Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata
Abstract
:1. Introduction
2. Materials and Methods
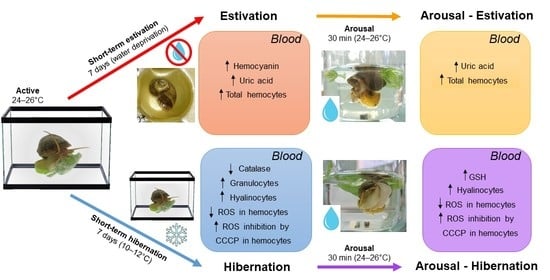
2.1. Animals and Experimental Conditions
2.2. Blood Withdrawal and Determination of Circulating Hemocyte Concentration
2.3. Biochemical Determinations
2.3.1. Total Protein Concentration
2.3.2. Hemocyanin
2.3.3. Lactate
2.3.4. Uric Acid
2.3.5. Reduced Glutathione (GSH)
2.3.6. Enzymatic Activities
2.4. Intracellular ROS Production
2.4.1. Hemocyte Exposure to DCF
2.4.2. Flow Cytometry: Hemocyte Gating and Analyses
2.5. ROS Inhibition by CCCP
2.6. Statistics
3. Results
3.1. Changes in the Blood Induced by Activity–Dormancy–Arousal Cycles
3.2. Changes in Circulating Hemocytes
3.3. Production of ROS by Circulating Hemocytes
3.4. ROS Inhibition by CCCP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Storey, K.; Storey, J. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q. Rev. Biol. 1990, 65, 145–174. [Google Scholar] [CrossRef] [PubMed]
- Storey, K. Turning down the fires of life: Metabolic regulation of hibernation and estivation. Mol. Mech. Metab. Arrest 2001, 126, S90. [Google Scholar] [CrossRef] [Green Version]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant defenses and animal adaptation to oxygen availability during environmental stress. In Cell and Molecular Responses to Stress; Storey, K.B., Storey, J.M., Eds.; Elsevier Press: Amsterdam, Germany, 2001; Volume 2—Protein Adaptations and Signal Transduction, pp. 263–287. [Google Scholar]
- Storey, K.B. Regulation of hypometabolism: Insights into epigenetic controls. J. Exp. Biol. 2015, 218, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2019; Volume 1. [Google Scholar]
- Hyman, L.H. Mollusca I. In The Invertebrates; McGraw-Hill: New York, NY, USA, 1967; Volume VI. [Google Scholar]
- Prashad, B. Anatomy of the Common Indian Apple-Snail, Pila Globosa; Zoological Survey of India: Kolkata, India, 1925.
- Coles, G. The termination of aestivation in the large fresh-water snail Pila ovata (ampulariidae)—I. Changes in oxygen uptake. Comp. Biochem. Physiol. 1968, 25, 517–522. [Google Scholar] [CrossRef]
- Burky, A.; Pacheco, J.; Pereyra, E. Temperature, water, and respiratory regimes of an amphibious snail, Pomacea urceus (Müller), from the Venezuelan savannah. Biol. Bull. 1972, 143, 304–316. [Google Scholar] [CrossRef]
- Hayes, K.; Burks, R.; Castro-Vazquez, A.; Darby, P.; Heras, H.; Martín, P.; Qiu, J.-W.; Thiengo, S.; Wada, T.; Yusa, Y.; et al. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 2015, 58, 245–302. [Google Scholar] [CrossRef] [Green Version]
- Giraud-Billoud, M.; Vega, I.A.; Rinaldi Tosi, M.E.; Abud, M.A.; Calderón, M.L.; Castro-Vazquez, A. Antioxidant and molecular chaperone defenses during estivation and arousal in the South American apple-snail Pomacea canaliculata. J. Exp. Biol. 2013, 216, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Giraud-Billoud, M.; Castro-Vazquez, A.; Campoy-Diaz, A.D.; Giuffrida, P.M.; Vega, I.A. Tolerance to hypometabolism and arousal induced by hibernation in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Campoy-Diaz, A.D.; Dellagnola, F.A.; Rodriguez, C.; Vega, I.A. Antioxidant Responses Induced by Short-Term Activity–Estivation–Arousal Cycle in Pomacea canaliculata. Front. Physiol. 2022, 13, 805168. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.C.; Campos, É.G.; Giraud-Billoud, M.; Storey, K.B.; Hermes-Lima, M. Commentary: On the merit of an early contributor of the “Preparation for Oxidative Stress” (POS) theory. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 276, 111341. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernández, J.M.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty years of the ‘Preparation for Oxidative Stress’ (POS) theory: Ecophysiological advantages and molecular strategies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 234, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comp. Biochem. Physiol.-Part B Biochem. Mol. Biol. 1998, 120, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.C.; Venancio, L.P.; Sabino, M.A.; Hermes-Lima, M. How widespread is preparation for oxidative stress in the animal kingdom? Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 200, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species a Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000. First published as special lift-out in Aliens; Volume 12, pp. 1–12. [Google Scholar]
