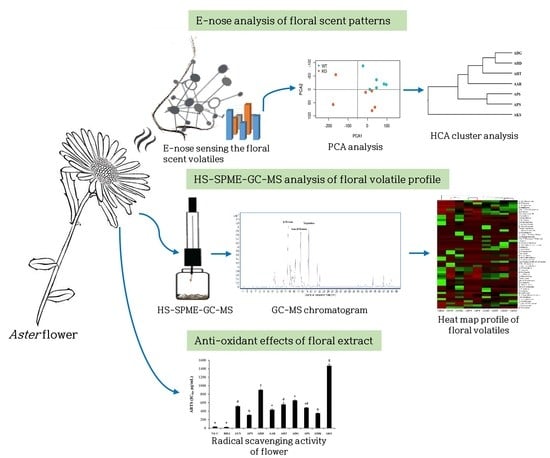
Analysis of Floral Scent and Volatile Profiles of Different Aster Species by E-nose and HS-SPME-GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation for E-nose Analysis
2.3. Floral Scent Analysis by E-nose
2.4. Floral Volatile Component Analysis by HS-SPME-GC-MS
2.5. Sample Preparation for DPPH, ABTS Radical Scavenging Assay
2.6. DPPH Radical Scavenging Assay
2.7. ABTS Radical Scavenging Assay
2.8. Statistical Analysis
3. Results
3.1. Optimization of Floral Fragrance Pattern Analysis in Flowering Stages of Aster yomena Using E-nose
3.2. Floral Scent Pattern Analysis of Aster Species Using E-nose
3.3. Evaluation of Volatile Compounds of Aster Species Using HS-SPME-GC-MS Analysis
3.4. PCA of Volatile Compounds of Different Aster Species
3.5. Cluster Analysis of Scent Patterns from E-nose and Volatile Compound Compositions from GC-MS
3.6. Antioxidant Activity of Flowers from Aster Species’ Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funk, V.A.; Susanna, A.; Stuessy, T.F.; Robinson, H. Classification of compositae. In Systematics, Evolution and Biogeography of the Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; IAPT: Vienna, Austria, 2009; pp. 171–189. [Google Scholar]
- Achika, J.I.; Arthur, D.E.; Gerald, I.; Adedayo, A. A Review on the Phytoconstituents and Related Medicinal Properties of Plants in the Asteraceae Family. IOSR-JAC 2014, 7, 01–08. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, S.; Park, Y.I.; Lee, J.; Kwon, K.H. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016, 148, 173–182. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, Y.Y.; Lee, H.I.; Lee, S.; Reaney, M.J.; Chung, M.J. Astragalin and isoquercitrin isolated from Aster scaber sup-press lps-induced neuroinflammatory responses in microglia and mice. Foods 2022, 11, 1505. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Choi, S.-I.; Han, X.; Men, X.; Jang, G.-W.; Kwon, H.-Y.; Kang, S.-R.; Han, J.-S.; Lee, O.-H. Radical Scavenging-Linked Anti-Adipogenic Activity of Aster scaber Ethanolic Extract and Its Bioactive Compound. Antioxidants 2020, 9, 1290. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Yao, K.; Hu, Z. Protective effect of Aster tataricus extract on retinal damage on the virtue of its antioxidant and anti-inflammatory effect in diabetic rat. Biomed. Pharmacother. 2017, 89, 617–622. [Google Scholar] [CrossRef]
- Yu, P.; Cheng, S.; Xiang, J.; Yu, B.; Zhang, M.; Zhang, C.; Xu, X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015, 164, 328–333. [Google Scholar] [CrossRef]
- Su, X.D.; Jang, H.-J.; Li, H.X.; Kim, Y.H.; Yang, S.Y. Identification of potential inflammatory inhibitors from Aster tataricus. Bioorg. Chem. 2019, 92, 103208. [Google Scholar] [CrossRef]
