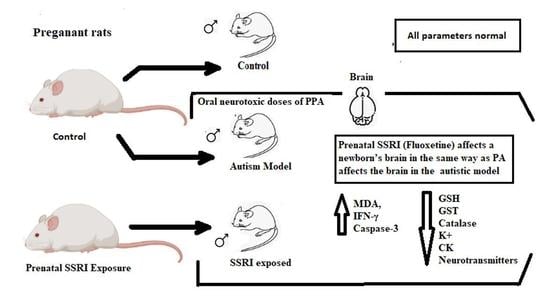
Prenatal SSRI Exposure Increases the Risk of Autism in Rodents via Aggravated Oxidative Stress and Neurochemical Changes in the Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain Tissue Collection
2.3. Biochemical Analyses
2.3.1. Lipid Peroxidation
2.3.2. Glutathione
2.3.3. Glutathione-S-Transferase
2.3.4. Catalase
2.3.5. Potassium
2.3.6. Neurotransmitter
2.3.7. Creatine Kinase
2.3.8. Caspase-3
2.3.9. Interferon Gamma
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Courtiol, E.; Castellanos, F.X.; Teixeira, C.M. Abnormal Serotonin Levels During Perinatal Development Lead to Behavioral Deficits in Adulthood. Front. Behav. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesch, K.-P.; Waider, J. Serotonin in the Modulation of Neural Plasticity and Networks: Implications for Neurodevelopmental Disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azmitia, E.C. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001, 56, 413–424. [Google Scholar] [CrossRef]
- Saldanha, D.; Kumar, N.; Ryali, V.; Srivastava, K.; Pawar, A. Serum Serotonin Abnormality in Depression. Med. J. Armed Forces India 2009, 65, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, O.M.; Morsy, S.M.Y.; Sleem, A.A. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI J. 2011, 10, 290–302, PMCID:PMC5611632. [Google Scholar] [PubMed]
- Ornoy, A.; Koren, G. SSRIs and SNRIs (SRI) in Pregnancy: Effects on the Course of Pregnancy and the Offspring: How Far Are We from Having All the Answers? Int. J. Mol. Sci. 2019, 20, 2370. [Google Scholar] [CrossRef] [Green Version]
- Rotem-Kohavi, N.; Williams, L.J.; Virji-Babul, N.; Bjornson, B.; Brain, U.; Werker, J.F.; Grunau, R.E.; Miller, S.; Oberlander, T.F. Alterations in Resting-State Networks Following In Utero Selective Serotonin Reuptake Inhibitor Exposure in the Neonatal Brain. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2018, 4, 39–49. [Google Scholar] [CrossRef]
- Yonkers, K.A.; Blackwell, K.A.; Glover, J.; Forray, A. Antidepressant Use in Pregnant and Postpartum Women. Annu. Rev. Clin. Psychol. 2014, 10, 369–392. [Google Scholar] [CrossRef] [Green Version]
- Pei, S.; Liu, L.; Zhong, Z.; Wang, H.; Lin, S.; Shang, J. Risk of prenatal depression and stress treatment: Alteration on serotonin system of offspring through exposure to Fluoxetine. Sci. Rep. 2016, 6, 33822. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, J.H.; Ilett, K.F.; Hackett, L.P.; Yapp, P.; Paech, M.; Begg, E.J. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br. J. Clin. Pharmacol. 1999, 48, 521–527. [Google Scholar] [CrossRef]
- Zengeler, K.E.; Shapiro, D.A.; Bruch, K.R.; Lammert, C.R.; Ennerfelt, H.; Lukens, J.R. SSRI treatment modifies the effects of maternal inflammation on in utero physiology and offspring neurobiology. Brain, Behav. Immun. 2023, 108, 80–97. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef] [Green Version]
