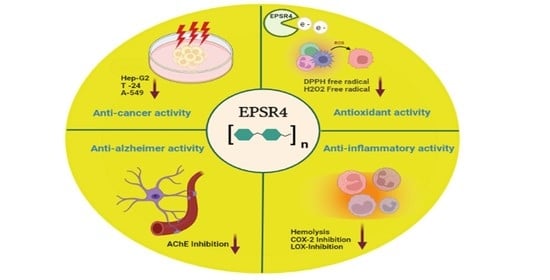
Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity
Abstract
:1. Introduction
2. Results
2.1. Identification of the Bacterial Isolates
2.2. Production and Purification of EPSR4
2.3. Molecular Weight and Chemical Composition of EPSR4
2.4. Structural Characterization of EPSR4 by FT-IR and UV-Vis Analysis
2.5. Morphological Analysis of EPSR4
2.6. Antioxidant Activity
2.7. Cytotoxic Activity against Different Cancer Cell Lines
2.8. Anti-Inflammatory Activity
2.9. Acetylcholinesterase (AChE) Inhibition
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Culture, Isolation and Identification of Bacterial Strains
4.3. Production and Fractionation of EPS
4.4. Structural Characterization of EPSR4
4.4.1. General Characterizations (Homogeneity, FT-IR, UV-Vis, Mw, PI)
4.4.2. Uronic Acid Analysis
4.4.3. Sulphate Analysis
4.4.4. Monosaccharide Composition Analysis
4.4.5. Morphological Analysis
4.5. Evaluation of Antioxidant Activity
4.5.1. DPPH Assay
4.5.2. Hydrogen Peroxide Scavenging (H2O2) Assay
4.6. Assessment of Cytotoxic Effects Using Different Cell Line
4.7. Assessment of the Anti-Inflammatory Activity
4.7.1. Lipoxygenase (LOX) Inhibition In Vitro
4.7.2. Cyclooxygenase (COX2) Inhibition In Vitro
4.7.3. Membrane Stabilization
4.8. Acetyl Cholinesterase Inhibition Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. Marine Carbohydrate-Based Compounds with Medicinal Properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.B.; Damonte, E.B. Interference in Dengue Virus Adsorption and Uncoating by Carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Elekhnawy, E.; Negm, W.A. The Potential Application of Probiotics for the Prevention and Treatment of COVID-19. Egypt. J. Med. Hum. Genet. 2022, 23, 36. [Google Scholar] [CrossRef]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Abo Nahas, H.H.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef]
- Vyas, T.K.; Vadnerker, P.; Bhavsar, R.; Kheni, K. Mushroom Exopolysaccharides: Their Antioxidant and Antiviral Properties to Boost Immunity Against COVID-19. In Immunity Boosting Functional Foods to Combat COVID-19; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-00-324260-4. [Google Scholar]
- Kang, Y.; Wang, Z.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and Potential Antitumor Activity of Polysaccharide from Gracilariopsis lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Vinsova, J.; Vavrikova, E. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular Characteristics and Anti-Inflammatory Activity of the Fucoidan Extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Morones-Ramírez, J.R. Bacterial Exopolysaccharides as Reducing and/or Stabilizing Agents during Synthesis of Metal Nanoparticles with Biomedical Applications. Int. J. Polym. Sci. 2018, 2018, e7045852. [Google Scholar] [CrossRef]
- Nwodo, U.; Green, E.; Okoh, A. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 4002. [Google Scholar] [CrossRef]
- Liu, G.; Liu, R.; Shan, Y.; Sun, C. Marine Bacterial Exopolysaccharide EPS11 Inhibits Migration and Invasion of Liver Cancer Cells by Directly Targeting Collagen I. J. Biol. Chem. 2021, 297, 101133. [Google Scholar] [CrossRef]
- Kirkland, S.C. Type I Collagen Inhibits Differentiation and Promotes a Stem Cell-like Phenotype in Human Colorectal Carcinoma Cells. Br. J. Cancer 2009, 101, 320–326. [Google Scholar] [CrossRef]
- Gutierrez, T.; Morris, G.; Green, D.H. Yield and Physicochemical Properties of EPS from Halomonas sp. Strain TG39 Identifies a Role for Protein and Anionic Residues (Sulfate and Phosphate) in Emulsification of n-Hexadecane. Biotechnol. Bioeng. 2009, 103, 207–216. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.; Reis, M.D.C.F.M. Advances in Bacterial Exopolysaccharides: From Production to Biotechnological Applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Choi, I.; Ko, S.; Lee, M.; Kim, H.; Yang, J.E.; Jeong, S.-G.; Lee, K.; Chang, J.; Kim, J.-C.; Park, H.W. Production, Characterization, and Antioxidant Activities of an Exopolysaccharide Extracted from Spent Media Wastewater after Leuconostoc Mesenteroides WiKim32 Fermentation. ACS Omega 2021, 6, 8171–8178. [Google Scholar] [CrossRef]