- Ng, T.; Liu, F.; Lu, Y.; Cheng, C.; Wang, Z. Antioxidant activity of compounds from the medicinal herb Aster tataricus. CBPC 2003, 136, 109–115. [Google Scholar] [CrossRef]
- Seo, S.W. Protective Effects of Ethanol Extract from Aster yomena on Acute Pancreatitis. JPPKM 2019, 33, 109–115. [Google Scholar] [CrossRef]
- Kim, S.O.; Jeong, J.S.; Choi, Y.H. Antioxidant and anti-inflammatory effects of ethanol extract of Aster yomena in RAW 264.7 macrophages. J. Life Sci. 2019, 29, 977–985. (In Korean) [Google Scholar]
- Chung, T.Y.; Eiserich, J.P.; Shibamoto, T. Volatile compounds isolated from edible Korean chamchwi (Aster scaber Thunb). J. Agric. Food Chem. 1993, 41, 1693–1697. [Google Scholar] [CrossRef]
- Choi, H.S. Comparison of the essential oil composition between Aster tataricus and A. koraiensis. Anal. Chem. 2012, 2, 138–151. [Google Scholar]
- Miyazawa, M.; Kawata, J.; Kohno, K.; Imai, M.; Ono, T. Essential Oil and Headspace Constituents from the Aerial Parts of Aster ageratoides Turcz. var. ovatus Nakai. J. Essent. Oil Res. 2008, 20, 9–11. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Steenhuisen, S.L.; Raguso, R.; Jürgens, A.; Johnson, S. Variation in scent emission among floral parts and inflorescence developmental stages in beetle-pollinated Protea species (Proteaceae). S. Afr. J. Bot. 2010, 76, 779–787. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wu, X.; Zhang, Y.; He, Z.; Zhang, Y.; Zhang, X.; Li, Q.; Huang, J.; Liu, Z. Puerh tea unique aroma: Volatile components, evaluation methods and metabolic mechanism of key odoractive compounds. Trends Food Sci. Technol. 2022, 124, 25–37. [Google Scholar] [CrossRef]
- Sanaeifar, A.; ZakiDizaji, H.; Jafari, A.; de la Guardia, M. Early detection of contamination and defect in foodstuffs by electronic nose: A review. TrAC 2017, 97, 257–271. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Namieśnik, J.; Wardencki, W. Food Analysis Using Artificial Senses. J. Agric. Food Chem. 2014, 62, 1423–1448. [Google Scholar] [CrossRef]
- Mohamed, E.I.; Linder, R.; Perriello, G.; Di Daniele, N.; Pöppl, S.; De Lorenzo, A. Predicting Type 2 diabetes using an electronic nose-based artificial neural network analysis. Diabetes Nutr. Metab. 2002, 15, 215–221. [Google Scholar]
- Berna, A. Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis. Sensors 2010, 10, 3882–3910. [Google Scholar] [CrossRef]
- Lee, Y.G.; Choi, W.S.; Yang, S.O.; Jeon, H.B.; Kim, H.G.; Fang, M.; Yi, T.H.; Kang, S.C.; Lee, Y.H.; Beak, N.I. Volatile profiles of five variants of Abeliophyllum distichum flowers using headspace solid-phase microextraction gas chromatography–mass spectrometry (HS-SPME-GC-MS) analysis. Plants 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Soejima, A.; Watanabe, K. Phylogenetic relationships of Japanese Aster (Asteraceae, Astereae) sensu lato based on chloroplast-DNA restriction site mutations. J. Plant Res. 1998, 111, 217–223. [Google Scholar] [CrossRef]
