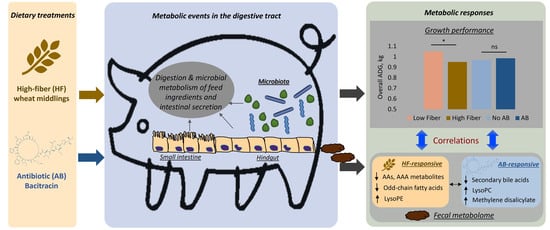
Identification of Independent and Shared Metabolic Responses to High-Fiber and Antibiotic Treatments in Fecal Metabolome of Grow–Finish Pigs
Abstract
:1. Introduction
2. Results
2.1. Effects of Fiber and Antibiotics (ABs) on Growth Performance of Grow–Finish Pigs
2.2. Targeted Analysis of Free Amino, Fatty, and Bile Acids in Fecal Samples
2.3. Untargeted Analysis of Fiber- and Antibiotics-Elicited Changes in the Fecal Metabolome
3. Discussion
3.1. Causes and Significances of WM Effects on Growth and Fecal Metabolome of Grow–Finish Pigs
3.1.1. Effects of WM on Growth Performanc
3.1.2. Effects of WM on Fecal Amino Acid Metabolites
3.1.3. Effects of WM on Fecal Lipids
3.2. Causes and Significances of Antibiotic Effects on Fecal Metabolome of Grow–Finish Pigs
3.2.1. Methylene Disalicylate as an Exposure Marker
3.2.2. Changes in Histidine and Bile Acids
3.2.3. Changes in Other Fecal Metabolites
3.3. Implications and Limitations of Observed Metabolic Changes from Feeding WM and Bacitracin
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals, Experimental Design, and Housing
4.3. Data and Sample Collection
4.4. Metabolomics
4.4.1. Sample Preparation
4.4.2. Chemical Derivatization
4.4.3. Conditions of LC-MS Analysis
4.4.4. Data Analysis and Visualization
4.4.5. Characterization, Quantification, and Pathway Analysis of Metabolite Markers
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaworski, N.W.; Lærke, H.N.; Bach Knudsen, K.E.; Stein, H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J. Anim. Sci. 2015, 93, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9.
- Salyer, J.A.; Derouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Nelssen, J.L.; Petry, D.B. Effects of dietary wheat middlings, distillers dried grains with solubles, and choice white grease on growth performance, carcass characteristics, and carcass fat quality of finishing pigs. J. Anim. Sci. 2012, 90, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.T.; Zhu, J.; Shurson, G.C.; Urriola, P.E.; Saqui-Salces, M. Decreased nutrient digestibility due to viscosity is independent of the amount of dietary fibre fed to growing pigs. Br. J. Nutr. 2022, 127, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.T.; Nienaber, J.A.; Hill, D.A.; Pond, W.G. Potential contribution of absorbed volatile fatty acids to whole-animal energy requirement in conscious swine. J. Anim. Sci. 1991, 69, 2001–2012. [Google Scholar] [CrossRef] [Green Version]
- Jha, R.; Berrocoso, J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Gallaher, D.D.; Locket, P.L.; Gallaher, C.M. Bile acid metabolism in rats fed two levels of corn oil and brans of oat, rye and barley and sugar beet fiber. J. Nutr. 1992, 122, 473–481. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Nørskov, N.P.; Bolvig, A.K.; Hedemann, M.S.; Laerke, H.N. Dietary fibers and associated phytochemicals in cereals. Mol. Nutr. Food Res. 2017, 61, 1600518. [Google Scholar] [CrossRef] [PubMed]
- Jacela, J.Y.; Derouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Renter, D.G.; Dritz, S.S. Feed additives for swine: Fact sheets-acidifiers and antibiotics. J. Swine Health Prod. 2009, 17, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Pi, Y.; Peng, Y.; Mu, C.-L.; Zhu, W.-Y. Time-course responses of ileal and fecal microbiota and metabolite profiles to antibiotics in cannulated pigs. Appl. Microbiol. Biotechnol. 2018, 102, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M. Antibiotics shape microbiota and weight gain across the animal kingdom. Anim. Front. 2016, 6, 8–14. [Google Scholar] [CrossRef]
- Proctor, A.; Phillips, G.J. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. Vet. Sci. 2019, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Yu, K.; Yu, M.; Zhang, C.; Su, Y.; Zhu, W. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids 2017, 49, 771–781. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, C.; Yang, Y.; Mu, C.; Su, Y.; Yu, K.; Zhu, W. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. J. Anim. Sci. Biotechnol. 2017, 8, 60. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Veterinary Feed Directive; FDA: Silver Spring, MD, USA, 2015; Volume 80, pp. 31708–31735.
