Association between Tryptophan Metabolism and Inflammatory Biomarkers in Dairy Cows with Ketosis
Abstract
:1. Introduction
2. Materials and Methods
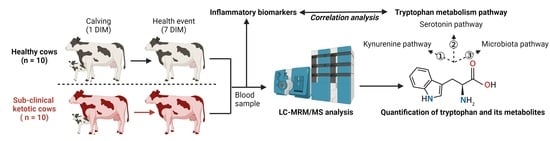
2.1. Animals and Study Design
2.2. Serum Markers Analyses
2.3. Quantification of Tryptophan and Its Metabolites
2.4. Statistical Analysis
3. Results
3.1. Alteration of Milk Yield, Dry Matter Intake, and Serum Markers in the Healthy and Ketotic Cows
3.2. Alteration in Tryptophan Metabolism
3.3. Association between Tryptophan Metabolism and Inflammatory Biomarkers during Ketosis Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of Dairy Cows During the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Mann, S.; Nydam, D.V.; Overton, T.R.; McArt, J.A. Short communication: Concentrations of nonesterified fatty acids and beta-hydroxybutyrate in dairy cows are not well correlated during the transition period. J. Dairy Sci. 2015, 98, 6284–6290. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A.A.; Gruys, E.; van Eerdenburg, F.J. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet. Q. 2005, 27, 117–126. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef] [Green Version]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and beta-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef]
- Tremblay, M.; Kammer, M.; Lange, H.; Plattner, S.; Baumgartner, C.; Stegeman, J.A.; Duda, J.; Mansfeld, R.; Dopfer, D. Identifying poor metabolic adaptation during early lactation in dairy cows using cluster analysis. J. Dairy Sci. 2018, 101, 7311–7321. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef] [Green Version]
- McArt, J.; Nydam, D.; Oetzel, G. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrenho, R.C.; Williamson, M.; Berke, O.; LeBlanc, S.J.; DeVries, T.J.; McBride, B.W.; Duffield, T.F. An investigation of blood, milk, and urine test patterns for the diagnosis of ketosis in dairy cows in early lactation. J. Dairy Sci. 2022, 105, 7719–7727. [Google Scholar] [CrossRef] [PubMed]
- Cascone, G.; Licitra, F.; Stamilla, A.; Amore, S.; Dipasquale, M.; Salonia, R.; Antoci, F.; Zecconi, A. Subclinical Ketosis in Dairy Herds: Impact of Early Diagnosis and Treatment. Front. Vet. Sci. 2022, 9, 895468. [Google Scholar] [CrossRef]
- Berge, A.C.; Vertenten, G. A field study to determine the prevalence, dairy herd management systems, and fresh cow clinical conditions associated with ketosis in western European dairy herds. J. Dairy Sci. 2014, 97, 2145–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steeneveld, W.; Amuta, P.; van Soest, F.J.S.; Jorritsma, R.; Hogeveen, H. Estimating the combined costs of clinical and subclinical ketosis in dairy cows. PLoS ONE 2020, 15, e0230448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cainzos, J.M.; Andreu-Vazquez, C.; Guadagnini, M.; Rijpert-Duvivier, A.; Duffield, T. A systematic review of the cost of ketosis in dairy cattle. J. Dairy Sci. 2022, 105, 6175–6195. [Google Scholar] [CrossRef]
- Raboisson, D.; Mounie, M.; Khenifar, E.; Maigne, E. The economic impact of subclinical ketosis at the farm level: Tackling the challenge of over-estimation due to multiple interactions. Prev. Vet. Med. 2015, 122, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Leroy, J.; Opsomer, G. Metabolic Stress in the Transition Period of Dairy Cows: Focusing on the Prepartum Period. Animals 2020, 10, 1419. [Google Scholar] [CrossRef]
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, H.B.; Schoenberg, K.M.; et al. Inflammatory biomarkers are associated with ketosis in periparturient Holstein cows. Res. Vet. Sci. 2016, 109, 81–85. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, Y.; Xiao, J.; Qian, B.; You, L.; Zhang, Y.; Yu, S.; Zong, X.; Cao, S. Serum adipokines play different roles in type I and II ketosis. Asian-Australas. J. Anim. Sci. 2020, 33, 1930–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodzki, P.; Marczuk, J.; Lisiecka, U.; Szczubial, M.; Brodzki, A.; Gorzkos, H.; Kulpa, K. Comparative evaluation of cytokine and acute-phase protein concentrations in sera of dairy cows with subclinical and clinical ketosis as a different view of the causes of the disease. Vet. World 2021, 14, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, D.A. Biochemistry of tryptophan in health and disease. Mol. Asp. Med. 1983, 6, 101–197. [Google Scholar] [CrossRef] [PubMed]