- Noyes, R.D.; Rieseberg, L.H. ITS sequence data support a single origin for North American Astereae (Asteraceae) and reflect deep geographic divisions in Aster sl. Am. J. Bot. 1999, 86, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Yukawa, T.; Kondo, K. Molecular phylogenetic analysis of members of Chrysanthemum and its related genera in the tribe Anthemideae, the Asteraceae in East Asia on the basis of the internal transcribed spacer (ITS) region and the ex-ternal transcribed spacer (ETS) region of nrDNA. Chromosome Bot. 2009, 4, 25–36. [Google Scholar] [CrossRef]
- Nogueira, P.C.D.L.; Bittrich, V.; Shepherd, G.J.; Lopes, A.V.; Marsaioli, A.J. The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae). Phytochemistry 2001, 56, 443–452. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Srinivasan, A.; Ahn, M.S.; Jo, G.S.; Suh, J.N.; Seo, K.H.; Kim, W.H.; Kang, Y.I.; Lee, Y.R.; Choi, Y.J. Analysis of relative scent intensity, volatile compounds and gene expression in Freesia “Shiny Gold”. Plants 2020, 9, 1597. [Google Scholar] [CrossRef]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and Antioxidant Scavenging Assay. DPPH Microplate Protoc. 2012, 7–9. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, 147–153. [Google Scholar] [CrossRef]
- Huang, Y.; Li, F.; Xia, Y.; Chen, K. Scent profiling of Cymbidium ensifolium by electronic nose. Sci. Hortic. 2011, 128, 306–310. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSuschem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Rao, V.S.; Menezes, A.M.S.; Viana, G.S.B. Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 1990, 42, 877–888. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259. [Google Scholar]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Chemother. Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Chen, G.; Gong, W.C.; Ge, J.; Dunn, B.L.; Sun, W.B. Floral scents of typical Buddleja species with different pollination syndromes. Biochem. Syst. Ecol. 2012, 44, 173–178. [Google Scholar] [CrossRef]
- Raguso, R.A.; Thompson, J.N.; Campbell, D.R. Improving our chemistry: Challenges and opportunities in the interdisciplinary study of floral volatiles. Nat. Prod. Rep. 2015, 32, 893–903. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Chemicals; Montclair: Newark, NJ, USA, 1969; pp. 2510–2659. [Google Scholar]
- Chang, K.-M.; Kim, G.-H. Constituents of the Essential Oil from Eclipta prostrata L. Prev. Nutr. Food Sci. 2009, 14, 168–171. [Google Scholar] [CrossRef]
