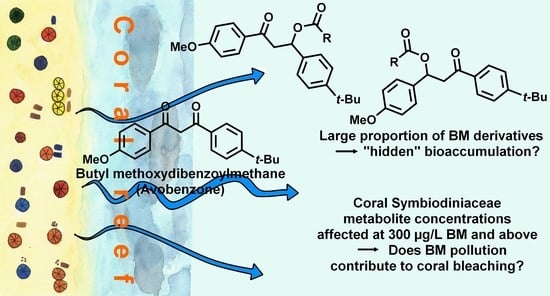
On the Fate of Butyl Methoxydibenzoylmethane (Avobenzone) in Coral Tissue and Its Effect on Coral Metabolome
Abstract
:1. Introduction
2. Material and Methods
2.1. Pocillopora damicornis
2.2. Coral Exposure, Extraction, Metabolomic Profiling, and Analyses
2.3. Standard C16:0-Dihydrobm (7)
2.4. Predicted Toxicity of BM Derivatives Compared to BM
3. Results and Discussion
3.1. Annotation of BM Derivatives
3.2. Annotation of Coral Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifan, H.; Klein, D.; Morse, A.N. UV Filters Are an Environmental Threat in the Gulf of Mexico: A Case Study of Texas Coastal Zones. Oceanologia 2016, 58, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Labille, J.; Slomberg, D.; Catalano, R.; Robert, S.; Apers-Tremelo, M.-L.; Boudenne, J.-L.; Manasfi, T.; Radakovitch, O. Assessing UV Filter Inputs into Beach Waters during Recreational Activity: A Field Study of Three French Mediterranean Beaches from Consumer Survey to Water Analysis. Sci. Total Environ. 2020, 706, 136010. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Bishop, E.; Diaz-Cruz, M.S.; Haghshenas, S.A.; Stien, D.; Rodrigues, A.M.S.; Woodley, C.M.; Sunyer-Caldú, A.; Doust, S.N.; Espero, W.; et al. Oxybenzone Contamination from Sunscreen Pollution and Its Ecological Threat to Hanauma Bay, Oahu, Hawaii, U.S.A. Chemosphere 2022, 291, 132880. [Google Scholar] [CrossRef]
- Downs, C.A.; Diaz-Cruz, M.S.; White, W.T.; Rice, M.; Jim, L.; Punihaole, C.; Dant, M.; Gautam, K.; Woodley, C.M.; Walsh, K.O.; et al. Beach Showers as Sources of Contamination for Sunscreen Pollution in Marine Protected Areas and Areas of Intensive Beach Tourism in Hawaii, USA. J. Hazard. Mater. 2022, 438, 129546. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Fauth, J.E.; Segal, R.; Bronstein, O.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; Kushmaro, A.; et al. Toxicological Effects of the Sunscreen UV Filter, Benzophenone-2, on Planulae and in Vitro Cells of the Coral, Stylophora pistillata. Ecotoxicology 2014, 23, 175–191. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of Inorganic UV Filters Contained in Sunscreen Products on Tropical Stony Corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ma, C.Y.; Yiu, S.K.F.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Toxicological Effects of Two Organic Ultraviolet Filters and a Related Commercial Sunscreen Product in Adult Corals. Environ. Pollut. 2019, 245, 462–471. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative Toxicities of Four Benzophenone Ultraviolet Filters to Two Life Stages of Two Coral Species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef]
- Stien, D.; Clergeaud, F.; Rodrigues, A.M.S.; Lebaron, K.; Pillot, R.; Romans, P.; Fagervold, S.; Lebaron, P. Metabolomics Reveal That Octocrylene Accumulates in Pocillopora damicornis Tissues as Fatty Acid Conjugates and Triggers Coral Cell Mitochondrial Dysfunction. Anal. Chem. 2019, 91, 990–995. [Google Scholar] [CrossRef]
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and Toxicological Effects of UV-Filters on Marine Species. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–46. [Google Scholar]
- Stien, D.; Suzuki, M.; Rodrigues, A.M.S.; Yvin, M.; Clergeaud, F.; Thorel, E.; Lebaron, P. A Unique Approach to Monitor Stress in Coral Exposed to Emerging Pollutants. Sci. Rep. 2020, 10, 9601. [Google Scholar] [CrossRef] [PubMed]
- Thorel, E.; Clergeaud, F.; Rodrigues, A.M.S.; Lebaron, P.; Stien, D. A Comparative Metabolomics Approach Demonstrates That Octocrylene Accumulates in Stylophora Pistillata Tissues as Derivatives and That Octocrylene Exposure Induces Mitochondrial Dysfunction and Cell Senescence. Chem. Res. Toxicol. 2022, 35, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Republic of Palau RPPL No. 10–30: The Responsible Tourism Education Act of 2018. Available online: https://www.palaugov.pw/documents/rppl-no-10-30-the-responsible-tourism-education-act-of-2018/ (accessed on 11 October 2022).