- Tako, M.; Kitajima, S.; Yogi, T.; Uechi, K.; Onaga, M.; Tamaki, Y.; Uechi, S. Structure-Function Relationship of a Gellan Family of Polysaccharide, S-198 Gum, Produced by Alcaligenes ATCC31853. Adv. Biol. Chem. 2016, 6, 55–69. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Jiao, W. Chapter Seven—Marine Glycan-Derived Therapeutics in China. In Progress in Molecular Biology and Translational Science; Glycans and Glycosaminoglycans as Clinical Biomarkers and Therapeutics—Part B; Zhang, L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 163, pp. 113–134. [Google Scholar]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Olaimat, A.; Esposito, G.; Itsaranuwat, P.; Osaili, T.; Obaid, R.; Kizhakkayil, J.; Liu, S. Physicochemical, Bioactive and Rheological Properties of an Exopolysaccharide Produced by a Probiotic Pediococcus Pentosaceus M41. Carbohydr. Polym. 2020, 229, 115462. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, T.; Yu, Q.; Nie, S.; Gong, D.; Xiong, T.; Xie, M. Exopolysaccharides from Lactobacillus Plantarum NCU116 Induce C-Jun Dependent Fas/Fasl-Mediated Apoptosis via TLR2 in Mouse Intestinal Epithelial Cancer Cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhao, X.; Ma, K.; Chen, X.; Zhang, C.; Wang, G.; Dong, M.; Rui, X.; Zhang, Q.; et al. Isolation, Purification, Characterization and Immunostimulatory Activity of an Exopolysaccharide Produced by Lactobacillus Pentosus LZ-R-17 Isolated from Tibetan Kefir. Int. J. Biol. Macromol. 2020, 158, 408–419. [Google Scholar] [CrossRef]
- Lin, S.M.; Baek, C.Y.; Jung, J.-H.; Kim, W.S.; Song, H.-Y.; Lee, J.H.; Ji, H.J.; Zhi, Y.; Kang, B.S.; Bahn, Y.-S.; et al. Antioxidant Activities of an Exopolysaccharide (DeinoPol) Produced by the Extreme Radiation-Resistant Bacterium Deinococcus radiodurans. Sci. Rep. 2020, 10, 55. [Google Scholar] [CrossRef]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and Antibacterial Activities of Exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef]
- Gangalla, R.; Macha, B.; Kasarla, S.; Eerla, R.; Thampu, R.K. Anti-inflammatory activity of the exopolysaccharides (eps) produced from polluted soil. Int. J. Pharm. Biol. Sci. 2018, 8, 623–631. [Google Scholar]
- Paynich, M.L.; Jones-Burrage, S.E.; Knight, K.L. Exopolysaccharide from Bacillus subtilis Induces Anti-Inflammatory M2 Macrophages That Prevent T Cell-Mediated Disease. J. Immunol. 2017, 198, 2689–2698. [Google Scholar] [CrossRef]
- Kim, S.-W.; Ahn, S.-G.; Seo, W.-T.; Kwon, G.-S.; Park, Y.-H. Rheological Properties of a Novel High Viscosity Polysaccharide, A49-Pol, Produced by Bacillus polymyxa. J. Microbiol. Biotechnol. 1998, 8, 178–181. [Google Scholar]
- Sharaf, M.; Arif, M.; Khan, S.; Abdalla, M.; Shabana, S.; Chi, Z.; Liu, C. Co-Delivery of Hesperidin and Clarithromycin in a Nanostructured Lipid Carrier for the Eradication of Helicobacter Pylori In Vitro. Bioorganic Chem. 2021, 112, 104896. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Lu, H.; Wang, S.; Han, J.; Xiang, H.; Jin, C. An Acidic Exopolysaccharide from Haloarcula hispanica ATCC33960 and Two Genes Responsible for Its Synthesis. Archaea 2017, 2017, 5842958. [Google Scholar] [CrossRef]
