Changes in Bone Marrow Fatty Acids Early after Ovariectomy-Induced Osteoporosis in Rats and Potential Functions
Abstract
:1. Introduction
2. Results
2.1. The Evaluation of Trabecular Bone Microarchitecture after OVX Surgery
2.2. Differential Bone Marrow Fatty Acids after OVX Surgery
2.3. Bone Marrow Fatty Acids Levels Were Correlated with Trabecular Bone Microarchitecture
2.4. Bone Marrow Fatty Acids Levels Were Correlated with Bone Metabolism-Related Parameters and NOS
3. Discussion
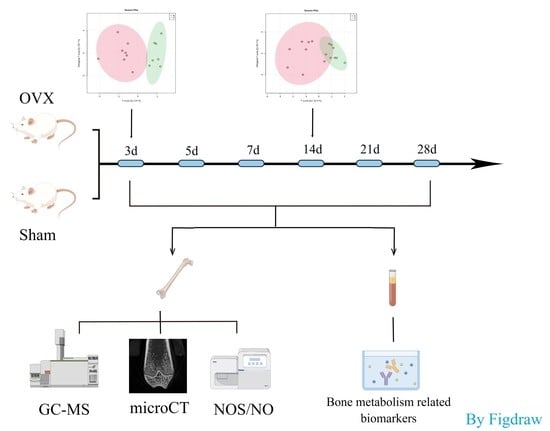
4. Materials and Methods
4.1. Animals and Establishment of Osteoporotic Rat Model
4.2. Specimen Collection
4.3. Bone Microstructure Measurement
4.4. Gas Chromatography/Mass Spectrometry
4.5. Determination of Serum CTX-1, OCN and PINP Levels
4.6. NO Measurement and NOS Enzymatic Activity Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Tao, Y.; Hyman, M.E.; Li, J.; Chen, Y. Osteoporosis in china. Osteoporos. Int. 2009, 20, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xia, W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch. Osteoporos. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tao, L.; Tao, Z.; Meng, Y.; Zhou, S.; Chen, J.; Yang, K.; Da, W.; Zhu, Y. Fecal and Serum Metabolomic Signatures and Microbial Community Profiling of Postmenopausal Osteoporosis Mice Model. Front. Cell. Infect. Microbiol. 2020, 10, 535310. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Yavropoulou, M.P.; Makras, P. Postmenopausal osteoporosis coexisting with other metabolic diseases: Treatment considerations. Maturitas 2021, 147, 19–25. [Google Scholar] [CrossRef] [PubMed]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef]
- During, A.; Coutel, X.; Bertheaume, N.; Penel, G.; Olejnik, C. Long Term Ovariectomy-Induced Osteoporosis is Associated with High Stearoyl-CoA Desaturase Indexes in Rat Femur. Calcif. Tissue Int. 2020, 106, 315–324. [Google Scholar] [CrossRef]
- Griffith, J.F.; Yeung, D.K.; Ahuja, A.T.; Choy, C.W.; Mei, W.Y.; Lam, S.S.; Lam, T.; Chen, Z.-Y.; Leung, P.C. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone 2009, 44, 1092–1096. [Google Scholar] [CrossRef]
- Chiu, H.H.; Kuo, C.H. Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Li, X.; Zhang, D.; Chen, H.; Chao, Y.; Wu, K.; Dong, X.; Su, J. Integrative Bone Metabolomics-Lipidomics Strategy for Pathological Mechanism of Postmenopausal Osteoporosis Mouse Model. Sci. Rep. 2018, 8, 16456. [Google Scholar] [CrossRef]
- Yao, Y.; Yan, L.; Chen, H.; Wu, N.; Wang, W.; Wang, D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar] [CrossRef]
- Si, Z.; Zhou, S.; Shen, Z.; Luan, F. High-Throughput Metabolomics Discovers Metabolic Biomarkers and Pathways to Evaluating the Efficacy and Exploring Potential Mechanisms of Osthole Against Osteoporosis Based on UPLC/Q-TOF-MS Coupled With Multivariate Data Analysis. Front. Pharmacol. 2020, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.M.; Rodríguez, J.P. Is fatty acid composition of human bone marrow significant to bone health? Bone 2019, 118, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Tyagi, S.; Myers, D.; Duque, G. Good, Bad, or Ugly: The Biological Roles of Bone Marrow Fat. Curr. Osteoporos. Rep. 2018, 16, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Zhang, K.; Wei, Y.; Hua, W.; Gao, Y.; Li, X.; Ye, L. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2020, 53, e12735. [Google Scholar] [CrossRef] [Green Version]
- Drosatos-Tampakaki, Z.; Drosatos, K.; Siegelin, Y.; Gong, S.; Khan, S.; Van Dyke, T.; Goldberg, I.J.; Schulze, P.C.; Schulze-Späte, U. Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. J. Bone Miner. Res. 2014, 29, 1183–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wauquier, F.; Léotoing, L.; Philippe, C.; Spilmont, M.; Coxam, V.; Wittrant, Y. Pros and cons of fatty acids in bone biology. Prog. Lipid Res. 2015, 58, 121–145. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Nitric oxide and bone. Ann. N. Y. Acad. Sci. 2010, 1192, 391–403. [Google Scholar] [CrossRef]
