pathol. 2005, 167, 651–661. [Google Scholar] [CrossRef]
- Devaney, J.M.; Greene, C.M.; Taggart, C.C.; Carroll, T.P.; O’Neill, S.J.; McElvaney, N.G. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003, 544, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Britigan, B.E.; Hayek, M.B.; Doebbeling, B.N.; Fick, R.B., Jr. Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect. Immun. 1993, 61, 5049–5055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, S.L.; Jovic, S.; Kurut, A.; Andersson, C.; Gela, A.; Bjartell, A.; Morgelin, M.; Olin, A.I.; Lund, M.; Egesten, A. High expression of midkine in the airways of patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 935–942. [Google Scholar] [CrossRef]
- Voynow, J.A.; Fischer, B.M.; Malarkey, D.E.; Burch, L.H.; Wong, T.; Longphre, M.; Ho, S.B.; Foster, W.M. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, 1293–1302. [Google Scholar] [CrossRef] [Green Version]
- Clancy, D.M.; Sullivan, G.P.; Moran, H.B.T.; Henry, C.M.; Reeves, E.P.; McElvaney, N.G.; Lavelle, E.C.; Martin, S.J. Extracellular Neutrophil Proteases Are Efficient Regulators of IL-1, IL-33, and IL-36 Cytokine Activity but Poor Effectors of Microbial Killing. Cell Rep. 2018, 22, 2937–2950. [Google Scholar] [CrossRef] [Green Version]
- Vandivier, R.; Fadok, V.A.; Hoffmann, P.R.; Bratton, D.L.; Penvari, C.; Brown, K.K.; Brain, J.D.; Accurso, F.J.; Henson, P.M. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 2002, 109, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Amitani, R.; Wilson, R.; Rutman, A.; Read, R.; Ward, C.; Burnett, D.; Stockley, R.A.; Cole, P.J. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 1991, 4, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.L.; Fischer, B.M.; Kummarapurugu, A.B.; Zheng, S.; Kennedy, T.P.; Rao, N.V.; Foster, W.M.; Voynow, J.A. 2-O, 3-O-desulfated heparin inhibits neutrophil elastase-induced HMGB-1 secretion and airway inflammation. Am. J. Respir. Cell Mol. Biol. 2014, 50, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Tosi, M.F.; Zakem, H.; Berger, M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J. Clin. Invest. 1990, 86, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fick, R.B., Jr.; Naegel, G.P.; Squier, S.U.; Wood, R.E.; Gee, J.B.; Reynolds, H.Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J. Clin. Invest. 1984, 74, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Hirche, T.O.; Crouch, E.C.; Espinola, M.; Brokelman, T.J.; Mecham, R.P.; DeSilva, N.; Cooley, J.; Remold-O’Donnell, E.; Belaaouaj, A. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J. Biol. Chem. 2004, 279, 27688–27698. [Google Scholar] [CrossRef] [Green Version]
- Rubio, F.; Cooley, J.; Accurso, F.J.; Remold-O’Donnell, E. Linkage of neutrophil serine proteases and decreased surfactant protein-A (SP-A) levels in inflammatory lung disease. Thorax 2004, 59, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Le Gars, M.; Descamps, D.; Roussel, D.; Saussereau, E.; Guillot, L.; Ruffin, M.; Tabary, O.; Hong, S.-S.; Boulanger, P.; Paulais, M.; et al. Neutrophil Elastase Degrades Cystic Fibrosis Transmembrane Conductance Regulator via Calpains and Disables Channel Function In Vitro and In Vivo. Am. J. Respir. Crit. Care Med. 2013, 187, 170–179. [Google Scholar] [CrossRef]
- Caldwell, R.A.; Boucher, R.C.; Stutts, M.J. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, B.M.; Domowicz, D.A.L.; Zheng, S.; Carter, J.L.; McElvaney, N.G.; Taggart, C.; Lehmann, J.R.; Voynow, J.A.; Ghio, A.J. Neutrophil Elastase Increases Airway Epithelial Nonheme Iron Levels. Clin. Transl. Sci. 2009, 2, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Doring, G.; Frank, F.; Boudier, C.; Herbert, S.; Fleischer, B.; Bellon, G. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J. Immunol. 1995, 154, 4842–4850. [Google Scholar] [PubMed]