- Kanmani, P.; Kumar, R.S.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and Purification of a Novel Exopolysaccharide from Lactic Acid Bacterium Streptococcus phocae PI80 and Its Functional Characteristics Activity In Vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Liu, J.-C.; Yang, X.-D.; Kennedy, J.F. Purification, Structural Analysis and Hydroxyl Radical-Scavenging Capacity of a Polysaccharide from the Fruiting Bodies of Russula Virescens. Process Biochem. 2010, 45, 874–879. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and Physical Characteristics and Antioxidant Activities of the Exopolysaccharide Produced by Tibetan Kefir Grains during Milk Fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Shang, N.; Xu, R.; Li, P. Structure Characterization of an Exopolysaccharide Produced by Bifidobacterium Animalis RH. Carbohydr. Polym. 2013, 91, 128–134. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Gonçalves, F.; Barros, A.S.; Delgadillo, I. Fourier Transform Infrared Spectroscopy and Chemometric Analysis of White Wine Polysaccharide Extracts. J. Agric. Food Chem. 2002, 50, 3405–3411. [Google Scholar] [CrossRef]
- Saravanan, C.; Shetty, P.K.H. Isolation and Characterization of Exopolysaccharide from Leuconostoc Lactis KC117496 Isolated from Idli Batter. Int. J. Biol. Macromol. 2016, 90, 100–106. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Zhang, H.; Chen, W.; Ai, L.; Guo, B. Partial Characterization and Immunostimulatory Activity of Exopolysaccharides from Lactobacillus Rhamnosus KF5. Carbohydr. Polym. 2014, 107, 51–56. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Jin, Z.; Wang, J.; Xu, X. Nitrite Oxide and Inducible Nitric Oxide Synthase Were Regulated by Polysaccharides Isolated from Glycyrrhiza Uralensis Fisch. J. Ethnopharmacol. 2008, 118, 59–64. [Google Scholar] [CrossRef]
- Imam, N.G.; Aquilanti, G.; Azab, A.A.; Ali, S.E. Correlation between Structural Asymmetry and Magnetization in Bi-Doped LaFeO3 Perovskite: A Combined XRD and Synchrotron Radiation XAS Study. J. Mater. Sci. Mater. Electron. 2021, 32, 3361–3376. [Google Scholar] [CrossRef]
- Jiang, G.; Gan, L.; Li, X.; He, J.; Zhang, S.; Chen, J.; Zhang, R.; Xu, Z.; Tian, Y. Characterization of Structural and Physicochemical Properties of an Exopolysaccharide Produced by Enterococcus Sp. F2 From Fermented Soya Beans. Front. Microbiol. 2021, 12, 744007. [Google Scholar] [CrossRef]
- Vollrath, A.; Kretzer, C.; Beringer-Siemers, B.; Shkodra, B.; Czaplewska, J.A.; Bandelli, D.; Stumpf, S.; Hoeppener, S.; Weber, C.; Werz, O.; et al. Effect of Crystallinity on the Properties of Polycaprolactone Nanoparticles Containing the Dual FLAP/MPEGS-1 Inhibitor BRP-187. Polymers 2021, 13, 2557. [Google Scholar] [CrossRef]
- Fukushi, Y.; Yoshino, H.; Ishikawa, J.; Sagisaka, M.; Kashiwakura, I.; Yoshizawa, A. Effects of Liquid Crystallinity on Anticancer Activity of Benzoate Derivatives Possessing a Terminal Hydroxyl Group. Liq. Cryst. 2014, 41, 1873–1878. [Google Scholar] [CrossRef]
- Hu, D.; Su, F.; Yang, G.; Wang, J.; Zhang, Y. Purification, Structural Characterization, and Anti-Inflammatory Effects of a Novel Polysaccharide Isolated from Orostachys Fimbriata. Molecules 2021, 26, 7116. [Google Scholar] [CrossRef]
- Gülçin, I.; Beydemir, S.; Alici, H.A.; Elmastaş, M.; Büyükokuroğlu, M.E. In Vitro Antioxidant Properties of Morphine. Pharm. Res. 2004, 49, 59–66. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural Polysaccharides Exhibit Anti-Tumor Activity by Targeting Gut Microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef]
- Jaismy Jacob, P.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer Anti-Inflammatory Therapy through Dual COX-2/5-LOX Inhibitors: A Structure-Based Approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Sharaf, M.; Hamouda, H.I.; Shabana, S.; Khan, S.; Arif, M.; Rozan, H.E.; Abdalla, M.; Chi, Z.; Liu, C. Design of Lipid-Based Nanocarrier for Drug Delivery Has a Double Therapy for Six Common Pathogens Eradication. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 625, 126662. [Google Scholar] [CrossRef]