- Casper, R.C.; Fleisher, B.E.; Lee-Ancajas, J.C.; Gilles, A.; Gaylor, E.; DeBattista, A.; Hoyme, H. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J. Pediatr. 2003, 142, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, L.H.; Henriksen, T.B.; Olsen, J. Fetal exposure to antidepressantand normal milestone development at 6 and 19 months of age. Pediatrics 2010, 125, e600–e608. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Grunau, R.E.; Fitzgerald, C.; Papsdorf, M.; Rurak, D.; Riggs, W. Pain reactivity in 2-month-old infants after prenatal and postnatal selectiveserotonin reuptake inhibitor medication exposure. Pediatrics 2005, 115, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Grether, J.K.; Yoshida, C.K.; Odouli, R.; Hendrick, V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011, 68, 1104–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, C.; Anacker, A.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [Green Version]
- De Grandis, E.; Serrano, M.; Pérez-Dueñas, B.; Ormazábal, A.; Montero, R.; Veneselli, E.; Pineda, M.; González, V.; Sanmartí, F.; Fons, C.; et al. Cerebrospinal fluid alterations of the serotonin product, 5-hydroxyindolacetic acid, in neurological disorders. J. Inherit. Metab. Dis. 2010, 33, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; De Bildt, A.; Van Lang, N.D.; Den Boer, J.A.; Minderaa, R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55. [Google Scholar] [CrossRef] [PubMed]
- Malwane, M.I.; Nguyen, E.B.; Trejo, S.; Kim, E.Y.; Cucalón-Calderón, J.R. A Delayed Diagnosis of Autism Spectrum Disorder in the Setting of Complex Attention Deficit Hyperactivity Disorder. Cureus 2022, 14, e25825. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Yang, T.; Chen, L.; Dai, Y.; Jia, F.; Hao, Y.; Li, L.; Zhang, J.; Wu, L.; Ke, X.; Yi, M.; et al. Vitamin A Status Is More CommonlyAssociated with Symptoms and Neurodevelopment in Boys with Autism Spectrum Disorders-A Multicenter Study in China. Front. Nutr. 2022, 9, 851980. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Kapra, O.; Rotem, R.; Gross, R. The Association Between Prenatal Exposure to Antidepressants and Autism: Some Research and Public Health Aspects. Front. Psychiatry 2020, 11, 55740. [Google Scholar] [CrossRef]
- Mathew, S.; Bichenapally, S.; Khachatryan, V.; Muazzam, A.; Hamal, C.; Velugoti, L.S.D.R.; Tabowei, G.; Gaddipati, G.N.; Mukhtar, M.; Alzubaidee, M.J.; et al. Role of Serotoninergic Antidepressants in the Development of Autism Spectrum Disorders: A Systematic Review. Cureus 2022, 14, e28505. [Google Scholar] [CrossRef]
- Sujan, A.C.; Öberg, A.S.; Quinn, P.D.; D’Onofrio, B.M. Annual Research Review: Maternal antidepressant use during pregnancy and offspring neurodevelopmental problems—A critical review and recommendations for future research. J. Child Psychol. Psychiatry 2018, 60, 356–376. [Google Scholar] [CrossRef] [Green Version]
- El-Ansary, A.K.; Ben Bacha, A.; Kotb, M. Etiology of autistic features: The persisting neurotoxic effects of propionic acid. J. Neuroinflammation 2012, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiji, K.N.; Muralidharan, P. Evaluation of the protective effect of Clitoria ternatea L. against propionic acid induced autistic spectrum disorders in rat model. Bull. Natl. Res. Cent. 2022, 46, 71. [Google Scholar] [CrossRef]
- Abuaish, S.; Al-Otaibi, N.M.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; AlShawakir, Y.A.; Aabed, K.; El-Ansary, A. Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism. Brain Sci. 2021, 11, 1038. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Van Donkelaar, E.; Abrams, Z.; Houbart, V.; Fillet, M.; Steinbusch, H.W.M.; Charlier, T.D. Fluoxetine Dose and Administration Method Differentially Affect Hippocampal Plasticity in Adult Female Rats. Neural Plast. 2014, 2014, 123026. [Google Scholar] [CrossRef]