- Hai-Yan, S.; Zhen-Qiu, L.; Hong, W.; Lan-Qing, M.; Ben-Ye, L.; Fang, Y.; Guo-Feng, L.; He-Chun, Y. Advances in sesquiterpene synthases (cyclases) of Artemisia annua. Chin. J. Biotechnol. 2007, 23, 976–981. [Google Scholar]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a sesquiterpene from the essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidanbaram, K.; Thangavelu, L.; Nordin, R.; et al. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Farooq, A.; Choudhary, I.; Rahman, A.-U.; Tahara, S.; Başer, K.H.C.; Demirci, F. Detoxification of Terpinolene by Plant Pathogenic Fungus Botrytis cinerea. Z. Nat. C 2002, 57, 863–866. [Google Scholar] [CrossRef]
- Grassmann, J.; Hippeli, S.; Spitzenberger, R.; Elstner, E. The monoterpene terpinolene from the oil of Pinus mugo L. in concert with α-tocopherol and β-carotene effectively prevents oxidation of LDL. Phytomedicine 2005, 12, 416–423. [Google Scholar] [CrossRef]
- Long, Q.; Li, Z.; Han, B.; Gholam Hosseini, H.; Zhou, H.; Wang, S.; Luo, D. Discrimination of two cultivars of Alpinia offici-narum hance using an electronic nose and gas chromatography-mass spectrometry coupled with chemometrics. Sensors 2019, 19, 572. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Y.; Zhang, Q.; Liu, X.; Li, F.; Chen, K. Fragrance discrimination of Chinese Cymbidium species and cultivars using an electronic nose. Sci. Hortic. 2014, 172, 271–277. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Lee, S.; Cho, E.J.; Kim, H.Y. Determination of radical scavenging activity of Aster yomena (Kitam.) Honda. JKAIS 2018, 19, 402–407. (In Korean) [Google Scholar]
- Kim, M.H.; Nugroho, A.; Choi, J.W.; Park, H.J. The extract of Aster glehni leaves rich in caffeoylquinic acids prevents athero-genic index, oxidative stress, and body weight increase in high-fat diet-induced rats. Korean J. Pharmacogn. 2011, 42, 54–60. (In Korean) [Google Scholar]
- Lee, J.Y.; Park, J.Y.; Kim, H.D.; Lee, S.E.; Lee, J.H.; Lee, Y.; Seo, K.H. Anti-oxidant and anti-adipocyte differentiation of Aster glehni and Aster yomena. J. Nutr. Health 2019, 52, 250–257. (In Korean) [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]








| No | RI a | Compounds | Relative Content (%) b ± SD | ID d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AYN | APN | AHD | AAR | AHT | ADG | APS | AMK | AKS | ||||
| Fatty acid esters | ||||||||||||
| 1 | 850 | ethyl isovalerate | 2.28 ± 0.33 c | 1.07 ± 0.49 | MS, RI | |||||||
| 2 | 1229 | cis-3-hexenyl valerate | 0.32 ± 0.14 | MS, RI | ||||||||
| Monoterpenes | ||||||||||||
| 3 | 928 | (-)-α-pinene | 16.77 ± 2.69 | 1.37 ± 0.22 | 5.06 ± 1.18 | 2.66 ± 0.38 | 17.65 ± 3.15 | 1.61 ± 0.22 | 0.83 ± 0.08 | MS, RI, STD | ||
| 4 | 941 | camphene | 0.47 ± 0.08 | 1.86 ± 0.61 | 0.96 ± 0.17 | 2.01 ± 0.21 | 0.92 ± 0.08 | 1.02 ± 0.09 | MS, RI | |||
| 5 | 968 | β-thujene | 2.27 ± 0.32 | 2.75 ± 0.30 | 2.62 ± 0.41 | 3.86 ± 0.20 | 5.66 ± 0.31 | 10.66 ± 1.15 | 0.66 ± 0.07 | MS, RI | ||