- Dutta, N.; Sarotra, P.; Gupta, S.; Aggarwal, R.; Agnihotri, N. Mechanism of Action of Celecoxib on Normal and Acid-Challenged Gastric Mucosa. Exp. Toxicol. Pathol. 2009, 61, 353–361. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant Capacity and Phenolic Contents of Some Mediterranean Medicinal Plants and Their Potential Role in the Inhibition of Cyclooxygenase-1 and Acetylcholinesterase Activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Petrovic, N.; Murray, M. Using N,N,N’,N’-Tetramethyl-p-Phenylenediamine (TMPD) to Assay Cyclooxygenase Activity In Vitro. Methods Mol. Biol. 2010, 594, 129–140. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical Composition, Antioxidative and Anti-Inflammatory Activity of Extracts Prepared from Aerial Parts of Oenothera biennis L. and Oenothera paradoxa Hudziok Obtained after Seeds Cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef]
- Shinde, U.A.; Phadke, A.S.; Nair, A.M.; Mungantiwar, A.A.; Dikshit, V.J.; Saraf, M.N. Membrane Stabilizing Activity—A Possible Mechanism of Action for the Anti-Inflammatory Activity of Cedrus Deodara Wood Oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of Cholinergic Abnormalities with Senile Plaques and Mental Test Scores in Senile Dementia. Br. Med. J. 1978, 2, 1457–1459. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, Properties, and Industrial Food Application of Lactic Acid Bacteria-Derived Exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Welman, A.; Maddox, I.; Archer, R. Exopolysaccharide and Extracellular Metabolite Production by Lactobacillus Delbrueckii Subsp. Bulgaricus, Grown on Lactose in Continuous Culture. Biotechnol. Lett. 2003, 25, 1515–1520. [Google Scholar] [CrossRef]
- Xie, J.-H.; Xie, M.-Y.; Nie, S.-P.; Shen, M.-Y.; Wang, Y.-X.; Li, C. Isolation, Chemical Composition and Antioxidant Activities of a Water-Soluble Polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, J.; Yang, H.; Yang, Z.; Xue, H.; Wu, F.; Wang, Z.; Xie, L.; Chen, Y. Acetylation Modification and Antioxidant Activity of Polysaccharides from Agrocybe cylindracea. Food Meas. 2022, 16, 1911–1919. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.-W.; Fang, L.; Gao, X.-D.; Tan, R.-X. Free Radical Scavenging and Antioxidant Activities of EPS2, an Exopolysaccharide Produced by a Marine Filamentous Fungus Keissleriella sp. YS 4108. Life Sci. 2004, 75, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dong, M.; Wang, W.; Shuo, H.; Rui, X.; Chen, X.; Jiang, M.; Zhang, Q.; Wu, J.; Li, W. Structural Characterization and Antioxidant Property of Released Exopolysaccharides from Lactobacillus delbrueckii ssp. Bulgaricus SRFM-1. Carbohydr. Polym. 2017, 173, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Ding, D.; Gao, J.; Hao, L.; Guo, X.; Liu, Y. Structural Characterization and Antioxidant Activity of a Novel High-Molecular-Weight Polysaccharide from Ziziphus jujuba cv. Muzao. Food Meas. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Seo, B.-J.; Bajpai, V.K.; Rather, I.A.; Park, Y.-H. Partially Purified Exopolysaccharide from Lactobacillus Plantarum YML009 with Total Phenolic Content, Antioxidant and Free Radical Scavenging Efficacy. Indian J. Pharm. Educ. Res. 2015, 49, 282–292. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Tao, X.; Wei, H. Characterization and Sulfated Modification of an Exopolysaccharide from Lactobacillus Plantarum ZDY2013 and Its Biological Activities. Carbohydr. Polym. 2016, 153, 25–33. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El-Newary, S.A. Production, Structural and Biochemical Characterization Relevant to Antitumor Property of Acidic Exopolysaccharide Produced from Bacillus Sp. NRC5. Arch. Microbiol. 2021, 203, 4337–4350. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Sungur, T.; Aslim, B.; Karaaslan, C.; Aktas, B. Impact of Exopolysaccharides (EPSs) of Lactobacillus Gasseri Strains Isolated from Human Vagina on Cervical Tumor Cells (HeLa). Anaerobe 2017, 47, 137–144. [Google Scholar] [CrossRef]