- Choo, J.M.; Kanno, T.; Zain, N.M.M.; Leong, L.E.X.; Abell, G.C.J.; Keeble, J.E.; Bruce, K.D.; Mason, A.J.; Rogers, G.B. Divergent relationships between fecal microbiota and metabolome following distinct antibiotic-induced disruptions. Am. Soc. Microbiol. 2017, 2, e00005-17. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Microbiome-metabolomics analysis investigating the impacts of dietary starch types on the composition and metabolism of colonic microbiota in finishing pigs. Front. Microbiol. 2019, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Sherry, P.A.; Harrison, P.C.; Fahey, G.C. Dietary fiber and antibiotic interactions on nutritional and metabolic parameters in the growing pig. J. Anim. Sci. 1981, 53, 1309–1315. [Google Scholar] [CrossRef]
- Ravindran, V.; Kornegay, E.T.; Webb, K.E. Effect of fiber and virginiamycin on nutrient absorption, nutrient retention and rate of passage in growing swine. J. Anim. Sci. 1984, 59, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Yan, H.; Almeida, V.V.; Adeola, O.; Ajuwon, K.M. Effects of dietary resistant starch content on nutrient and energy digestibility and fecal metabolomic profile in growing pigs. J. Anim. Sci. 2016, 94, 364–368. [Google Scholar] [CrossRef]
- Moeser, A.J.; Van Kempen, T.A.T.G. Dietary fibre level and enzyme inclusion affect nutrient digestibility and excreta characteristics in grower pigs. J. Sci. Food Agric. 2002, 82, 1606–1613. [Google Scholar] [CrossRef]
- Feoli, C.; Monge, C.R.; Jones, C.L.; Starkey, C.W.; Hancock, J.D. Effects of xylanase and wheat middlings in diets for finishing pigs. J. Anim. Sci. 2006, 84 (Suppl. 1), 429. [Google Scholar] [CrossRef] [Green Version]
- Lenis, N.P.; Bikker, P.; van der Meulen, J.; van Diepen, J.T.M.; Bakker, J.; Jongbloed, A.W. Effect of dietary neutral detergent fiber on ileal digestibility and portal flux of nitrogen and amino acids and on nitrogen utilization in growing pigs. J. Anim. Sci 1996, 74, 2687–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windmueller, H.G.; Spaeth, A.E. Source and fate of circulating citrulline. Am. J. Physiol. Metab. 1981, 4, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.J.; Wang, L.X.; Yang, H.S.; Hu, A.; Yin, Y.L. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal 2019, 13, 2727–2735. [Google Scholar] [CrossRef] [Green Version]
- Berkeveld, M.; Langendijk, P.; Verheijden, J.H.M.; Taverne, M.A.M.; van Nes, A.; van Haard, P.; Koets, A.P. Citrulline and intestinal fatty acid-binding protein: Longitudinal markers of postweaning small intestinal function in pigs? J. Anim. Sci. 2008, 86, 3440–3449. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.R.; Schneeman, B.O. Effects of dietary wheat bran on rat colonic structure and mucosal cell growth. J. Nutr. 1981, 111, 798–803. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Crenshaw, J.D. Effects of dietary fiber on intestinal growth, cell proliferation, and morphology in growing pigs. J. Anim. Sci. 1994, 72, 2270–2278. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Y.; Petrofsky, K.; Liu, J.; Cheng, Y.; Ruan, R.; Chen, C. Double-edged metabolic effects from Short-Term Feeding of Functionalized Wheat Bran to Mouse Revealed by Metabolomic Profiling. J. Agric. Food Chem. 2021, 69, 6543–6555. [Google Scholar] [CrossRef]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as functional food ingredients: A review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Liang, S.