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, H.H.; Shannon, P.; Suzuki, T.; Hong, O.V.E.; Moraes, T.; Abreu, M.T.H.; Cherepanov, V.; Wang, X.; Chow, C.-W.; Downey, G.P. Leukocyte elastase induces epithelial apoptosis: Role of mitochondrial permeability changes and Akt. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karandashova, S.; Kummarapurugu, A.B.; Zheng, S.; Chalfant, C.E.; Voynow, J.A. Neutrophil elastase increases airway ceramide levels via upregulation of serine palmitoyltransferase. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, 206–214. [Google Scholar] [CrossRef]
- Krotova, K.; Khodayari, N.; Oshins, R.; Aslanidi, G.; Brantly, M.L. Neutrophil elastase promotes macrophage cell adhesion and cytokine production through the integrin-Src kinases pathway. Sci Rep. 2020, 10, 15874–15884. [Google Scholar] [CrossRef]
- Fischer, B.M.; Cuellar, J.G.; Byrd, A.S.; Rice, A.B.; Bonner, J.C.; Martin, L.D.; Voynow, J.A. ErbB2 activity is required for airway epithelial repair following neutrophil elastase exposure. FASEB J. 2005, 19, 1374–1376. [Google Scholar] [CrossRef]
- Geraghty, P.; Rogan, M.P.; Greene, C.M.; Boxio, R.M.; Poiriert, T.; O’Mahony, M.; Belaaouaj, A.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J. Immunol. 2007, 178, 5871–5878. [Google Scholar] [CrossRef] [Green Version]
- Jackson, P.L.; Xu, X.; Wilson, L.; Weathington, N.M.; Clancy, J.P.; Blalock, J.E.; Gaggar, A. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: Implications to cystic fibrosis proteolytic dysfunction. Mol. Med. 2010, 16, 159–166. [Google Scholar] [CrossRef]
- Fischer, B.M.; Zheng, S.; Fan, R.; Voynow, J.A. Neutrophil elastase inhibition of cell cycle progression in airway epithelial cells in vitro is mediated by p27kip1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, 762–768. [Google Scholar] [CrossRef]
- Fischer, B.M.; Wong, J.K.; Degan, S.; Kummarapurugu, A.B.; Zheng, S.; Haridass, P.; Voynow, J.A. Increased expression of senescence markers in cystic fibrosis airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Weldon, S.; McNally, P.; McElvaney, N.G.; Elborn, J.S.; McAuley, D.F.; Wartelle, J.; Belaaouaj, A.; Levine, R.L.; Taggart, C.C. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. 2009, 183, 8148–8156. [Google Scholar] [CrossRef] [Green Version]
- Roghanian, A.; Drost, E.M.; MacNee, W.; Howie, S.E.; Sallenave, J.M. Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase. Am. J. Respir. Crit. Care Med. 2006, 174, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Janoff, A.; Sloan, B.; Weinbaum, G.; Damiano, V.; Sandhaus, R.A.; Elias, J.; Kimbel, P. Experimental emphysema induced with purified human neutrophil elastase: Tissue localization of the instilled protease. Am. Rev. Respir. Dis. 1977, 115, 461–478. [Google Scholar] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell. Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leavell, K.J.; Peterson, M.W.; Gross, T.J. The role of fibrin degradation products in neutrophil recruitment to the lung. Am. J. Respir. Cell Mol. Biol. 1996, 14, 53–60. [Google Scholar] [CrossRef]
- Rose, M.C.; Voynow, J.A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef] [Green Version]
- Button, B.; Cai, L.H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef] [Green Version]
- Boucher, R.C. Muco-Obstructive Lung Diseases. N. Engl. J. Med. 2019, 380, 1941–1953. [Google Scholar] [CrossRef]