- Ooi, V.E.; Liu, F. Immunomodulation and Anti-Cancer Activity of Polysaccharide-Protein Complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal Mushrooms as a Source of Antitumor and Immunomodulating Polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, Anti-Inflammatory and Antiproliferative Activities of Natural and Sulfonated Exo-Polysaccharides from Streptococcus Thermophilus ASCC 1275. J. Food Sci. 2016, 81, M1167–M1176. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Structural Characterization and Bioactivity of Released Exopolysaccharides from Lactobacillus Plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–78. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Liu, S.; Zhang, X.; Chen, P.; Li, W.; Xu, X.; Tian, W. Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus Thermophilus CH9. Molecules 2018, 23, E2898. [Google Scholar] [CrossRef]
- Li, W.; Tang, W.; Ji, J.; Xia, X.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Dong, M. Characterization of a Novel Polysaccharide with Anti-Colon Cancer Activity from Lactobacillus Helveticus MB2-1. Carbohydr. Res. 2015, 411, 6–14. [Google Scholar] [CrossRef]
- Bhatia, M.; Moochhala, S. Role of Inflammatory Mediators in the Pathophysiology of Acute Respiratory Distress Syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Margolles, A.; Wells, J.M.; Ruas-Madiedo, P. Exopolysaccharides Synthesized by Bifidobacterium Animalis Subsp. Lactis Interact with TLR4 in Intestinal Epithelial Cells. Anaerobe 2019, 56, 98–101. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El Awady, M.E. Production, Characterization and Biological Activities of Acidic Exopolysaccharide from Marine Bacillus Amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017, 10, 652–662. [Google Scholar] [CrossRef]
- Asker, M.; Mahmoud, M.; Ibrahim, A.; Mohamed, S.S. Inhibitory Effect of Exopolysaccharide from Achromobacter Piechaudii NRC2 against Cyclooxygenases and Acetylcholinesterase with Evaluation of Its Antioxidant Properties and Structure Elucidation. Der Pharm. Lett. 2015, 7, 129–141. [Google Scholar]
- Rossner, S.; Ueberham, U.; Schliebs, R.; Perez-Polo, J.R.; Bigl, V. The Regulation of Amyloid Precursor Protein Metabolism by Cholinergic Mechanisms and Neurotrophin Receptor Signaling. Prog. Neurobiol. 1998, 56, 541–569. [Google Scholar] [CrossRef]
- Oliva, C.; Vargas, J.; Inestrosa, N. Wnts in Adult Brain: From Synaptic Plasticity to Cognitive Deficiencies. Front. Cell. Neurosci. 2013, 7, 224. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kazui, H.; Matsumoto, K.; Nakano, Y.; Yasuda, M.; Mori, E. Does Donepezil Treatment Slow the Progression of Hippocampal Atrophy in Patients With Alzheimer’s Disease? Am. J. Psychiatry 2005, 162, 676–682. [Google Scholar] [CrossRef]
- Pasinetti, G.M. Cyclooxygenase and Inflammation in Alzheimer’s Disease: Experimental Approaches and Clinical Interventions. J. Neurosci. Res. 1998, 54, 1–6. [Google Scholar] [CrossRef]
- Shene, C.; Canquil, N.; Bravo, S.; Rubilar, M. Production of the Exopolysaccharides by Streptococcus Thermophilus: Effect of Growth Conditions on Fermentation Kinetics and Intrinsic Viscosity. Int. J. Food Microbiol. 2008, 124, 279–284. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Lu, L.; Liu, Y.; Wang, F.; Xiao, M. Isolation, Structural Characterization and Immunological Activity of an Exopolysaccharide Produced by Bacillus Licheniformis 8-37-0-1. Bioresour. Technol. 2010, 101, 5528–5533. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Mu, H.; Liang, X.; Guan, H. Structure and Protective Effect of Exopolysaccharide from P. Agglomerans Strain KFS-9 against UV Radiation. Microbiol. Res. 2007, 162, 124–129. [Google Scholar] [CrossRef]