- Bhat, R.S.; GezeerY, A.E.; Bachan, A.B.; AlonazI, M.A.; Alsuhaibani, L.S.; El-Ansary, A. Prenatal exposure to the fluoride containing psychiatric drug fluoxetine and anti-oxidative alterations in the neonatal rat brain. Biocell 2019, 43, 65–71. [Google Scholar]
- Ruiz-Larrea, M.B.; Leal, A.M.; Liza, M.; Lacort, M.; de Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 1994, 59, 383–388. [Google Scholar] [CrossRef]
- Beutle, E.; Duran, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Chance, B. Catalases and peroxidess, part II. Special methods. Methods Biochem. Anal. 1954, 1, 408. [Google Scholar]
- Terri, A.E.; Sesin, P.G. Determination of potassium in blood serum. Am. Soc. Clin. Pathol. 1958, 29, 86–89. [Google Scholar] [CrossRef]
- Zagrodzka, J.; Romaniuk, A.; Wieczorek, M.; Boguszewski, P. Bicuculline administration into ventromedial hypothalamus: Effects on fear and regional brainmonoamines and GABA concentrations in rats. Acta Neurobiol. Exp. 2000, 60, 333–343. [Google Scholar]
- Boukhris, T.; Sheehy, O.; Mottron, L.; Bérard, A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016, 170, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, H.K.; Hussain-Shamsy, N.; Lunsky, Y.; Dennis, C.L.E.; Vigod, S.N. The Association between antenatal exposure to selective serotonin reuptake inhibitors and autism: A systematic review and meta-analysis. J. Clin. Psychiatry 2017, 78, e48. [Google Scholar] [CrossRef] [PubMed]
- Gidaya, N.B.; Lee, B.K.; Burstyn, I.; Yudell, M.; Mortensen, E.L.; Newschaffer, C.J. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J. Autism. Dev. Disord. 2014, 44, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Lee, B.K.; Dalman, C.; Golding, J.; Lewis, G.; Magnusson, C. Parental depression, maternalantidepressant use during pregnancy, and risk of autism spectrum disorders:population based case-control study. BMJ 2013, 346, f2059. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.H. News Feature: Better models for brain disease. Proc. Natl. Acad. Sci. USA 2016, 113, 5461–5464. [Google Scholar] [CrossRef] [Green Version]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [Green Version]
- Pugsley, K.; Scherer, S.W.; Bellgrove, M.A.; Hawi, Z. Environmental exposures associated with elevated risk for autism spectrum disorder may augment the burden of deleterious de novo mutations among probands. Mol. Psychiatry 2021, 27, 710–730. [Google Scholar] [CrossRef]
- Parker, W.; Hornik, C.D.; Bilbo, S.; Holzknecht, Z.E.; Gentry, L.; Rao, R.; Lin, S.S.; Herbert, M.R.; Nevison, C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J. Int. Med. Res. 2017, 45, 407–438. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [Green Version]
- Quaak, I.; Brouns, M.R.; Van de Bor, M. The dynamics of autism spectrum disorders: How neurotoxic compounds and neurotransmitters interact. Int. J. Environ. Res. Public Health 2013, 10, 3384–3408. [Google Scholar] [CrossRef] [Green Version]
- Janecka, M.; Kodesh, A.; Levine, S.Z.; Lusskin, S.I.; Viktorin, A.; Rahman, R.; Buxbaum, J.D.; Schlessinger, A.; Sandin, S.; Reichenberg, A. Association of Autism Spectrum Disorder With Prenatal Exposure to Medication Affecting Neurotransmitter Systems. JAMA Psychiatry 2018, 75, 1217–1224. [Google Scholar] [CrossRef]