- Jiang, X.; Zheng, P.; Soto, I.; Haubrock, P.J.; Chen, J.; Ji, L. Global economic costs and knowledge gaps of invasive gastropods. Ecol. Indic. 2022, 145, 109614. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Burela, S.; Martín, P.R. Influence of water temperature on the activity of the freshwater snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) at its southernmost limit (Southern Pampas, Argentina). J. Therm. Biol. 2010, 35, 77–84. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Abud, M.A.; Cueto, J.A.; Vega, I.A.; Castro-Vazquez, A. Uric acid deposits and estivation in the invasive apple-snail, Pomacea canaliculata. Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2011, 158, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Koch, E.; Vega, I.; Gamarra-Luques, C.; Castro-Vazquez, A. Urate cells and tissues in the South American apple snail Pomacea canaliculata. J. Molluscan Stud. 2008, 74, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Vega, I.; Giraud-Billoud, M.; Koch, E.; Gamarra-Luques, C.; Castro-Vazquez, A. Uric acid accumulation within intracellular corpuscles of the midgut gland in Pomacea canaliculata (Caenogastropoda, Ampullariidae). Veliger 2007, 48, 276–283. [Google Scholar]
- Huiping, Y. Immunological assays of hemocytes in molluscan bivalves as biomarkers to evaluate stresses for aquaculture. In Proceedings of the 47th UJNR. Bulletin of Japan Fisheries Research and Education Agency, Naha, Japan, 12–13 November 2019; pp. 31–45. [Google Scholar]
- Rodriguez, C.; Vega, I.A.; Castro-Vazquez, A. A Dissenters’ View on Apple Snail Immunobiology. Front. Immunol. 2022, 13, 879122. [Google Scholar] [CrossRef]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Assessment of the kidney and lung as immune barriers and hematopoietic sites in the invasive apple snail Pomacea canaliculata. PeerJ 2018, 6, e5789. [Google Scholar] [CrossRef] [Green Version]
- Cueto, J.A.; Rodriguez, C.; Vega, I.A.; Castro-Vazquez, A. Immune defenses of the invasive apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Phagocytic hemocytes in the circulation and the kidney. PLoS ONE 2015, 10, e0123964. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Simon, V.; Conget, P.; Vega, I.A. Both quiescent and proliferating cells circulate in the blood of the invasive apple snail Pomacea canaliculata. Fish Shellfish Immunol. 2020, 107, 95–103. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Behrens, J.W.; Elias, J.P.; Taylor, H.H.; Weber, R.E. The archaeogastropod mollusc Haliotis iris: Tissue and blood metabolites and allosteric regulation of haemocyanin function. J. Exp. Biol. 2002, 205, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Lester, P., Ed.; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. J. Biol. Chem. 1974, 249, 7130. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaghy, L.; Kraffe, E.; Le Goïc, N.; Lambert, C.; Volety, A.K.; Soudant, P. Reactive oxygen species in unstimulated hemocytes of the Pacific oyster Crassostrea gigas: A mitochondrial involvement. PLoS ONE 2012, 7, e46594. [Google Scholar] [CrossRef] [Green Version]
- Hégaret, H.; Wikfors, G.H.; Soudant, P. Flow cytometric analysis of haemocytes from eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation: II. Haemocyte functions: Aggregation, viability, phagocytosis, and respiratory burst. J. Exp. Mar. Biol. Ecol. 2003, 293, 249–265. [Google Scholar] [CrossRef]