- Fischer, B.M.; Voynow, J.A. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 2002, 26, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Byrd, A.S.; Fischer, B.M.; Grover, A.R.; Ghio, A.J.; Voynow, J.A. Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1. Free Radic. Biol. Med. 2007, 42, 1398–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, M.X.; Nadel, J.A. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J. Immunol. 2005, 175, 4009–4016. [Google Scholar] [CrossRef] [Green Version]
- Kohri, K.; Ueki, I.F.; Nadel, J.A. Neutrophil elastase induces mucin production by ligand-dependent epidermal growth factor receptor activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Kao, S.S.; Ramezanpour, M.; Bassiouni, A.; Wormald, P.J.; Psaltis, A.J.; Vreugde, S. The effect of neutrophil serine proteases on human nasal epithelial cell barrier function. Int. Forum Allergy Rhinol. 2019, 9, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Boxio, R.; Wartelle, J.; Nawrocki-Raby, B.; Lagrange, B.; Malleret, L.; Hirche, T.; Taggart, C.; Pacheco, Y.; Devouassoux, G.; Bentaher, A. Neutrophil elastase cleaves epithelial cadherin in acutely injured lung epithelium. Respir. Res. 2016, 17, 129–144. [Google Scholar] [CrossRef]
- Vermeer, P.D.; Einwalter, L.A.; Moninger, T.O.; Rokhlina, T.; Kern, J.A.; Zabner, J.; Welsh, M.J. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003, 422, 322–326. [Google Scholar] [CrossRef]
- Kim, K.C.; Wasano, K.; Niles, R.M.; Schuster, J.E.; Stone, P.J.; Brody, J.S. Human neutrophil elastase releases cell surface mucins from primary cultures of hamster tracheal epithelial cells. Proc. Natl. Acad. Sci. USA 1987, 84, 9304–9308. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.L.; Potts-Kant, E.N.; Ghio, A.J.; Fischer, B.M.; Foster, W.M.; Voynow, J.A. NAD(P)H quinone oxidoreductase 1 regulates neutrophil elastase-induced mucous cell metaplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-A.; Sharif, A.S.; Shiomi, T.; Kobzik, L.; Kasahara, D.I.; Tschumperlin, D.J.; Voynow, J.; Drazen, J.M. Human neutrophil elastase-mediated goblet cell metaplasia is attenuated in TACE-deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kuang, Z.; Walling, B.E.; Bhatia, S.; Sivaguru, M.; Chen, Y.; Gaskins, H.R.; Lau, G.W. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell Microbiol. 2012, 14, 401–415. [Google Scholar] [CrossRef]
- Ganesan, S.; Comstock, A.T.; Kinker, B.; Mancuso, P.; Beck, J.M.; Sajjan, U.S. Combined exposure to cigarette smoke and nontypeable Haemophilus influenzae drives development of a COPD phenotype in mice. Respir. Res. 2014, 15, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.G.; Van Dyken, S.J.; Wierenga, J.R.; Hotchkiss, J.A.; Harkema, J.R. Ozone exposure enhances endotoxin-induced mucous cell metaplasia in rat pulmonary airways. Toxicol. Sci. 2003, 74, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. CHEST 2018, 154, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Gendler, S.J.; Rose, M.C. Regulation of mucin genes in chronic inflammatory airway diseases. Am. J. Respir. Cell. Mol. Biol. 2006, 34, 661–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegner, H.; Ohlsson, K.; Toremalm, N.G.; von Mecklenburg, C. Effect of human leukocyte enzymes on tracheal mucosa and its mucociliary activity. Rhinology 1979, 17, 199–206. [Google Scholar]
- Karlinsky, J.B.; Snider, G.L. Animal models of emphysema. Am. Rev. Respir. Dis. 1978, 117, 1109–1133. [Google Scholar] [PubMed]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Chua, F.; Laurent, G.J. Neutrophil elastase: Mediator of extracellular matrix destruction and accumulation. Proc. Am. Thorac. Soc. 2006, 3, 424–427. [Google Scholar] [CrossRef]