- Nicely, W.B. Infrared Spectra of Carbohydrates. In Advances in Carbohydrate Chemistry; Wolfrom, M.L., Tipson, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1957; Volume 12, pp. 13–33. [Google Scholar]
- Jung, S.; Höltzel, A.; Ehlert, S.; Mora, J.-A.; Kraiczek, K.; Dittmann, M.; Rozing, G.P.; Tallarek, U. Impact of Conduit Geometry on the Performance of Typical Particulate Microchip Packings. Anal. Chem. 2009, 81, 10193–10200. [Google Scholar] [CrossRef]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; et al. A Polymer Tandem Solar Cell with 10.6% Power Conversion Efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of Uronic Acids without Interference from Neutral Sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A Note on the Determination of the Ester Sulphate Content of Sulphated Polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.C.; Phillips, G.O.; Williams, P.A. Fractionation and Characterization of Gum from Acacia Senegal. Food Hydrocoll. 1989, 3, 65–75. [Google Scholar] [CrossRef]
- Elmastaş, M.; Gülçin, İ.; Beydemir, Ş.; İrfan Küfrevioğlu, Ö.; Aboul-Enein, H.Y. A Study on the In Vitro Antioxidant Activity of Juniper (Juniperus Communis L.) Fruit Extracts. Anal. Lett. 2006, 39, 47–65. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of Cytotoxicity and Inhibition of Intercellular Communication by Antioxidant Catechins Isolated from Chinese Green Tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Gomha, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-Grafted-Poly(Vinylpyridine) as Basic Catalyst. Heterocycles 2015, 91, 1227. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Koch-Edelmann, S.; Banhart, S.; Saied, E.M.; Rose, L.; Aeberhard, L.; Laue, M.; Doellinger, J.; Arenz, C.; Heuer, D. The Cellular Ceramide Transport Protein CERT Promotes Chlamydia Psittaci Infection and Controls Bacterial Sphingolipid Uptake. Cell. Microbiol. 2017, 19, e12752. [Google Scholar] [CrossRef]
- Banhart, S.; Saied, E.M.; Martini, A.; Koch, S.; Aeberhard, L.; Madela, K.; Arenz, C.; Heuer, D. Improved Plaque Assay Identifies a Novel Anti-Chlamydia Ceramide Derivative with Altered Intracellular Localization. Antimicrob. Agents Chemother. 2014, 58, 5537–5546. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Bianchini, A.; Bainy, A.C.D. Kinetic and Toxicological Characteristics of Acetylcholinesterase from the Gills of Oysters (Crassostrea Rhizophorae) and Other Aquatic Species. Mar. Environ. Res. 2002, 54, 781–785. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral MiRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting MiRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Ezzat, S.F.; Elayat, W.M.; El-Kharashi, O.A.; El-Kareem, H.F.A.; Nahas, H.H.A.; Abdel-Wahab, B.A.; Alshawwa, S.Z.; Saleh, A.; Helmy, Y.A.; et al. Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting MiRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals 2022, 15, 832. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Wahab, B.A.; F. Abd El-Kareem, H.; Alzamami, A.; A. Fahmy, C.; H. Elesawy, B.; Mostafa Mahmoud, M.; Ghareeb, A.; El Askary, A.; H. Abo Nahas, H.; G. M. Attallah, N.; et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. https://doi.org/10.3390/metabo12080715
Abdel-Wahab BA, F. Abd El-Kareem H, Alzamami A, A. Fahmy C, H. Elesawy B, Mostafa Mahmoud M, Ghareeb A, El Askary A, H. Abo Nahas H, G. M. Attallah N, et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites. 2022; 12(8):715. https://doi.org/10.3390/metabo12080715
Chicago/Turabian StyleAbdel-Wahab, Basel A., Hanaa F. Abd El-Kareem, Ahmad Alzamami, Cinderella A. Fahmy, Basem H. Elesawy, Maged Mostafa Mahmoud, Ahmed Ghareeb, Ahmad El Askary, Hebatallah H. Abo Nahas, Nashwah G. M. Attallah, and et al. 2022. "Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity" Metabolites 12, no. 8: 715. https://doi.org/10.3390/metabo12080715