- Bass, D.; Parce, J.W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.; Thomas, M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J. Immunol. 1983, 130, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Salway, K.D.; Tattersall, G.J.; Stuart, J.A. Rapid upregulation of heart antioxidant enzymes during arousal from estivation in the Giant African snail (Achatina fulica). Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2010, 157, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Alencastro, A.; Hermes-Lima, M. Role of antioxidant defenses during estivation and anoxia exposure in the freshwater snail Biomphalaria tenagophila (Orbigny, 1835). Can. J. Zoology. 2003, 81, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Welker, A.F.; Moreira, D.C.; Hermes-Lima, M. Roles of catalase and glutathione peroxidase in the tolerance of a pulmonate gastropod to anoxia and reoxygenation. J. Comp. Physiol. B 2016, 186, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vasconcelos, G.; Cardoso, L.; Hermes-Lima, M. Seasonal modulation of free radical metabolism in estivating land snails Helix aspersa. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 165–174. [Google Scholar] [CrossRef]
- Nowakowska, A.; Caputa, M.; Rogalska, J. Defence against oxidative stress in two species of land snails (Helix pomatia and Helix aspersa) subjected to estivation. J. Exp. Zoology. Part A Ecol. Genet. Physiol. 2011, 315, 593–601. [Google Scholar] [CrossRef]
- English, T.E.; Storey, K.B. Freezing and anoxia stresses induce expression of metallothionein in the foot muscle and hepatopancreas of the marine gastropod Littorina littorea. J. Exp. Biol. 2003, 206, 2517–2524. [Google Scholar] [CrossRef] [Green Version]
- Pannunzio, T.; Storey, K. Antioxidant defenses and lipid peroxidation during anoxia stress and aerobic recovery in the marine gastropod Littorina littorea. J. Exp. Mar. Biol. Ecol. 1998, 221, 277–292. [Google Scholar] [CrossRef]
- Storey, K.B.; Lant, B.; Anozie, O.O.; Storey, J.M. Metabolic mechanisms for anoxia tolerance and freezing survival in the intertidal gastropod, Littorina littorea. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 448–459. [Google Scholar] [CrossRef]
- Nowakowska, A.; Caputa, M.; Rogalska, J. Natural aestivation and antioxidant defence in Helix pomatia: Effect of acclimation to various external conditions. J. Molluscan Stud. 2010, 76, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Nowakowska, A.; Swiderska-Kolacz, G.; Rogalska, J.; Caputa, M. Antioxidants and oxidative stress in Helix pomatia snails during estivation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 481–486. [Google Scholar] [CrossRef]
- Nowakowska, A.; Świderska-Kołacz, G.; Rogalska, J.; Caputa, M. Effect of winter torpor upon antioxidative defence in Helix pomatia. Can. J. Zoology 2009, 87, 471–479. [Google Scholar] [CrossRef]
- Nowakowska, A.; Rogalska, J.; Caputa, M. Adaptability of antioxidant defence system in Helix pomatia snails: Effect of forced aestivation during early spring. J. Molluscan Stud. 2016, 82, 205–207. [Google Scholar]
- Pöhlmann, K.; Koenigstein, S.; Alter, K.; Abele, D.; Held, C. Heat-shock response and antioxidant defense during air exposure in Patagonian shallow-water limpets from different climatic habitats. Cell Stress Chaperones 2011, 16, 621–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermes-Lima, M.; Storey, K.B. Antioxidant defenses and metabolic depression in a pulmonate land snail. Am. J. Physiol. 1995, 268, 1386–1393. [Google Scholar] [CrossRef]
- Sun, J.; Mu, H.; Zhang, H.; Chandramouli, K.H.; Qian, P.-Y.; Wong, C.K.C.; Qiu, J.-W. Understanding the regulation of estivation in a freshwater snail through iTRAQ-based comparative proteomics. J. Proteome Res. 2013, 12, 5271–5280. [Google Scholar] [CrossRef]
- Suljević, D.; Muhić, A.; Islamagić, E.; Fočak, M. Temporal dependence between hibernation and posthibernation period according to biochemical profile of hemolymph in Helix pomatia Linnaeus, 1758. Acta Biol. Szeged. 2017, 61, 129–134. [Google Scholar]
- Vosloo, A.; Laas, A.; Vosloo, D. Differential responses of juvenile and adult South African abalone (Haliotis midae Linnaeus) to low and high oxygen levels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 192–199. [Google Scholar] [CrossRef]
- Meenakshi, V.R. Aestivation in the Indian apple snail Pila—I. Adaptation in natural and experimental conditions. Comp. Biochem. Physiol. 1964, 11, 379–386. [Google Scholar] [CrossRef]
