Surface-Coated Acupuncture Needles as Solid-Phase Microextraction Probes for In Vivo Analysis of Bioactive Molecules in Living Plants by Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Mass Spectrometry
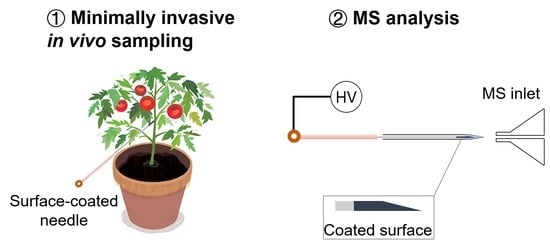
2.3. Fabrication of Surface-Coated Acupuncture Needles
2.4. SPME Via Surface-Coated Needles Coupled with NanoESI-MS Analysis
3. Results and Discussion
3.1. Characterization of the Surface-Coated Acupuncture Needles
3.2. Analytical Figures of Merit
3.3. Comparison of Needle-Tip Extraction/NanoESI with Conventional NanoESI
3.4. Identification of Bioactive Molecules in Living Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, G.A.; Galhena, A.S.; Fernandez, F.M. Ambient Sampling/Ionization Mass Spectrometry: Applications and Current Trends. Anal. Chem. 2011, 83, 4508–4538. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray Ionization–Principles and Practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Kebarle, P.; Verkerk, U.H. Electrospray: From Ions in Solution to Ions in the Gas Phase, What We Know Now. Mass Spectrom. Rev. 2009, 28, 898–917. [Google Scholar] [CrossRef] [PubMed]
- Wilm, M.S.; Mann, M. Electrospray and Taylor-Cone Theory, Dole’s Beam of Macromolecules at Last? Int. J. Mass Spectrom. 1994, 136, 167–180. [Google Scholar] [CrossRef]
- Wilm, M.; Neubauer, G.; Mann, M. Parent ion scans of unseparated peptide mixtures. Anal. Chem. 1996, 68, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Ouyang, G.F.; Vuckovic, D.; Pawliszyn, J. Nondestructive Sampling of Living Systems Using in Vivo Solid-Phase Microextraction. Chem. Rev. 2011, 111, 2784–2814. [Google Scholar] [CrossRef]
- Walles, M.; Gu, Y.; Dartiguenave, C.; Musteata, F.M.; Waldron, K.; Lubda, D.; Pawliszyn, J. Approaches for coupling solid-phase microextraction to nanospray. J. Chromatogr. A 2005, 1067, 197–205. [Google Scholar] [CrossRef]
- D’Agostino, P.A.; Chenier, C.L.; Hancock, J.R.; Lepage, C.R.J. Desorption electrospray ionisation mass spectrometric analysis of chemical warfare agents from solid-phase microextraction fibers. Rapid Commun. Mass Spectrom. 2007, 21, 543–549. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Aurand, C.; Shirey, R.; Laughlin, B.C.; Wiseman, J.M. Coupling Desorption Electrospray Ionization with Solid-Phase Microextraction for Screening and Quantitative Analysis of Drugs in Urine. Anal. Chem. 2010, 82, 7502–7508. [Google Scholar] [CrossRef]
- D’Agostino, P.A.; Hancock, J.R.; Chenier, C.L.; Lepage, C.R.J. Liquid chromatography electrospray tandem mass spectrometric and desorption electrospray ionization tandem mass spectrometric analysis of chemical warfare agents in office media typically collected during a forensic investigation. J. Chromatogr. A 2006, 1110, 86–94. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, Z.; Zhang, Y.; Bai, Y.; Liu, H. Online Coupling of In-Tube Solid-Phase Microextraction with Direct Analysis in Real Time Mass Spectrometry for Rapid Determination of Triazine Herbicides in Water Using Carbon-Nanotubes-Incorporated Polymer Monolith. Anal. Chem. 2014, 86, 4739–4747. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Sun, W.; Ding, L. Desorption Corona Beam Ionization Coupled with a Poly(dimethylsiloxane) Substrate: Broadening the Application of Ambient Ionization for Water Samples. Anal. Chem. 2010, 82, 9188–9193. [Google Scholar] [CrossRef]
- Chen, D.; Huang, Y.Q.; He, X.M.; Shi, Z.G.; Feng, Y.Q. Coupling carbon nanotube film microextraction with desorption corona beam ionization for rapid analysis of Sudan dyes (I–IV) and Rhodamine B in chilli oil. Analyst 2015, 140, 1731–1738. [Google Scholar] [CrossRef]