- Lesurtel, M.; Soll, C.; Graf, R.; Clavien, P.A. Role of serotonin in the hepato-gastroIntestinal tract: An old molecule for new perspectives. Cell Mol. Life Sci. 2008, 65, 940–952. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Vervoort, J.; Saccenti, E.; Kemp, B.; van Hoeij, R.J.; van Knegsel, A.T.M. Relationship between energy balance and metabolic profiles in plasma and milk of dairy cows in early lactation. J. Dairy Sci. 2020, 103, 4795–4805. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Xia, C.; Zhang, H.; Sun, L.; Gao, Y. Plasma metabolic profiling of dairy cows affected with clinical ketosis using LC/MS technology. Vet. Q. 2014, 34, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Laporta, J.; Moore, S.A.; Peters, M.W.; Peters, T.L.; Hernandez, L.L. Short communication: Circulating serotonin (5-HT) concentrations on day 1 of lactation as a potential predictor of transition-related disorders. J. Dairy Sci. 2013, 96, 5146–5150. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.; Luo, Z.Z.; Luo, Q.; Yang, Q.W.; Huang, Y.X.; Zhao, X.X.; Zhang, Y.; Cao, S.Z. Plasma metabolome alteration in dairy cows with left displaced abomasum before and after surgical correction. J. Dairy Sci. 2021, 104, 8177–8187. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Yong, K.; Huang, Y.X.; Zhang, C.S.; Shen, L.H.; Jiang, H.; Yang, X.; Zhang, Y.; Cao, S.Z. Effects of replacement of corn silage and distillers’ grain diets with hay during prepartum on the health and production performance of dairy heifers postpartum. J. Anim. Physiol. Anim. Nutr. 2022, 106, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.T.; Ghassemi Nejad, J.; Moon, J.O.; Lee, H.G. Dietary supplementation of acetate-conjugated tryptophan alters feed intake, milk yield and composition, blood profile, physiological variables, and heat shock protein gene expression in heat-stressed dairy cows. J. Therm. Biol. 2021, 98, 102949. [Google Scholar] [CrossRef]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.P.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367–1377. [Google Scholar] [CrossRef]
- Loor, J.J.; Everts, R.E.; Bionaz, M.; Dann, H.M.; Morin, D.E.; Oliveira, R.; Rodriguez-Zas, S.L.; Drackley, J.K.; Lewin, H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007, 32, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.; Kang, S.; Han, M.; Lee, J.; Chung, H.; Oh, S.I.; Kim, S.; Park, J. Predicting ketosis during the transition period in Holstein Friesian cows using hematological and serum biochemical parameters on the calving date. Sci. Rep. 2022, 12, 853. [Google Scholar] [CrossRef]
- Turk, R.; Podpečan, O.; Mrkun, J.; Flegar-Meštrić, Z.; Perkov, S.; Zrimšek, P. The Effect of Seasonal Thermal Stress on Lipid Mobilisation, Antioxidant Status and Reproductive Performance in Dairy Cows. Reprod. Domest. Anim. 2015, 50, 595–603. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. The omics side of fatty liver: A holistic approach for a commonly occurring peripartal disease. In Periparturient Diseases of Dairy Cows: A Systems Biology Approach; Ametaj, B.N., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 223–246. [Google Scholar]
- Grummer, R.R. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef]
- Mezzetti, M.; Bionaz, M.; Trevisi, E. Interaction between inflammation and metabolism in periparturient dairy cows. J. Anim. Sci. 2020, 98, S155–S174. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Tóthová, C.; Nagy, O.; Kováč, G. Acute phase proteins and their use in the diagnosis of diseases in ruminants: A review. J. Vet. Med. 2018, 59, 163–180. [Google Scholar]