- Goines, P.E.; Ashwood, P. Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicology Teratol. 2012, 36, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Goines, P.E.; Croen, L.A.; Braunschweig, D.; Yoshida, C.K.; Grether, J.; Hansen, R.; Kharrazi, M.; Ashwood, P.; Van de Water, J. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol. Autism 2011, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Robinson-Agramonte, M.D.L.A.; García, E.N.; Guerra, J.F.; Hurtado, Y.V.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]


| Parameters | Groups | N | Min. | Max. | Mean ± S.D. | Percent Change | p Value a | p Value b |
|---|---|---|---|---|---|---|---|---|
| MDA (µmoles/mL) | Control | 10 | 0.31 | 0.35 | 0.330 ± 0.014 | 100.00 | 0.001 | |
| PPA | 10 | 0.45 | 0.47 | 0.460 ± 0.009 | 139.39 | 0.001 | ||
| FLX | 10 | 0.35 | 0.37 | 0.360 ± 0.009 | 109.09 | 0.001 | ||
| GSH (ug/mL) | Control | 10 | 33.20 | 37.55 | 36.19 ± 1.66 | 100.00 | 0.001 | |
| PPA | 10 | 28.99 | 31.20 | 30.04 ± 0.83 | 83.00 | 0.001 | ||
| FLX | 10 | 15.60 | 17.60 | 16.45 ± 0.86 | 45.45 | 0.001 | ||
| Catalase (U/dl) | Control | 10 | 5.25 | 7.50 | 6.23 ± 0.87 | 100.00 | 0.001 | |
| PPA | 10 | 4.20 | 5.30 | 4.67 ± 0.45 | 75.01 | 0.002 | ||
| FLX | 10 | 3.89 | 5.20 | 4.59 ± 0.56 | 73.73 | 0.001 | ||
| GST (U/mL) | Control | 10 | 13.22 | 18.22 | 15.47 ± 1.71 | 100.00 | 0.001 | |
| PPA | 10 | 11.22 | 13.80 | 12.55 ± 0.97 | 81.11 | 0.002 | ||
| FLX | 10 | 7.10 | 9.60 | 8.56 ± 0.84 | 55.33 | 0.001 | ||
| K+ (mmol/L) | Control | 10 | 5.10 | 6.33 | 5.60 ± 0.53 | 100.00 | 0.001 | |
| PPA | 10 | 4.31 | 5.22 | 4.82 ± 0.34 | 86.01 | 0.010 | ||
| FLX | 10 | 2.50 | 3.44 | 2.94 ± 0.35 | 52.43 | 0.001 | ||
| CK (IU/L) | Control | 10 | 250.33 | 311.80 | 284.91 ± 22.10 | 100.00 | 0.001 | |
| PPA | 10 | 275.55 | 300.22 | 287.07 ± 10.38 | 100.76 | 0.954 | ||
| FLX | 10 | 320.77 | 340.89 | 330.94 ± 7.17 | 116.16 | 0.001 | ||
| IF γ (pg/100 mg) | Control | 10 | 87.50 | 90.44 | 89.18 ± 1.15 | 100.00 | 0.001 | |
| PPA | 10 | 111.44 | 115.33 | 113.50 ± 1.42 | 127.27 | 0.001 | ||
| FLX | 10 | 90.40 | 99.55 | 93.10 ± 3.28 | 104.39 | 0.013 | ||
| Norepinephrine (ng/100 mg) | Control | 10 | 4.55 | 5.50 | 5.05 ± 0.32 | 100.00 | 0.001 | |
| PPA | 10 | 3.09 | 3.88 | 3.46 ± 0.27 | 68.55 | 0.001 | ||
| FLX | 10 | 3.99 | 5.11 | 4.82 ± 0.42 | 95.45 | 0.416 | ||
| Serotonin (ng/100 mg) | Control | 10 | 5.95 | 7.10 | 6.63 ± 0.39 | 100.00 | 0.001 | |
| PPA | 10 | 3.99 | 4.42 | 4.23 ± 0.14 | 63.87 | 0.001 | ||
| FLX | 10 | 4.50 | 5.02 | 4.77 ± 0.18 | 72.03 | 0.001 | ||
| Dopamine (ng/100 mg) | Control | 10 | 20.97 | 22.50 | 21.88 ± 0.50 | 100.00 | 0.001 | |
| PPA | 10 | 14.59 | 16.00 | 15.08 ± 0.51 | 68.92 | 0.001 | ||
| FLX | 10 | 15.17 | 17.09 | 16.08 ± 0.76 | 73.48 | 0.001 | ||
| Caspase-3 (pg/100 mg) | Control | 10 | 108.40 | 113.18 | 110.83 ± 1.76 | 100.00 | 0.001 | |
| PPA | 10 | 138.90 | 145.30 | 142.56 ± 2.40 | 128.62 | 0.001 | ||
| FLX | 10 | 132.99 | 135.15 | 134.03 ± 0.93 | 120.93 | 0.001 |
| Dependent Variable | Predictor Variable | Coefficient | p Value | Adjusted R2 | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| MDA | IF γ | 0.005 | 0.000 | 0.950 | 0.004 | 0.006 |
| K+ | GSH | 0.134 | 0.000 | 0.887 | 0.110 | 0.159 |
| CK | −0.017 | 0.010 | −0.029 | −0.005 | ||