- Hilliard, T.N.; Regamey, N.; Shute, J.K.; Nicholson, A.G.; Alton, E.W.F.W.; Bush, A.; Davies, J.C. Airway remodelling in children with cystic fibrosis. Thorax 2007, 62, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-Y.; Ho, S.-C.; Lin, H.-C.; Lin, S.-M.; Liu, C.-Y.; Huang, C.-D.; Wang, C.-H.; Chung, K.F.; Kuo, H.-P. Neutrophil-Derived Elastase Induces TGF-β1 Secretion in Human Airway Smooth Muscle via NF-κB Pathway. Am. J. Respir. Cell Mol. Biol. 2006, 35, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Moraes, T.J.; Vachon, E.; Ginzberg, H.H.; Huang, T.-T.; Matthay, M.A.; Hollenberg, M.D.; Marshall, J.; McCulloch, C.A.G.; Abreu, M.T.H.; et al. Proteinase-Activated Receptor-1 Mediates Elastase-Induced Apoptosis of Human Lung Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2005, 33, 231–247. [Google Scholar] [CrossRef] [Green Version]
- Aoshiba, K.; Tsuji, T.; Yamaguchi, K.; Itoh, M.; Nakamura, H. The danger signal plus DNA damage two-hit hypothesis for chronic inflammation in COPD. Eur. Respir. J. 2013, 42, 1689–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Walsh, D.; Greene, C.; Carroll, T.; Taggart, C.; Gallagher, P.; O’neill, S.; McElvaney, N. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J. Biol. Chem. 2001, 276, 35494–35499. [Google Scholar] [CrossRef] [Green Version]
- Liou, T.G.; Adler, F.R.; Keogh, R.H.; Li, Y.; Jensen, J.L.; Walsh, W.; Packer, K.; Clark, T.; Carveth, H.; Chen, J.; et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS ONE 2012, 7, e42748. [Google Scholar] [CrossRef]
- Chirico, V.; Lacquaniti, A.; Leonardi, S.; Grasso, L.; Rotolo, N.; Romano, C.; Di Dio, G.; Lionetti, E.; David, A.; Arrigo, T.; et al. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: High-mobility group box 1 (HMGB1) between inflammation and infection. Clin. Microbiol. Infect. 2015, 21, 368.e1–368.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferhani, N.; Letuve, S.; Kozhich, A.; Thibaudeau, O.; Grandsaigne, M.; Maret, M.; Dombret, M.C.; Sims, G.P.; Kolbeck, R.; Coyle, A.J.; et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Karandashova, S.; Kummarapurugu, A.; Zheng, S.; Kang, L.; Sun, S.; Rubin, B.K.; Voynow, J.A. Neutrophil elastase correlates with increased sphingolipid content in cystic fibrosis sputum. Pediatr. Pulmonol. 2018, 53, 872–880. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Weldon, S.; McAuley, D.F.; Mall, M.A.; Taggart, C.C. Targeting Proteases in Cystic Fibrosis Lung Disease. Paradigms, Progress, and Potential. Am. J. Respir. Crit. Care Med. 2020, 201, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garratt, L.W.; Sutanto, E.N.; Ling, K.M.; Looi, K.; Iosifidis, T.; Martinovich, K.M.; Shaw, N.C.; Kicic-Starcevich, E.; Knight, D.A.; Ranganathan, S.; et al. Australian Respiratory Early Surveillance Team for Cystic F. Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur. Respir. J. 2015, 46, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [Green Version]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Rogan, M.P.; Taggart, C.C.; Greene, C.M.; Murphy, P.G.; O’Neill, S.J.; McElvaney, N.G. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 2004, 190, 1245–1253. [Google Scholar] [CrossRef]
- Le-Barillec, K.; Si-Tahar, M.; Balloy, V.; Chignard, M. Proteolysis of monocyte CD14 by human leukocyte elastase inhibits lipopolysaccharide-mediated cell activation. J. Clin. Invest. 1999, 103, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Roghanian, A.; Williams, S.E.; Sheldrake, T.A.; Brown, T.I.; Oberheim, K.; Xing, Z.; Howie, S.E.; Sallenave, J.M. The antimicrobial/elastase inhibitor elafin regulates lung dendritic cells and adaptive immunity. Am. J. Respir. Cell Mol. Biol. 2006, 34, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; Schechter, M.S.; Voynow, J.A. Cystic Fibrosis. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R., St. Geme, J.W., III, Blum, N.J., Shah, S.S., Tasker, R.C., Wilson, K.M., Behrman, R.E., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2020; pp. 2282–2297. [Google Scholar]