- Kováč, G.; Tóthová, C.; Nagy, O.; Seidel, H.; Konvičná, J. Acute phase proteins and their relation to energy metabolites in dairy cows during the pre-and postpartal period. J. Acta Vet. Brno 2009, 78, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied. Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Guo, Y.S.; Tao, J.Z.; Xu, L.H.; Wei, F.H.; He, S.H. Identification of disordered metabolic networks in postpartum dairy cows with left displacement of the abomasum through integrated metabolomics and pathway analyses. J. Vet. Med. Sci. 2020, 82, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Hayaishi, O. Properties and function of indoleamine 2,3-dioxygenase. J. Biochem. 1976, 79, 13–21. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Connor, T.J.; Starr, N.; O’Sullivan, J.B.; Harkin, A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-gamma? Neurosci. Lett. 2008, 441, 29–34. [Google Scholar] [CrossRef]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1609–1616. [Google Scholar] [CrossRef]
- Larsson, M.K.; Faka, A.; Bhat, M.; Imbeault, S.; Goiny, M.; Orhan, F.; Oliveros, A.; Stahl, S.; Liu, X.C.; Choi, D.S.; et al. Repeated LPS Injection Induces Distinct Changes in the Kynurenine Pathway in Mice. Neurochem. Res. 2016, 41, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Millischer, V.; Heinzl, M.; Faka, A.; Resl, M.; Trepci, A.; Klammer, C.; Egger, M.; Dieplinger, B.; Clodi, M.; Schwieler, L. Intravenous administration of LPS activates the kynurenine pathway in healthy male human subjects: A prospective placebo-controlled cross-over trial. J. Neuroinflam. 2021, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Vecsei, L.; Szalardy, L.; Fulop, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; Zhao, Y.; Yang, L.; Cheng, J.; Hua, R.; Zhang, Z.; Li, Q. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J. Pineal Res. 2020, 69, e12690. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horst, E.A.; Kvidera, S.K.; Abuajamieh, M.; Mayorga, E.J.; Al-Qaisi, M.; Baumgard, L.H. Short communication: Ketosis, feed restriction, and an endotoxin challenge do not affect circulating serotonin in lactating dairy cows. J. Dairy Sci. 2019, 102, 11736–11743. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Bao, L.; Qiu, M.; Feng, L.; Chen, L.; Liu, Z.; Duan, S.; Zhao, Y.; Wu, K.; Zhang, N.; et al. Dietary Tryptophan-Mediated Aryl Hydrocarbon Receptor Activation by the Gut Microbiota Alleviates Escherichia coli-Induced Endometritis in Mice. Microbiol. Spectr. 2022, 10, e0081122. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Macfarlane, G.T. Enumeration of human colonic bacteria producing phenolic and indolic compounds: Effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J. Appl. Bacteriol. 1996, 81, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, R.; Wyatt, C.M. Manipulating the gut microbiome to decrease uremic toxins. Kidney Int. 2017, 91, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; Dinatale, B.C.; Murray, I.A.; Flaveny, C.A.; Liu, Q.; Laurenzana, E.M.; Lin, J.M.; Strom, S.C.; Omiecinski, C.J.; Amin, S.; et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010, 49, 393–400. [Google Scholar] [CrossRef] [Green Version]




| Item | Healthy Cows (n = 10) | Sub-Clinical Ketotic Cows (n = 10) |
|---|---|---|
| Calf weight (kg), female | 37.90 ± 0.84 | 38.20 ± 0.97 |
| Calving ease score | 1.10 ± 0.09 | 1.20 ± 0.13 |
| Age (month) 1 | 27.34 ± 1.06 | 28.08 ± 0.94 |
| Day of pregnancy (d) | 275.40 ± 1.06 | 274.50 ± 0.47 |
| Body condition score 2 | 3.50 ± 0.04 | 3.60 ± 0.06 |
| BHBA concentration at 1 DIM | 0.63 ± 0.03 | 0.72 ± 0.04 |
| BHBA concentration at 7 DIM | 0.89 ± 0.05 | 2.36 ± 0.09 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Yong, K.; Du, Z.; Huang, Y.; Zhou, T.; Ma, L.; Yao, X.; Shen, L.; Yu, S.; Yan, Z.; et al. Association between Tryptophan Metabolism and Inflammatory Biomarkers in Dairy Cows with Ketosis. Metabolites 2023, 13, 333. https://doi.org/10.3390/metabo13030333
Luo Z, Yong K, Du Z, Huang Y, Zhou T, Ma L, Yao X, Shen L, Yu S, Yan Z, et al. Association between Tryptophan Metabolism and Inflammatory Biomarkers in Dairy Cows with Ketosis. Metabolites. 2023; 13(3):333. https://doi.org/10.3390/metabo13030333
Chicago/Turabian StyleLuo, Zhengzhong, Kang Yong, Zhenlong Du, Yixin Huang, Tao Zhou, Li Ma, Xueping Yao, Liuhong Shen, Shumin Yu, Zuoting Yan, and et al. 2023. "Association between Tryptophan Metabolism and Inflammatory Biomarkers in Dairy Cows with Ketosis" Metabolites 13, no. 3: 333. https://doi.org/10.3390/metabo13030333