| 6 | 991 | β-myrcene | 41.46 ± 3.43 | 13.52 ± 0.52 | 9.58 ± 0.40 | 18.98 ± 1.58 | 15.70 ± 1.11 | 9.86 ± 1.87 | 22.67 ± 0.41 | 38.94 ± 0.48 | 5.84 ± 0.42 | MS, RI, STD |
| 7 | 998 | α-phellandrene | 1.09 ± 0.13 | 5.49 ± 1.76 | 3.20 ± 0.29 | 4.83 ± 0.24 | 5.21 ± 0.22 | 3.31 ± 0.37 | 3.21 ± 0.23 | 0.72 ± 0.31 | MS, RI | |
| 8 | 1004 | 3-carene | 4.09 ± 0.63 | MS, RI, STD | ||||||||
| 9 | 1010 | 4-carene | 1.57 ± 0.29 | 1.26 ± 0.23 | 2.59 ± 0.24 | 3.08 ± 0.15 | 1.95 ± 0.19 | 1.25 ± 0.08 | MS, RI | |||
| 10 | 1019 | p-cymene | 0.69 ± 0.03 | 3.78 ± 0.51 | 3.52 ± 0.06 | MS, RI | ||||||
| 11 | 1022 | D-limonene | 3.66 ± 1.06 | 31.66 ± 3.52 | 12.86 ± 1.28 | 6.14 ± 0.38 | 26.74 ± 0.89 | 14.20 ± 1.20 | 37.93 ± 0.97 | 4.49 ± 0.25 | MS, RI, STD | |
| 12 | 1034 | (Z)-β-ocimene | 0.70 ± 0.23 | 0.74 ± 0.06 | 0.53 ± 0.03 | 0.44 ± 0.05 | 0.59 ± 0.16 | 3.55 ± 0.12 | MS, RI | |||
| 13 | 1047 | trans-β-ocimene | 19.41 ± 4.26 | 0.79 ± 0.24 | 23.04 ± 0.31 | 12.43 ± 0.75 | 14.67 ± 2.20 | 16.45 ± 3.81 | 7.47 ± 0.65 | 2.41 ± 0.20 | MS, RI | |
| 14 | 1052 | γ-terpinene | 0.77 ± 0.04 | 0.91 ± 0.08 | 0.70 ± 0.05 | 1.46 ± 0.07 | 1.60 ± 0.07 | 0.42 ± 0.05 | 0.25 ± 0.03 | MS, RI | ||
| 15 | 1065 | isoterpinolene | 0.12 ± 0.02 | MS, RI | ||||||||
| 16 | 1083 | terpinolene | 0.91 ± 0.11 | 14.74 ± 1.53 | 0.90 ± 0.01 | 1.63 ± 0.09 | 1.24 ± 0.11 | 8.08 ± 0.61 | MS, RI, STD | |||
| 17 | 1101 | durene | 0.15 ± 0.00 | 0.27 ± 0.06 | MS, RI | |||||||
| 18 | 1114 | p-mentha-1,5,8-triene | 0.32 ± 0.04 | 0.14 ± 0.01 | MS, RI | |||||||
| 19 | 1122 | allo-ocimene | 2.50 ± 0.47 | MS, RI | ||||||||
| 20 | 1123 | 1,3,8-p-menthatriene | 5.59 ± 0.35 | 1.70 ± 0.48 | 5.74 ± 1.22 | 1.88 ± 0.13 | MS, RI | |||||
| 21 | 1134 | neo-allo-ocimene | 1.35 ± 0.30 | 1.48 ± 0.12 | 1.35 ± 0.06 | 0.87 ± 0.15 | 1.33 ± 0.28 | 0.46 ± 0.03 | 0.71 ± 0.08 | MS, RI | ||
| 22 | 1227 | thymol methyl ether | 0.23 ± 0.05 | MS, RI | ||||||||
| 23 | 1276 | L-bornyl acetate | 0.13 ± 0.05 | MS, RI | ||||||||
| Sesquiterpenes | ||||||||||||
| 24 | 1340 | α-cubebene | 0.40 ± 0.03 | 0.22 ± 0.06 | 0.29 ± 0.09 | 0.94 ± 0.07 | 1.47 ± 0.32 | MS, RI | ||||
| 25 | 1357 | cyclosativene | 0.21 ± 0.01 | 0.21 ± 0.06 | 0.27 ± 0.04 | 2.08 ± 0.77 | MS, RI | |||||
| 26 | 1366 | ylangene | 0.98 ± 0.36 | 0.42 ± 0.08 | 0.22 ± 0.07 | 0.70 ± 0.20 | 1.29 ± 0.04 | 1.66 ± 0.43 | MS, RI | |||
| 27 | 1371 | daucene | 0.64 ± 0.44 | MS, RI | ||||||||
| 28 | 1375 | β-bourbonene | 0.14 ± 0.03 | MS, RI | ||||||||
| 29 | 1381 | β-cubebene | 0.27 ± 0.01 | 0.19 ± 0.06 | 0.69 ± 0.02 | MS, RI | ||||||
| 30 | 1384 | β-elemene | 1.22 ± 0.10 | 4.38 ± 1.51 | MS, RI | |||||||
| 31 | 1400 | α-gurjunene | 0.59 ± 0.41 | MS, RI | ||||||||
| 32 | 1410 | caryophyllene | 2.59 ± 1.68 | 2.39 ± 0.50 | 6.25 ± 0.33 | 1.45 ± 0.29 | 4.44 ± 1.04 | 0.34 ± 0.06 | 2.25 ± 0.11 | 24.54 ± 5.05 | MS, RI, STD | |
| 33 | 1420 | β-copaene | 1.03 ± 0.51 | 0.69 ± 0.03 | 0.39 ± 0.13 | 0.52 ± 0.18 | 0.20 ± 0.05 | 0.66 ± 0.06 | 2.53 ± 0.22 | MS, RI | ||