- Hoegger, M.J.; Fischer, A.J.; McMenimen, J.D.; Ostedgaard, L.S.; Tucker, A.J.; Awadalla, M.A.; Moninger, T.O.; Michalski, A.S.; Hoffman, E.A.; Zabner, J.; et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014, 345, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Ermund, A.; Meiss, L.N.; Dolan, B.; Bahr, A.; Klymiuk, N.; Hansson, G.C. The mucus bundles responsible for airway cleaning are retained in cystic fibrosis and by cholinergic stimulation. Eur. Respir. J. 2018, 52, 800457. [Google Scholar] [CrossRef]
- Rosen, B.H.; Evans, T.I.A.; Moll, S.R.; Gray, J.S.; Liang, B.; Sun, X.; Zhang, Y.; Jensen-Cody, C.W.; Swatek, A.M.; Zhou, W.; et al. Infection Is Not Required for Mucoinflammatory Lung Disease in CFTR-Knockout Ferrets. Am. J. Respir. Crit Care Med. 2018, 197, 1308–1318. [Google Scholar] [CrossRef]
- Sly, P.D.; Gangell, C.L.; Chen, L.; Ware, R.S.; Ranganathan, S.; Mott, L.S.; Murray, C.P.; Stick, S.M.; AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013, 368, 1963–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenow, T.; Mok, L.C.; Turkovic, L.; Berry, L.J.; Sly, P.D.; Ranganathan, S.; Tiddens, H.; Stick, S.M. The cumulative effect of inflammation and infection on structural lung disease in early cystic fibrosis. Eur. Respir. J. 2019, 54, 801771. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Kapsner, R.; Osberg, I.; Sontag, M.K.; Accurso, F.J. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am. J. Respir. Crit. Care Med. 2001, 164, 1425–1431. [Google Scholar] [CrossRef]
- Nakamura, H.; Yoshimura, K.; McElvaney, N.G.; Crystal, R.G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Invest. 1992, 89, 1478–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taggart, C.; Coakley, R.J.; Greally, P.; Canny, G.; O’Neill, S.J.; McElvaney, N.G. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruscia, E.M.; Bonfield, T.L. Cystic Fibrosis Lung Immunity: The Role of the Macrophage. J. Innate Immun. 2016, 8, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.D.; McCullagh, B.N.; McCray, P.B. NETs and CF Lung Disease: Current Status and Future Prospects. Antibiotics 2015, 4, 62–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzenreiter, R.; Kienberger, F.; Marcos, V.; Schilcher, K.; Krautgartner, W.D.; Obermayer, A.; Huml, M.; Stoiber, W.; Hector, A.; Griese, M.; et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cyst. Fibros. 2012, 11, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef] [Green Version]
- Abboud, R.T.; Vimalanathan, S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int. J. Tuberc. Lung Dis. 2008, 12, 361–367. [Google Scholar]
- Barnes, P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2009, 41, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Stănescu, D.; Sanna, A.; Veriter, C.; Kostianev, S.; Calcagni, P.G.; Fabbri, L.M.; Maestrelli, P. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 1996, 51, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, R.A.; Peebles, C.; Ward, J.A.; Daraker, A.; Angco, G.; Broberg, P.; Pierrou, S.; Lund, J.; Holgate, S.T.; Davies, D.E.; et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax 2004, 59, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Beasley, V.; Joshi, P.V.; Singanayagam, A.; Molyneaux, P.L.; Johnston, S.L.; Mallia, P. Lung microbiology and exacerbations in COPD. Int J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 555–569. [Google Scholar]
- Thulborn, S.J.; Mistry, V.; Brightling, C.E.; Moffitt, K.L.; Ribeiro, D.; Bafadhel, M. Neutrophil elastase as a biomarker for bacterial infection in COPD. Respir. Res. 2019, 20, 170–177. [Google Scholar] [CrossRef]