- Jamal, S.A.; Hamilton, C.J. Nitric oxide donors for the treatment of osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 86–92. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, J.; Zhou, Z.; Weng, Z.; Xu, Y.; Liu, L.; Zhang, W.; Yang, Y.; Luo, J.; Wang, X. Near-Infrared Light and Upconversion Nanoparticle Defined Nitric Oxide-Based Osteoporosis Targeting Therapy. ACS Nano 2021, 15, 13692–13702. [Google Scholar] [CrossRef]
- Bercea, C.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 2007, 89, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotto, S.; Suzuki, Y.; Persichini, T.; Colasanti, M.; Suzuki, H.; Cantoni, O. Cross-talk between NO and arachidonic acid in inflammation. Curr. Med. Chem. 2007, 14, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Palomba, L.; Persichini, T.; Mazzone, V.; Colasanti, M.; Cantoni, O. Inhibition of nitric-oxide synthase-I (NOS-I)-dependent nitric oxide production by lipopolysaccharide plus interferon-gamma is mediated by arachidonic acid. Effects on NFkappaB activation and late inducible NOS expression. J. Biol. Chem. 2004, 279, 29895–29901. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Nitric oxide as the mediator of the antiosteoporotic actions of estrogen, statins, and essential fatty acids. Exp. Biol. Med. 2002, 227, 88–93. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Oh, S.R.; Sul, O.J.; Kim, Y.Y.; Kim, H.J.; Yu, R.; Suh, J.H.; Choi, H.S. Saturated fatty acids enhance osteoclast survival. J. Lipid Res. 2010, 51, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.-O.; Jin, W.J.; Kim, B.; Kim, H.-H.; Lee, Z.H. Myristoleic acid inhibits osteoclast formation and bone resorption by suppressing the RANKL activation of Src and Pyk2. Eur. J. Pharmacol. 2015, 768, 189–198. [Google Scholar] [CrossRef]
- Kuroda, T.; Ohta, H.; Onoe, Y.; Tsugawa, N.; Shiraki, M. Intake of omega-3 fatty acids contributes to bone mineral density at the hip in a younger Japanese female population. Osteoporos. Int. 2017, 28, 2887–2891. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Song, J.; Jing, Z.; Hu, W.; Yu, J.; Cui, X. α-Linolenic Acid Inhibits Receptor Activator of NF-κB Ligand Induced (RANKL-Induced) Osteoclastogenesis and Prevents Inflammatory Bone Loss via Downregulation of Nuclear Factor-KappaB-Inducible Nitric Oxide Synthases (NF-κB-iNOS) Signaling Pathways. Med. Sci. Monit. 2017, 23, 5056–5069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Q.; Tian, Y.; Han, L.; Wang, K.; Wang, J.; Xue, C. The opposite effects of Antarctic krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 2020, 11, 7048–7060. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Haag, M.; Kruger, M.C. Effects of arachidonic acid, docosahexaenoic acid, prostaglandin E(2) and parathyroid hormone on osteoprotegerin and RANKL secretion by MC3T3-E1 osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Haag, M.; Kruger, M.C. Effects of arachidonic acid and docosahexaenoic acid on differentiation and mineralization of MC3T3-E1 osteoblast-like cells. Cell Biochem. Funct. 2009, 27, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, A.E.; Kruger, M.; Coetzee, M. Free fatty acid receptor 4-β-arrestin 2 pathway mediates the effects of different classes of unsaturated fatty acids in osteoclasts and osteoblasts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 281–289. [Google Scholar] [CrossRef]