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Microbial synthesis of functional odd-chain fatty acids: A review. World J. Microbiol. Biotechnol. 2020, 36, 35. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Furuzono, T.; Yamakuni, K.; Li, Y.; Kim, Y.; Takahashi, H.; Ohue-Kitano, R.; Jheng, H.; Takahashi, N.; Kano, Y.; et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017, 31, 5036–5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Jia, H.-M.; Zhou, C.; Yang, Y.; Sun, L.-L.; Zou, Z.-M. Urinary and fecal metabonomics study of the protective effect of Chaihu-Shu-Gan-San on antibiotic-induced gut microbiota dysbiosis in rats. Sci. Rep. 2017, 7, 46551. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, M.; Zhou, M.; Wu, L.; Yang, H.; Huang, L.; Chen, C. Isolation and genomic characterization of five novel strains of Erysipelotrichaceae from commercial pigs. BMC Microbiol. 2021, 21, 125. [Google Scholar] [CrossRef]
- Peng, T.; Royer, A.L.; Guitton, Y.; Le Bizec, B.; Dervilly-Pinel, G. Serum-based metabolomics characterization of pigs treated with ractopamine. Metabolomics 2017, 13, 77. [Google Scholar] [CrossRef]
- Davies, J.M.; Hua, H.-U.; Dheer, R.; Martinez, M.; Bhattacharya, S.K.; Abreu, M.T. Stool phospholipid signature is altered by diet and tumors. PLoS ONE 2014, 9, e114352. [Google Scholar] [CrossRef]
- Usui, N.; Ray, C.J.; Drongowski, R.A.; Coran, A.G.; Harmon, C.M. The effect of phospholipids and mucin on bacterial internalization in an enterocyte-cell culture model. In Pediatric Surgery International; Springer: Berlin/Heidelberg, Germany, 1999; Volume 15, pp. 150–154. [Google Scholar]
- Saqui-Salces, M.; Huang, Z.; Ferrandis Vila, M.; Li, J.; Mielke, J.A.; Urriola, P.E.; Shurson, G.C. Modulation of intestinal cell differentiation in growing pigs is dependent on the fiber source in the diet. J. Anim. Sci. 2017, 95, 1179–1190. [Google Scholar] [CrossRef]
- Vila, M.F.; Trudeau, M.P.; Hung, Y.T.; Zeng, Z.; Urriola, P.E.; Shurson, G.C.; Saqui-Salces, M. Dietary fiber sources and non-starch polysaccharide-degrading enzymes modify mucin expression and the immune profile of the swine ileum. PLoS ONE 2018, 13, e0207196. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [Green Version]
- Rickard, J.W.; Allee, G.L.; Rincker, P.J.; Gruber, S.L.; Puls, C.L.; Carr, S.N. Effect of narasin (Skycis) or zinc bacitracin (Albac) inclusion on the growth performance and carcass characteristics of finishing pigs sent for slaughter using a 3-phase marketing strategy. Transl. Anim. Sci. 2017, 1, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, J.B.; Love, P.W.; Knotts, L.L. High pressure liquid chromatographic determination of bacitracin in premix feeds and finished feeds: Collaborative study. J. Assoc. Off. Anal. Chem. 1982, 65, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. CompTox Chemicals Dashboard [Salicylic Acid, Methylene Ester]. Available online: https://comptox.epa.gov/dashboard/chemical/details/DTXSID50196503 (accessed on 1 June 2022).