- Hautamaki, R.D.; Kobayashi, D.K.; Senior, R.M.; Shapiro, S.D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997, 277, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.; Cervantes-Laurean, D.; Kim, G.; McElvaney, N.G.; Wehr, N.; Moss, J.; Levine, R.L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 2000, 275, 27258–27265. [Google Scholar] [CrossRef]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- King, P.T. The pathophysiology of bronchiectasis. Int. J. Chron. Obstruct. Pulmon. Dis. 2009, 4, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Brower, K.S.; Del Vecchio, M.T.; Aronoff, S.C. The etiologies of non-CF bronchiectasis in childhood: A systematic review of 989 subjects. BMC Pediatr. 2014, 14, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Shum, H.; Tipoe, G.L.; Leung, R.; Lam, W.K.; Ooi, G.C.; Tsang, K.W. Macrophages, neutrophils and tumour necrosis factor-alpha expression in bronchiectatic airways in vivo. Respir. Med. 2001, 95, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuschillo, S.; De Felice, A.; Balzano, G. Mucosal inflammation in idiopathic bronchiectasis: Cellular and molecular mechanisms. Eur. Respir. J. 2008, 31, 396–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulton, B.C.; Barker, A.F. Pathogenesis of Bronchiectasis. Clin. Chest Med. 2012, 33, 211–217. [Google Scholar] [CrossRef]
- Tsang, K.W.; Chan, K.; Ho, P.; Zheng, L.; Ooi, G.C.; Ho, J.C.; Lam, W. Sputum elastase in steady-state bronchiectasis. CHEST 2000, 117, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Smith, M.P.; McHugh, B.J.; Doherty, C.; Govan, J.R.; Hill, A.T. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2012, 186, 657–665. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Moffitt, K.L.; Suarez-Cuartin, G.; Sibila, O.; Finch, S.; Furrie, E.; Dicker, A.; Wrobel, K.; Elborn, J.S.; Walker, B.; et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2017, 195, 1384–1393. [Google Scholar] [CrossRef] [Green Version]
- Stockley, R.; De Soyza, A.; Gunawardena, K.; Perrett, J.; Forsman-Semb, K.; Entwistle, N.; Snell, N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 2013, 107, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Watt, A.P.; Brown, V.; Courtney, J.; Kelly, M.; Garske, L.; Elborn, J.S.; Ennis, M. Neutrophil apoptosis, proinflammatory mediators and cell counts in bronchiectasis. Thorax 2004, 59, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Shoemark, A.; Cant, E.; Carreto, L.; Smith, A.; Oriano, M.; Keir, H.R.; Perea, L.; Canto, E.; Terranova, L.; Vidal, S.; et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur. Respir. J. 2019, 53, 900303. [Google Scholar] [CrossRef] [Green Version]
- Voynow, J.A. New bronchopulmonary dysplasia and chronic lung disease. Paediatr. Respir. Rev. 2017, 24, 17–18. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Feng, R.; Schmidt, B.; Aschner, J.L.; Ballard, R.A.; Hamvas, A.; Reynolds, A.M.; Shaw, P.A.; Jobe, A.H. Prematurity, Respiratory Outcomes P. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann. Am. Thorac. Soc. 2015, 12, 1822–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Marter, L.J.; Dammann, O.; Allred, E.N.; Leviton, A.; Pagano, M.; Moore, M.; Martin, C. Developmental Epidemiology Network I. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J. Pediatr. 2002, 140, 171–176. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.P. Inflammation and bronchopulmonary dysplasia: A continuing story. Semin. Fetal Neonatal Med. 2006, 11, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.T.; Plosa, E.J.; Sucre, J.M.S.; Meer Rvd Dave, S.; Gutor, S.; Nichols, D.S.; Gulleman, P.M.; Jetter, C.S.; Han, W.; Xin, M.; et al. Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J. Clin. Invest. 2021, 131, e139481. [Google Scholar] [CrossRef]