- Van Heerden, B.; Kasonga, A.; Kruger, M.C.; Coetzee, M. Palmitoleic Acid Inhibits RANKL-Induced Osteoclastogenesis and Bone Resorption by Suppressing NF-κB and MAPK Signalling Pathways. Nutrients 2017, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- van’t Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Kho, J.; Dawson, B.; Jiang, M.-M.; Chen-Evenson, Y.; Ali, S.; Burrage, L.C.; Grover, M.; Palmer, D.J.; Turner, D.L.; et al. Nitric oxide modulates bone anabolism through regulation of osteoblast glycolysis and differentiation. J. Clin. Investig. 2021, 131, e138935. [Google Scholar] [CrossRef]
- Evans, D.M.; Ralston, S.H. Nitric oxide and bone. J. Bone Miner. Res. 1996, 11, 300–305. [Google Scholar] [CrossRef]
- Liu, H.; Rosen, C.J. Nitric oxide and bone: The phoenix rises again. J. Clin. Investig. 2021, 131, e147072. [Google Scholar] [CrossRef]
- Palomino, O.M.; Giordani, V.; Chowen, J.; Alfonso, S.F.; Goya, L. Physiological Doses of Oleic and Palmitic Acids Protect Human Endothelial Cells from Oxidative Stress. Molecules 2022, 27, 5217. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.M.; O’Kane, F.; McConville, M.; Devine, A.B.; McVeigh, G.E. Platelet redox balance in diabetic patients with hypertension improved by n-3 fatty acids. Diabetes Care 2013, 36, 998–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, B.A.; Li, Y.; Seifert, M. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: Actions on bone mineral and serum biomarkers in ovariectomized rats. J. Nutr. Biochem. 2006, 17, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.; Bhattacharya, A.; Rahman, M.; Kang, J.X.; Fernandes, G. Endogenously produced n-3 fatty acids protect against ovariectomy induced bone loss in fat-1 transgenic mice. J. Bone Miner. Metab. 2010, 28, 617–626. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Le Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]






| Day | Fatty Acid | OVX Group (Mean ± SEM) | Sham Group (Mean ± SEM) | p Value | VIP Value |
|---|---|---|---|---|---|
| 3rd day | Palmitoleate | 0.8749 ± 0.1389 | 1.430 ± 0.1916 | 0.040 | 2.69 |
| Myristate | 0.7399 ± 0.03411 | 0.9102 ± 0.06195 | 0.033 | 2.08 | |
| Arachidonate | 6.414 ± 0.5745 | 4.341 ± 0.6434 | 0.037 | 1.53 | |
| 14th day | Palmitoleate | 2.099 ± 0.2807 | 6.868 ± 2.200 | 0.038 | 1.73 |
| Myristate | 1.028 ± 0.0602 | 2.241 ± 0.5614 | 0.038 | 1.66 | |
| Alpha linolenate | 0.4549 ± 0.0600 | 1.415 ± 0.4345 | 0.036 | 1.65 | |
| Stearate | 30.38 ± 1.737 | 25.76 ± 0.8923 | 0.046 | 1.16 | |
| Eicosenoate | 0.3501 ± 0.01103 | 0.6707 ± 0.1521 | 0.045 | 1.05 |
| Time | Fatty Acid | Bone Microstructure | Bone Metabolism Related Parameters | ||||
|---|---|---|---|---|---|---|---|
| BMD | BV/TV | Tb.Sp | CTX-1 | OCN | NOS | ||
| 3rd day | Myristate | ||||||
| Palmitoleate | −0.51 | ||||||
| Arachidonate | 0.6 | −0.76 | |||||
| 14th day | Myristate | 0.62 | 0.78 | −0.67 | |||
| Palmitoleate | 0.83 | ||||||
| Stearate | −0.86 | ||||||
| Alpha linolenate | −0.56 | ||||||
| Eicosenoate | −0.65 | 0.74 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Tang, C.; Chen, J.; Tang, H.; Zhang, L.; Tang, G. Changes in Bone Marrow Fatty Acids Early after Ovariectomy-Induced Osteoporosis in Rats and Potential Functions. Metabolites 2023, 13, 36. https://doi.org/10.3390/metabo13010036
Wang S, Tang C, Chen J, Tang H, Zhang L, Tang G. Changes in Bone Marrow Fatty Acids Early after Ovariectomy-Induced Osteoporosis in Rats and Potential Functions. Metabolites. 2023; 13(1):36. https://doi.org/10.3390/metabo13010036
Chicago/Turabian StyleWang, Sizhu, Cuisong Tang, Jieying Chen, Huan Tang, Lin Zhang, and Guangyu Tang. 2023. "Changes in Bone Marrow Fatty Acids Early after Ovariectomy-Induced Osteoporosis in Rats and Potential Functions" Metabolites 13, no. 1: 36. https://doi.org/10.3390/metabo13010036