- Cheng, S.; Zhu, L.; Faden, H.S. Interactions of bile acids and the gut microbiota: Learning from the differences in Clostridium difficile infection between children and adults. Physiol. Genom. 2019, 51, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Hylemon, P.B. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7α-dehydroxylating intestinal bacterium. J. Lipid Res. 2012, 53, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, M.P.; Zhou, Y.; Leite, F.L.; Gomez, A.; Urriola, P.E.; Shurson, G.C.; Chen, C.; Isaacson, R.E. Fecal hyodeoxycholic acid is correlated with tylosin-induced microbiome changes in growing pigs. Front. Vet. Sci. 2018, 5, 196. [Google Scholar] [CrossRef]
- Chan, G.; Farzan, A.; Prescott, J.F.; Friendship, R. How do swine practitioners and veterinary pathologists arrive at a diagnosis of Clostridium perfringens type A enteritis in neonatal piglets? Can. Vet. J. 2013, 54, 504–506. [Google Scholar]
- Argiolas, A.; Pisano, J.J. Facilitation of phospholipase as activity by mastoparans, a new class of mast cell degranulating peptides from wasp venom. J. Biol. Chem. 1983, 258, 13697–13702. [Google Scholar] [CrossRef]
- Hung, Y.-T.; Hu, Q.; Faris, R.J.; Guo, J.; Urriola, P.E.; Shurson, G.C.; Chen, C.; Saqui-Salces, M. Analysis of gastrointestinal responses revealed both shared and specific targets of zinc oxide and carbadox in weaned pigs. Antibiotics 2020, 9, 463. [Google Scholar] [CrossRef]
- Chen, C.; Gonzalez, F.J.; Idle, J.R. LC-MS-based metabolomics in drug metabolism. Drug Metab. Rev. 2007, 39, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yao, D.; Chen, C.; Lu, Y.; Yao, D.; Chen, C. 2-Hydrazinoquinoline as a derivatization agent for LC-MS-based metabolomic investigation of diabetic ketoacidosis. Metabolites 2013, 3, 993–1010. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Treatment Group 1 | SE 2 | p-Values 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| AF + LF | AB + LF | AF + HF | AB + HF | AB 4 | Fiber | AB × Fiber 4 | ||
| Body weight, kg | 0.16 | <0.01 | 0.49 | |||||
| Initial | 25.04 | 24.45 | 25.50 | 24.34 | 2.02 | |||
| Phase 1 (d0–21) | 44.15 | 41.80 | 43.13 | 41.00 | 2.03 | |||
| Phase 2 (d21–42) | 64.90 | 63.94 | 63.43 | 61.98 | 2.03 | |||
| Phase 3 (d42–70) | 93.81 | 94.15 | 90.15 | 90.50 | 2.03 | |||