| 34 | 1427 | γ-elemene | 2.18 ± 0.16 | 1.38 ± 0.67 | MS, RI | |||||||
| 35 | 1429 | α-bergamotene | 0.27 ± 0.02 | 0.92 ± 0.14 | MS, RI | |||||||
| 36 | 1430 | calarene | 0.21 ± 0.01 | 0.18 ± 0.05 | MS, RI | |||||||
| 37 | 1445 | humulene | 0.20 ± 0.12 | 1.05 ± 0.06 | 1.39 ± 0.36 | MS, RI | ||||||
| 38 | 1452 | (E)-β-farnesene | 0.51 ± 0.34 | 4.31 ± 0.96 | MS, RI | |||||||
| 39 | 1455 | cis-muurola-4(15),5-diene | 0.90 ± 0.03 | 0.65 ± 0.26 | 0.17 ± 0.04 | 0.32 ± 0.04 | MS, RI | |||||
| 40 | 1471 | γ-muurolene | 0.40 ± 0.21 | 1.54 ± 0.27 | 0.84 ± 0.26 | 1.03 ± 0.35 | 0.36 ± 0.09 | 0.79 ± 0.07 | 0.93 ± 0.19 | MS, RI | ||
| 41 | 1474 | germacrene D | 0.55 ± 0.38 | 3.82 ± 0.39 | 1.97 ± 0.63 | 2.76 ± 0.86 | 0.78 ± 0.21 | 0.39 ± 0.09 | 1.68 ± 0.15 | MS, RI | ||
| 42 | 1477 | α-curcumene | 2.04 ± 0.55 | MS, RI | ||||||||
| 43 | 1479 | γ-gurjunene | 0.19 ± 0.02 | MS, RI | ||||||||
| 44 | 1488 | valencene | 0.72 ± 0.24 | 0.89 ± 0.32 | 0.28 ± 0.07 | 0.80 ± 0.06 | 0.95 ± 0.21 | MS, RI | ||||
| 45 | 1494 | α-muurolene | 0.87 ± 0.43 | 0.67 ± 0.22 | 0.71 ± 0.24 | 0.33 ± 0.08 | 1.05 ± 0.05 | 1.45 ± 0.23 | MS, RI | |||
| 46 | 1501 | 10-epizonarene | 0.23 ± 0.12 | 0.16 ± 0.06 | MS, RI | |||||||
| 47 | 1504 | β-bisabolene | 7.06 ± 1.75 | MS, RI | ||||||||
| 48 | 1508 | γ-cadinene | 1.27 ± 0.19 | 0.71 ± 0.23 | 0.83 ± 0.29 | 0.27 ± 0.07 | 0.40 ± 0.00 | 1.05 ± 0.20 | MS, RI | |||
| 49 | 1519 | β-cadinene | 0.58 ± 0.34 | 2.26 ± 0.24 | 1.42 ± 0.41 | 1.55 ± 0.53 | 0.56 ± 0.14 | 0.33 ± 0.07 | 2.82 ± 0.06 | 6.82 ± 2.17 | MS, RI | |
| 50 | 1527 | 1,4-cadinadiene | 0.19 ± 0.06 | 0.27 ± 0.01 | 0.74 ± 0.12 | MS, RI | ||||||
| 51 | 1532 | α-cadinene | 0.28 ± 0.10 | 0.32 ± 0.12 | 0.19 ± 0.01 | MS, RI | ||||||
| Others | ||||||||||||
| 52 | 1074 | 2-ethyl-p-xylene | 0.48 ± 0.05 | MS, RI | ||||||||
| 78.60 | 79.73 | 84.31 | 88.80 | 91.90 | 86.38 | 87.39 | 88.18 | 72.24 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.-Y.; Ahn, M.-S.; Mekapogu, M.; Jung, J.-A.; Song, H.-Y.; Lim, S.-H.; Jin, J.-S.; Kwon, O.-K. Analysis of Floral Scent and Volatile Profiles of Different Aster Species by E-nose and HS-SPME-GC-MS. Metabolites 2023, 13, 503. https://doi.org/10.3390/metabo13040503
Song S-Y, Ahn M-S, Mekapogu M, Jung J-A, Song H-Y, Lim S-H, Jin J-S, Kwon O-K. Analysis of Floral Scent and Volatile Profiles of Different Aster Species by E-nose and HS-SPME-GC-MS. Metabolites. 2023; 13(4):503. https://doi.org/10.3390/metabo13040503
Chicago/Turabian StyleSong, Seung-Yeop, Myung-Suk Ahn, Manjulatha Mekapogu, Jae-A Jung, Hyun-Young Song, So-Hyeon Lim, Jong-Sik Jin, and Oh-Keun Kwon. 2023. "Analysis of Floral Scent and Volatile Profiles of Different Aster Species by E-nose and HS-SPME-GC-MS" Metabolites 13, no. 4: 503. https://doi.org/10.3390/metabo13040503