- Meenakshi, V. Studies on the Physiology of Pila virens (Lamarck) with Special Reference to Aestivation. Ph. D. Thesis, Annamalai University, Chidambaram, India, 1956. [Google Scholar]
- Barros, R.M.; Cruz-Höfling, M.A.; Matsuura, M.S. Functional and dissociation properties and structural organization of the hemocyanin of Ampullaria canaliculata (Gastropoda, Mollusca). Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 105, 725–730. [Google Scholar] [CrossRef]
- Chiumiento, I.R.; Ituarte, S.; Sun, J.; Qiu, J.W.; Heras, H.; Dreon, M.S. Hemocyanin of the caenogastropod Pomacea canaliculata exhibits evolutionary differences among gastropod clades. PLoS ONE 2020, 15, e0228325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, J. The effects of hypoxia on hemocyanin regulation in Cancer magister: Possible role of hypoxia-inducible factor-1. J. Exp. Mar. Biol. Ecol. 2010, 386, 77–85. [Google Scholar] [CrossRef]
- Zheng, C.; Zhao, Q.; Li, E.; Zhao, D.; Sun, S. Role of hypoxia in the behaviour, physiology, immunity and response mechanisms of crustaceans: A review. Rev. Aquac. 2022, 14, 676–687. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef]
- Bhunia, A.S.; Mukherjee, S.; Bhunia, N.S.; Ray, M.; Ray, S. Immunological resilience of a freshwater Indian mollusc during aestivation and starvation. Aquac. Rep. 2016, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Suljevic, D.; Islamagic, E.; Hamzic, A.; Zubcevic, N.; Alijagic, A. Hibernation perturbs the number of hemocytes and causes hematological turnover: Basal traits to understand season-dependent physiological variations in Helix pomatia (Gastropoda: Helicidae). Turk. J. Zoology. 2019, 43, 243–249. [Google Scholar] [CrossRef]
- Lambert, C.; Soudant, P.; Choquet, G.; Paillard, C. Measurement of Crassostrea gigas hemocyte oxidative metabolism by flow cytometry and the inhibiting capacity of pathogenic vibrios. Fish Shellfish Immunol. 2003, 15, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Donaghy, L.; Hong, H.-K.; Jauzein, C.; Choi, K.-S. The known and unknown sources of reactive oxygen and nitrogen species in haemocytes of marine bivalve molluscs. Fish Shellfish Immunol. 2015, 42, 91–97. [Google Scholar] [CrossRef]
- Kajino, N.; Choi, K.-S.; Hong, H.-K. Flow cytometric characterization of the hemocytes of sea hares from tidal pools in Jeju Island off the south coast of Korea. Fish Shellfish Immunol. 2022, 122, 409–418. [Google Scholar] [CrossRef]




| Control | Estivation | Arousal-Est | Hibernation | Arousal-Hib | |
|---|---|---|---|---|---|
| Proteins | 5.7 ± 0.5 | 6.3 ± 0.5 | 5.2 ± 0.5 | 4.9 ± 0.4 | 6.5 ± 0.9 |
| Hemocyanin | 0.40 ± 0.03 | 0.65 ± 0.06 * | 0.53 ± 0.04 | 0.39 ± 0.04 | 0.46 ± 0.07 |
| Lactate | 8.9 ± 1.2 | 9.1 ± 0.9 | 11.1 ± 3.6 | 6.3 ± 1.0 | 8.7 ± 1.6 |
| Uric Acid | 5.3 ± 0.3 | 12.1 ± 3.6 * | 7.2 ± 0.5 * | 8.5 ± 1.8 | 6.4 ± 0.4 |
| GSH | 2.5 ± 0.6 | 2.4 ± 0.3 | 3.8 ± 0.4 | 3.8 ± 0.6 | 5.9 ± 0.6 * |
| SOD | 10.0 ± 1.5 | 12.0 ± 1.6 | 16.5 ± 1.4 | 7.4 ± 1.1 | 8.0 ± 1.6 |
| CAT | 1.5 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.5 ± 0.2 * | 0.9 ± 0.2 |
| GST | 25.5 ± 2.7 | 22.9 ± 2.1 | 31.9 ± 3.6 | 25.2 ± 6.2 | 31.4 ± 4.3 |
| Control | Hibernation | Arousal | |
|---|---|---|---|
| CAT | |||
| Gill | 9.8 ± 1.5 | 14.0 ± 1.7 | 13.8 ± 4.1 |
| Digestive gland | 65.8 ± 18.9 | 341.3 ± 94.8 * | 187.0 ± 39.7 |
| Lung | 15.2 ± 2.9 | 7.8 ± 3.4 | 5.5 ± 0.5 |
| GST | |||
| Gill | 89.8 ± 29.5 | 471.5 ± 52.2 * | 1118.0 ± 126.6 * |
| Digestive gland | 168.0 ± 53.8 | 848.7 ± 197.7 | 1538.0 ± 291.7 * |
| Lung | 65.2 ± 7.4 | 338.4 ± 151.9 * | 961.1 ± 179.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, C.; Campoy-Diaz, A.D.; Giraud-Billoud, M. Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata. Metabolites 2023, 13, 289. https://doi.org/10.3390/metabo13020289
Rodriguez C, Campoy-Diaz AD, Giraud-Billoud M. Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata. Metabolites. 2023; 13(2):289. https://doi.org/10.3390/metabo13020289
Chicago/Turabian StyleRodriguez, Cristian, Alejandra D. Campoy-Diaz, and Maximiliano Giraud-Billoud. 2023. "Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata" Metabolites 13, no. 2: 289. https://doi.org/10.3390/metabo13020289