| Phase 4 (d70–98) | 122.75 | 124.72 | 118.41 | 118.41 | 2.03 | |||
| Average daily feed intake (ADFI), kg | 0.08 | 0.81 | 0.65 | |||||
| Phase 1 (d0–21) | 1.66 | 1.62 | 1.63 | 1.59 | 0.2 | |||
| Phase 2 (d21–42) | 2.28 | 2.38 | 2.29 | 2.30 | 0.2 | |||
| Phase 3 (d42–70) | 2.82 | 2.98 | 2.84 | 2.97 | 0.2 | |||
| Phase 4 (d70–98) | 3.33 | 3.36 | 3.46 | 3.44 | 0.2 | |||
| Overall | 2.52 | 2.58 | 2.54 | 2.58 | 0.19 | |||
| Average daily gain (ADG), kg | 0.16 | <0.01 | 0.88 | |||||
| Phase 1 (d0–21) | 0.89 | 0.81 | 0.83 | 0.81 | 0.05 | |||
| Phase 2 (d21–42) | 1.01 | 1.06 | 0.97 | 0.99 | 0.05 | |||
| Phase 3 (d42–70) | 1.03 | 1.08 | 0.96 | 1.01 | 0.05 | |||
| Phase 4 (d70–98) | 1.06 | 1.09 | 1.02 | 1.03 | 0.05 | |||
| Overall | 1.00 | 1.01 | 0.94 | 0.96 | 0.05 | |||
| Gain efficiency (ADG/ADFI) | 0.70 | <0.01 | 0.53 | |||||
| Phase 1 (d0–21) | 0.53 | 0.51 | 0.51 | 0.50 | 0.008 | |||
| Phase 2 (d21–42) | 0.44 | 0.45 | 0.43 | 0.44 | 0.008 | |||
| Phase 3 (d42–70) | 0.37 | 0.37 | 0.34 | 0.35 | 0.008 | |||
| Phase 4 (d70–98) | 0.32 | 0.33 | 0.30 | 0.30 | 0.008 | |||
| Overall | 0.42 | 0.41 | 0.39 | 0.40 | 0.006 | |||
| Treatment Group 1 | SE 2 | p-Values 3 | ADG (r Value) 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AF + LF | AB + LF | AF + HF | AB + HF | AB 4 | Fiber | AB × Fiber 4 | |||
| Amino acids, µg/g | |||||||||
| Alanine | 72.28 | 72.52 | 49.39 | 50.61 | 5.17 | 0.89 | <0.01 | 0.92 | 0.15 |
| Arginine | 0.94 | 1.84 | 1.23 | 1.24 | 0.35 | 0.19 | 0.66 | 0.21 | 0.02 |
| Aspartic acid | 317 | 403 | 275 | 312 | 49 | 0.14 | 0.11 | 0.55 | 0.23 |
| Citrulline | 19.14 | 24.27 | 14.01 | 16.14 | 2.90 | 0.15 | 0.01 | 0.56 | 0.20 |
| Glutamic acid | 835 | 898 | 724 | 816 | 75 | 0.27 | 0.17 | 0.84 | 0.17 |
| Glutamine | 0.71 | 0.74 | 0.23 | 0.12 | 0.14 | 0.75 | <0.01 | 0.57 | 0.28 |
| Glycine | 61.38 | 69.35 | 46.55 | 59.73 | 13.6 | 0.44 | 0.37 | 0.85 | −0.04 |
| Histidine | 2.00 | 3.36 | 1.94 | 2.09 | 0.29 | 0.01 | 0.02 | 0.04 | 0.18 |
| Leucine/Isoleucine | 21.97 | 19.13 | 11.55 | 12.36 | 2.26 | 0.65 | <0.01 | 0.42 | 0.08 |
| Lysine | 193 | 214 | 143 | 159 | 15 | 0.21 | <0.01 | 0.87 | 0.25 |
| Methionine | 3.56 | 3.54 | 2.49 | 2.15 | 0.35 | 0.60 | <0.01 | 0.65 | 0.16 |
| Ornithine | 6.91 | 5.61 | 2.94 | 6.64 | 1.98 | 0.54 | 0.46 | 0.21 | 0.05 |
| Phenylalanine | 14.81 | 15.16 | 8.55 | 10.45 | 2.11 | 0.59 | 0.01 | 0.71 | 0.07 |