| CK | K+ | −16.297 | 0.000 | 0.922 | −22.599 | −9.996 |
| Catalase | 10.859 | 0.000 | 6.435 | 15.283 | ||
| GST | −3.047 | 0.021 | −5.552 | −0.542 | ||
| IF γ | MDA | 187.386 | 0.000 | 0.950 | 165.202 | 209.569 |
| Serotonin | Caspase-3 | −0.076 | 0.000 | 0.950 | −0.085 | −0.067 |
| Dopamine | Caspase-3 | −0.192 | 0.000 | 0.958 | −0.222 | −0.163 |
| GST | 0.202 | 0.005 | 0.072 | 0.331 | ||
| Caspase-3 | Serotonin | −5.793 | 0.002 | 0.984 | −9.090 | −2.495 |
| Dopamine | −0.800 | 0.209 | −2.107 | 0.507 | ||
| MDA | 82.120 | 0.000 | 44.826 | 119.413 | ||
| K+ | −2.005 | 0.011 | −3.478 | −0.532 | ||
| Parameters | Groups | AUC | Cut-off Value | Sensitivity % | Specificity % | p Value | 95% CI |
|---|---|---|---|---|---|---|---|
| MDA (µmoles/mL) | PPA | 1.000 | 0.400 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 0.972 | 0.345 | 100.0% | 83.3% | 0.006 | 0.889–1.056 | |
| GSH (ug/mL) | PPA | 1.000 | 32.200 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 1.000 | 25.400 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Catalase (U/dl) | PPA | 0.972 | 5.370 | 100.0% | 83.3% | 0.006 | 0.889–1.056 |
| FLX | 1.000 | 5.225 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| GST (U/mL) | PPA | 0.958 | 14.100 | 100.0% | 83.3% | 0.008 | 0.851–1.065 |
| FLX | 1.000 | 11.410 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| K+ (mmol/L) | PPA | 0.917 | 5.050 | 83.3% | 100.0% | 0.016 | 0.742–1.091 |
| FLX | 1.000 | 4.270 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| CK (IU/L) | PPA | 0.528 | 288.980 | 66.7% | 50.0% | 0.873 | 0.168–0.888 |
| FLX | 1.000 | 316.285 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| IF g (pg/100 mg) | PPA | 1.000 | 100.940 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 0.972 | 90.300 | 100.0% | 83.3% | 0.006 | 0.889–1.056 | |
| Norepinephrine (ng/100 mg) | PPA | 1.000 | 4.215 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 0.694 | 5.030 | 83.3% | 66.7% | 0.262 | 0.377–1.012 | |
| Serotonin (ng/100 mg) | PPA | 1.000 | 5.185 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 1.000 | 5.485 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Dopamine (ng/100 mg) | PPA | 1.000 | 18.485 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 1.000 | 19.030 | 100.0% | 100.0% | 0.004 | 1.000–1.000 | |
| Caspase-3 (pg/100 mg) | PPA | 1.000 | 126.040 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
| FLX | 1.000 | 123.085 | 100.0% | 100.0% | 0.004 | 1.000–1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, R.S.; Alonazi, M.; Al-Daihan, S.; El-Ansary, A. Prenatal SSRI Exposure Increases the Risk of Autism in Rodents via Aggravated Oxidative Stress and Neurochemical Changes in the Brain. Metabolites 2023, 13, 310. https://doi.org/10.3390/metabo13020310
Bhat RS, Alonazi M, Al-Daihan S, El-Ansary A. Prenatal SSRI Exposure Increases the Risk of Autism in Rodents via Aggravated Oxidative Stress and Neurochemical Changes in the Brain. Metabolites. 2023; 13(2):310. https://doi.org/10.3390/metabo13020310
Chicago/Turabian StyleBhat, Ramesa Shafi, Mona Alonazi, Sooad Al-Daihan, and Afaf El-Ansary. 2023. "Prenatal SSRI Exposure Increases the Risk of Autism in Rodents via Aggravated Oxidative Stress and Neurochemical Changes in the Brain" Metabolites 13, no. 2: 310. https://doi.org/10.3390/metabo13020310