- Gomez-Rios, G.A.; Pawliszyn, J. Development of Coated Blade Spray Ionization Mass Spectrometry for the Quantitation of Target Analytes Present in Complex Matrices. Angew. Chem.-Int. Ed. 2014, 53, 14503–14507. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Nishidate, K.; Mori, K.; Asakawa, D.; Suzuki, S. Development of probe electrospray using a solid needle. Rapid Commun. Mass Spectrom. 2007, 21, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K. Fundamentals and Application of Probe Electrospray Ionization. Bunseki Kagaku 2010, 59, 95–105. [Google Scholar] [CrossRef]
- Gong, X.Y.; Zhao, Y.Y.; Cai, S.Q.; Fu, S.J.; Yang, C.D.; Zhang, S.C.; Zhang, X.R. Single Cell Analysis with Probe ESI-Mass Spectrometry: Detection of Metabolites at Cellular and Subcellular Levels. Anal. Chem. 2014, 86, 3809–3816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gong, X.; Si, X.; Wei, Z.; Yang, C.; Zhang, S.; Zhang, X. Coupling a solid phase microextraction (SPME) probe with ambient MS for rapid enrichment and detection of phosphopeptides in biological samples. Analyst 2015, 140, 2599–2602. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Maschino, A.C.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Witt, C.M.; Linde, K.; Acupuncture, T. Acupuncture for Chronic Pain Individual Patient Data Meta-analysis. Arch. Intern. Med. 2012, 172, 1444–1453. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Y.; Wan, X.; Han, B.; Yu, W.; Liang, Q.; Xiang, J.; Wang, Z.; Liu, Y.; Qian, Y.; et al. Rapid Screening of Proanthocyanidins from the Roots of Ephedra sinica Stapf and its Preventative Effects on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis. Metabolites 2022, 12, 957. [Google Scholar] [CrossRef]
- Ivanović, S.; Simić, K.; Lekić, S.; Jadranin, M.; Vujisić, L.; Gođevac, D. Plant Metabolomics as a Tool for Detecting Adulterants in Edible Plant: A Case Study of Allium ursinum. Metabolites 2022, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Ishikawa, C.; Umeda, M.; Tarumoto, Y.; Okubo, M.; Tamura, Y.; Ono, H. Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements. Metabolites 2022, 12, 862. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.F.; Mata, A.T.; António, C. Mass spectrometry as a quantitative tool in plant metabolomics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150370. [Google Scholar] [CrossRef]
- Liu, J.J.; Wang, H.; Cooks, R.G.; Ouyang, Z. Leaf Spray: Direct Chemical Analysis of Plant Material and Living Plants by Mass Spectrometry. Anal. Chem. 2011, 83, 7608–7613. [Google Scholar] [CrossRef]






| Analyte | Linear Range (ng/mL) | Regression Equation | R2 | LOD (ng/mL) | Repeatability (%) | Accuracy (Mean ± SD), %, n = 3 | ||
|---|---|---|---|---|---|---|---|---|
| One Tip | Tip-Tip | 50 ng/mL | 500 ng/mL | |||||
| L-histidine | 50–5000 | y = 0.4218x + 0.0402 | 0.9991 | 3.0 | 3.4 | 7.6 | 97.5 ± 5.3 | 105.6 ± 5.8 |
| L-lysine | 10–1000 | y = 2.092x + 0.0371 | 0.9996 | 5.0 | 2.4 | 4.8 | 98.2 ± 2.2 | 103.5 ± 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Zhao, X.; Zhang, L.; Ma, M.; Ma, X. Surface-Coated Acupuncture Needles as Solid-Phase Microextraction Probes for In Vivo Analysis of Bioactive Molecules in Living Plants by Mass Spectrometry. Metabolites 2023, 13, 220. https://doi.org/10.3390/metabo13020220
Cheng H, Zhao X, Zhang L, Ma M, Ma X. Surface-Coated Acupuncture Needles as Solid-Phase Microextraction Probes for In Vivo Analysis of Bioactive Molecules in Living Plants by Mass Spectrometry. Metabolites. 2023; 13(2):220. https://doi.org/10.3390/metabo13020220
Chicago/Turabian StyleCheng, Huiyun, Xu Zhao, Lin Zhang, Mingying Ma, and Xiaoxiao Ma. 2023. "Surface-Coated Acupuncture Needles as Solid-Phase Microextraction Probes for In Vivo Analysis of Bioactive Molecules in Living Plants by Mass Spectrometry" Metabolites 13, no. 2: 220. https://doi.org/10.3390/metabo13020220