- State of Hawaii SB. No. 20. Available online: https://www.capitol.hawaii.gov/session2022/Bills/SB20_.PDF (accessed on 11 October 2022).
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Ansari, G.A.S.; Kaphalia, B.S.; Khan, M.F. Fatty Acid Conjugates of Xenobiotics. Toxicol. Lett. 1995, 75, 1–17. [Google Scholar] [CrossRef]
- Choi, Y.; Jeon, J.; Kim, S.D. Identification of Biotransformation Products of Organophosphate Ester from Various Aquatic Species by Suspect and Non-Target Screening Approach. Water Res. 2021, 200, 117201. [Google Scholar] [CrossRef]
- Mi, J.-N.; Han, Y.; Xu, Y.; Kou, J.; Wang, J.-R.; Jiang, Z.-H. New Immunosuppressive Sphingoid Base and Ceramide Analogues in Wild Cordyceps. Sci. Rep. 2016, 6, 38641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siskind, L.J.; Mullen, T.D.; Obeid, L.M. Chapter 148—The Role of Ceramide in Cell Regulation. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 1201–1211. ISBN 978-0-12-374145-5. [Google Scholar]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Tumor Suppressive Functions of Ceramide: Evidence and Mechanisms. Apoptosis 2015, 20, 689–711. [Google Scholar] [CrossRef]
- Timmins-Schiffman, E.; Roberts, S. Characterization of Genes Involved in Ceramide Metabolism in the Pacific Oyster (Crassostrea gigas). BMC Res. Notes 2012, 5, 502. [Google Scholar] [CrossRef] [Green Version]
- Marcellin-Gros, R.; Piganeau, G.; Stien, D. Metabolomic Insights into Marine Phytoplankton Diversity. Mar. Drugs 2020, 18, 78. [Google Scholar] [CrossRef] [Green Version]
- Sikorskaya, T.V.; Efimova, K.V.; Imbs, A.B. Lipidomes of Phylogenetically Different Symbiotic Dinoflagellates of Corals. Phytochemistry 2021, 181, 112579. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching Molecular Structure Databases with Tandem Mass Spectra Using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [Green Version]
- Johansen, J.E.; Svec, W.A.; Liaaen-Jensen, S.; Haxo, F.T. Carotenoids of the Dinophyceae. Phytochemistry 1974, 13, 2261–2271. [Google Scholar] [CrossRef]
- Fiksdahl, A.; Withers, N.; Liaaen-Jensen, S. Carotenoids of Heterosigma Akashiwo: A Chemosystematic Contribution. Biochem. Syst. Ecol. 1984, 12, 355–356. [Google Scholar] [CrossRef]
- Skjenstad, T.; Haxo, F.T.; Liaaen-Jensen, S. Carotenoids of Clam, Coral and Nudibranch Zooxanthellae in Aposymbiotic Culture. Biochem. Syst. Ecol. 1984, 12, 149–153. [Google Scholar] [CrossRef]
- Bjørnland, T. Chromatographic Separation and Spectrometric Characterization of Native Carotenoids from the Marine Dinoflagellate Thoracosphaera heimii. Biochem. Syst. Ecol. 1990, 18, 307–316. [Google Scholar] [CrossRef]
- Maoka, T.; Fujiwara, Y.; Hashimoto, K.; Akimoto, N. Carotenoids in Three Species of Corbicula Clams, Corbicula japonica, Corbicula sandai, and Corbicula Sp. (Chinese Freshwater Corbicula Clam). J. Agric. Food Chem. 2005, 53, 8357–8364. [Google Scholar] [CrossRef] [PubMed]
- Thorel, E.; Clergeaud, F.; Jaugeon, L.; Rodrigues, A.M.S.; Lucas, J.; Stien, D.; Lebaron, P. Effect of 10 UV Filters on the Brine Shrimp Artemia Salina and the Marine Microalga Tetraselmis sp. Toxics 2020, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Cmpd. # | tR (Min) | Exp. m/z | Ion Species | Th. m/z | Molecular Formula |
|---|---|---|---|---|---|