- Chapman, K.R.; Burdon, J.G.; Piitulainen, E.; Sandhaus, R.A.; Seersholm, N.; Stocks, J.M.; Stoel, B.C.; Huang, L.; Yao, Z.; Edelman, J.M.; et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 360–368. [Google Scholar] [CrossRef]
- Dirksen, A.; Piitulainen, E.; Parr, D.G.; Deng, C.; Wencker, M.; Shaker, S.B.; Stockley, R.A. Exploring the role of CT densitometry: A randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur. Respir. J. 2009, 33, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Dirksen, A.; Dijkman, J.H.; Madsen, F.; Stoel, B.; Hutchison, D.C.; Ulrik, C.S.; Skovgaard, L.T.; Kok-Jensen, A.; Rudolphus, A.; Seersholm, N.; et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am. J. Respir. Crit. Care Med. 1999, 160, 1468–1472. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.A.; Geraghty, P.; Holt, G.; Mendes, E.; Newby, P.R.; Ma, S.; Luna-Diaz, L.V.; Turino, G.M.; Stockley, R.A. The Biological Effects of Double-Dose Alpha-1 Antitrypsin Augmentation Therapy. A Pilot Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 318–326. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, N.G.; Hubbard, R.C.; Birrer, P.; Chernick, M.S.; Caplan, D.B.; Frank, M.M.; Crystal, R.G. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet 1991, 337, 392–394. [Google Scholar] [CrossRef]
- Martin, S.L.; Downey, D.; Bilton, D.; Keogan, M.T.; Edgar, J.; Elborn, J.S. Safety and efficacy of recombinant alpha(1)-antitrypsin therapy in cystic fibrosis. Pediatr. Pulmonol. 2006, 41, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Gaggar, A.; Chen, J.; Chmiel, J.F.; Dorkin, H.L.; Flume, P.A.; Griffin, R.; Nichols, D.; Donaldson, S.H. Inhaled Alpha1-Proteinase Inhibitor Therapy in Patients with Cystic Fibrosis. J. Cyst. Fibros. 2016, 15, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Zeiher, B.G.; Artigas, A.; Vincent, J.L.; Dmitrienko, A.; Jackson, K.; Thompson, B.T.; Bernard, G.; STRIVE Study Group. Neutrophil elastase inhibition in acute lung injury: Results of the STRIVE study. Crit. Care Med. 2004, 32, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Kuna, P.; Jenkins, M.; O’Brien, C.D.; Fahy, W.A. AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir. Med. 2012, 106, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Elborn, J.S.; Perrett, J.; Forsman-Semb, K.; Marks-Konczalik, J.; Gunawardena, K.; Entwistle, N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur. Respir. J. 2012, 40, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Jakimiuk, K.; Gesek, J.; Atanasov, A.G.; Tomczyk, M. Flavonoids as inhibitors of human neutrophil elastase. J. Enzyme Inhib. Med. Chem. 2021, 36, 1016–1028. [Google Scholar] [CrossRef]
- Granica, S.; Czerwinska, M.E.; Zyzynska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Zheng, S.; Kummarapurugu, A.B. Glycosaminoglycans as Multifunctional Anti-Elastase and Anti-Inflammatory Drugs in Cystic Fibrosis Lung Disease. Front. Pharmacol. 2020, 11, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Shute, J.K.; Calzetta, L.; Cardaci, V.; di Toro, S.; Page, C.P.; Cazzola, M. Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: A pilot study. Pulm. Pharmacol. Ther. 2018, 48, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummarapurugu, A.B.; Afosah, D.K.; Sankaranarayanan, N.V.; Navaz Gangji, R.; Zheng, S.; Kennedy, T.; Rubin, B.K.; Voynow, J.A.; Desai, U.R. Molecular principles for heparin oligosaccharide-based inhibition of neutrophil elastase in cystic fibrosis. J. Biol. Chem. 2018, 293, 12480–12490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummarapurugu, A.B.; Zheng, S.; Pulsipher, A.; Savage, J.R.; Ma, J.; Rubin, B.K.; Kennedy, T.P.; Voynow, J.A. Polysulfated Hyaluronan GlycoMira-1111 Inhibits Elastase and Improves Rheology in Cystic Fibrosis Sputum. Am. J. Respir. Cell Mol. Biol. 2021, 64, 260–267. [Google Scholar] [CrossRef]
- Morla, S.; Sankaranarayanan, N.V.; Afosah, D.K.; Kumar, M.; Kummarapurugu, A.B.; Voynow, J.A.; Desai, U.R. On the Process of Discovering Leads That Target the Heparin-Binding Site of Neutrophil Elastase in the Sputum of Cystic Fibrosis Patients. J. Med. Chem. 2019, 62, 5501–5511. [Google Scholar] [CrossRef]
- Pulsipher, A.; Qin, X.; Thomas, A.J.; Prestwich, G.D.; Oottamasathien, S.; Alt, J.A. Prevention of sinonasal inflammation by a synthetic glycosaminoglycan. Int. Forum Allergy Rhinol. 2017, 7, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Kummarapurugu, A.B.; Afosah, D.K.; Sankaranarayanan, N.V.; Boothello, R.S.; Desai, U.R.; Kennedy, T.; Voynow, J.A. 2-O, 3-O Desulfated Heparin Blocks High Mobility Group Box 1 Release by Inhibition of p300 Acetyltransferase Activity. Am. J. Respir. Cell Mol. Biol. 2017, 56, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.V.; Argyle, B.; Xu, X.; Reynolds, P.R.; Walenga, J.M.; Prechel, M.; Prestwich, G.D.; MacArthur, R.B.; Walters, B.B.; Hoidal, J.R.; et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am. J. Physiol. Cell Physiol. 2010, 299, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Mulloy, B. The non-anticoagulant promise of heparin and its mimetics. Curr. Opin. Pharmacol. 2019, 46, 50–54. [Google Scholar] [CrossRef]
- Alt, J.A.; Lee, W.Y.; Davis, B.M.; Savage, J.R.; Kennedy, T.P.; Prestwich, G.D.; Pulsipher, A. A synthetic glycosaminoglycan reduces sinonasal inflammation in a murine model of chronic rhinosinusitis. PLoS ONE 2018, 13, e0204709. [Google Scholar] [CrossRef]
| Airway Remodeling | Pro-Inflammatory Effects | Impaired Innate Immunity |
|---|---|---|
| Upregulates airway mucins, MUC5AC, MUC4, and MUC1 [13,14,15,16]; Triggers mucin secretion [17] | Activates TLR4 and upregulates IL-8 [18] | Degrades transferrin, lactoferrin [19] and midkine [20] |
| Stimulates goblet cell metaplasia a [21] | Activates IL-1α, IL-33, IL-36α, IL-36γ [22] | Cleaves phosphatidyl serine receptor resulting in efferocytosis failure [23] |
| Inhibits ciliary motility and injures cilia [24] | Activates High Mobility Group Box 1 [HMGB1] release [25] | Cleaves opsonins- C3bi [26], IgG [27], SP-D [28] SP-A [29]; Cleaves opsonin receptors [26] |
| Degrades CFTR [30] and activates ENaC [31] to promote airway dehydration | Increases cellular oxidative stress by releasing heme-free iron for uptake [32] | Cleaves lymphocyte receptors CD2, CD4, CD8 [33]; Cleaves neutrophil CXCR1 receptor [34] |
| Increases epithelial apoptosis [35] | Upregulates ceramide in vivo which mediates airway inflammation a [36] | Impairs macrophage phagocytic function [37] |
| Transiently down-regulates ErbB2 and suppresses epithelial proliferation [38] | Activates other proteases meprin alpha [16], matrix metalloprotease (MMP)-2 [39], MMP-9 [40], calpain-2 [30], Cathepsin B [39] | Impairs neutrophil E. coli and S. aureus killing [22] |
| Promotes epithelial cell cycle arrest [41] and senescence [42] | Degrades TIMP1 [40] and SLPI [43] | Blocks dendritic cell maturation [44] |
| Degrades extracellular matrix b [45] | Triggers NETs [46] and exosome release [47] | Cleaves fibrin degradation products that are chemotactic for PMN [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voynow, J.A.; Shinbashi, M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules 2021, 11, 1065. https://doi.org/10.3390/biom11081065
Voynow JA, Shinbashi M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules. 2021; 11(8):1065. https://doi.org/10.3390/biom11081065
Chicago/Turabian StyleVoynow, Judith A., and Meagan Shinbashi. 2021. "Neutrophil Elastase and Chronic Lung Disease" Biomolecules 11, no. 8: 1065. https://doi.org/10.3390/biom11081065