| 1 (BM) | 7.62/9.32 | 311.1642 | [M + H]+ | 311.1642 | C20H22O3 |
| 2 | 13.18 | 619.3762 | [M + Na]+ | 619.3758 | C40H52O4 |
| 3 | 13.49 | 595.3758 | [M + Na]+ | 595.3758 | C38H52O4 |
| 4 | 13.55 | 645.3916 | [M + Na]+ | 645.3914 | C42H54O4 |
| 5 | 13.86 | 621.3916 | [M + Na]+ | 621.3914 | C40H54O4 |
| 6 | 14.42 | 623.4072 | [M + Na]+ | 623.4071 | C40H56O4 |
| 7 a | 14.87 | 573.3916 | [M + Na]+ | 573.3914 | C36H54O4 |
| 8 | 14.94 | 573.3914 | [M + Na]+ | 573.3914 | C36H54O4 |
| 9 | 14.98 | 599.4071 | [M + Na]+ | 599.4071 | C38H56O4 |
| 10 | 15.91 | 601.4229 | [M + Na]+ | 601.4227 | C38H58O4 |
| 11 | 12.77 | 589.3864 | [M + Na]+ | 589.3863 | C36H54O5 |
| 12 | 10.39 | 655.3033 | [M + Na]+ | 655.3030 | C41H44O6 |
| 13 | 10.47 | 655.3034 | [M + Na]+ | 655.3030 | C41H44O6 |
| 14 b,c | 9.32 | 449.1370 | n.d. | n.d. | n.d. |
| 15 c | 13.69 | 283.1694 | [M + H]+ | 283.1693 | C19H22O2 |
| 16 c | 14.35 | 559.3760 | [M + Na]+ | 559.3758 | C35H52O4 |
| 17 c | 14.45 | 279.1744 | [M + H]+ | 279.1743 | C20H22O |
| Cmpd. # | tR (Min) | Exp. m/z | Species | Th. m/z | Molecular Formula | Cmpd Class a | Annotation b | Rel. Conc. (BM Conc.) c |
|---|---|---|---|---|---|---|---|---|
| 18 | 12.61 | 548.4674 | [M + H]+ | 548.4673 | C34H61NO4 | Ceramide | Cer(18/16) derivative | ↗ (300) |
| 19 | 13.08 | 562.4830 | [M + H]+ | 562.4830 | C35H63NO4 | Ceramide | Cer(19/16) derivative | ↗ (300) |
| 20 | 13.38 | 550.4832 | [M + H]+ | 550.4830 | C34H63NO4 | Ceramide | Cer(18/16) derivative | ↗ (1000) |
| 21 | 8.45 | 870.5731 | [M + H]+ | 870.5726 | C50H79NO11 | Choline deriv. | n.d. | ↗ (300) |
| 22 | 9.35 | 798.5731 | [M + H]+ | 798.5726 | C44H79NO11 | Choline deriv. | n.d. | ↗ (300) |
| 23 | 10.93 | 789.4553 | [M + Na]+ | 789.4548 | C45H66O10 | MGDG | MGDG 18:5/18:5 | ↗ (1000) |
| 24 | 11.23 | 791.4709 | [M + Na]+ | 791.4705 | C45H68O10 | MGDG | MGDG 18:5/18:4 | ↗ (300) |
| 25 | 11.66 | 817.4853 | [M + Na]+ | 817.4861 | C47H70O10 | MGDG | MGDG 18:5/20:5 | ↘ (300) |
| 26 | 12.31 | 769.4871 | [M + Na]+ | 769.4861 | C43H70O10 | MGDG | MGDG 18:5/16:1 | ↗ (300) |
| 27 | 12.67 | 771.5025 | [M + Na]+ | 771.5018 | C43H72O10 | MGDG | MGDG 18:4/16:1 | ↗ (300) |
| 28 | 11.13 | 613.3521 | [M + H]+ | 613.3524 | C39H48O6 | Carotenoid | Pyrrhoxanthin or isomer | ↘ (300) |
| 29 | 11.30 | 613.3525 | [M + H]+ | 613.3524 | C39H48O6 | Carotenoid | Pyrrhoxanthin or isomer | ↘ (1000) |
| 30 | 12.23 | 1070.6836 | [M + NH4]+ | 1070.6833 | C53H96O20 | n.d. | n.d. | ↗ (300) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clergeaud, F.; Giraudo, M.; Rodrigues, A.M.S.; Thorel, E.; Lebaron, P.; Stien, D. On the Fate of Butyl Methoxydibenzoylmethane (Avobenzone) in Coral Tissue and Its Effect on Coral Metabolome. Metabolites 2023, 13, 533. https://doi.org/10.3390/metabo13040533
Clergeaud F, Giraudo M, Rodrigues AMS, Thorel E, Lebaron P, Stien D. On the Fate of Butyl Methoxydibenzoylmethane (Avobenzone) in Coral Tissue and Its Effect on Coral Metabolome. Metabolites. 2023; 13(4):533. https://doi.org/10.3390/metabo13040533
Chicago/Turabian StyleClergeaud, Fanny, Maeva Giraudo, Alice M. S. Rodrigues, Evane Thorel, Philippe Lebaron, and Didier Stien. 2023. "On the Fate of Butyl Methoxydibenzoylmethane (Avobenzone) in Coral Tissue and Its Effect on Coral Metabolome" Metabolites 13, no. 4: 533. https://doi.org/10.3390/metabo13040533