| Proline | 36.34 | 38.03 | 31.42 | 36.49 | 4.43 | 0.44 | 0.46 | 0.70 | 0.05 |
| Serine | 10.86 | 12 | 7.70 | 8.46 | 1.56 | 0.51 | 0.02 | 0.90 | 0.12 |
| Taurine | 0.55 | 1.05 | 0.56 | 0.52 | 0.24 | 0.27 | 0.21 | 0.20 | 0.19 |
| Threonine | 11.60 | 13.71 | 7.83 | 8.55 | 1.10 | 0.20 | <0.01 | 0.53 | 0.17 |
| Tryptophan | 1.02 | 1.15 | 0.84 | 0.79 | 0.14 | 0.76 | 0.02 | 0.41 | 0.06 |
| Tyrosine | 20.03 | 24.45 | 11.23 | 16.09 | 3.35 | 0.17 | 0.01 | 0.95 | 0.06 |
| Valine | 38.53 | 33.84 | 24.35 | 25.06 | 3.68 | 0.59 | <0.01 | 0.46 | 0.07 |
| γ-Aminobutyric acid | 0.78 | 1.17 | 1.04 | 0.75 | 0.27 | 0.83 | 0.77 | 0.19 | 0.15 |
| Fatty acids, mg/g | |||||||||
| Acetic acid | 10.23 | 9.48 | 13.36 | 13.70 | 2.46 | 0.14 | 0.93 | 0.82 | −0.01 |
| Propionic acid | 6.21 | 6.29 | 8.72 | 8.46 | 1.74 | 0.18 | 0.96 | 0.92 | −0.02 |
| Butyric acid | 5.37 | 5.43 | 7.25 | 7.15 | 1.40 | 0.20 | 0.99 | 0.95 | −0.02 |
| Isovaleric acid | 3.56 | 4.29 | 3.60 | 4.21 | 0.80 | 0.41 | 0.98 | 0.95 | −0.02 |
| C6:0 | 0.06 | 0.09 | 0.09 | 0.07 | 0.02 | 0.85 | 0.96 | 0.28 | 0.01 |
| C8:0 | 0.001 | 0.001 | 0.001 | 0.001 | 0.0002 | 0.69 | 0.26 | 0.90 | 0.06 |
| C12:0 | 0.004 | 0.003 | 0.002 | 0.002 | 0.0003 | 0.27 | <0.01 | 0.18 | −0.11 |
| C14:0 | 0.08 | 0.07 | 0.06 | 0.06 | 0.01 | 0.56 | <0.01 | 0.14 | −0.15 |
| C15:0 | 0.18 | 0.15 | 0.11 | 0.11 | 0.01 | 0.13 | <0.01 | 0.07 | −0.09 |
| C16:0 | 2.22 | 2.21 | 2.12 | 2.45 | 0.19 | 0.39 | 0.73 | 0.35 | −0.04 |
| C16:1 | 0.007 | 0.007 | 0.010 | 0.011 | 0.001 | 0.24 | <0.01 | 0.71 | −0.02 |
| C18:0 | 2.22 | 2.09 | 1.98 | 2.39 | 0.19 | 0.47 | 0.88 | 0.16 | −0.03 |
| C18:1 | 1.71 | 2.02 | 2.15 | 2.26 | 0.15 | 0.14 | 0.02 | 0.48 | −0.02 |
| C18:2 | 2.17 | 2.93 | 2.95 | 2.92 | 0.23 | 0.10 | 0.09 | 0.08 | 0.09 |
| Bile acids, µg/g 6 | |||||||||
| CA | 0.40 | 0.37 | 0.21 | 0.20 | 0.05 | 0.70 | <0.01 | 0.89 | 0.04 |
| CDCA | 0.14 | 0.34 | 0.26 | 0.20 | 0.07 | 0.26 | 0.92 | 0.04 | 0.13 |
| DCA | 0.66 | 0.53 | 0.42 | 0.32 | 0.11 | 0.22 | 0.02 | 0.89 | 0.18 |
| HDCA | 671 | 571 | 720 | 383 | 70 | <0.01 | 0.32 | 0.09 | 0.10 |
| LCA | 549 | 385 | 509 | 310 | 58 | <0.01 | 0.32 | 0.76 | −0.09 |
| GCA | 0.17 | 0.21 | 0.09 | 0.15 | 0.04 | 0.21 | 0.09 | 0.75 | 0.21 |
| GCDCA | 0.04 | 0.2 | 0.10 | 0.14 | 0.04 | 0.02 | 0.92 | 0.14 | −0.01 |
| TCA | 0.15 | 0.15 | 0.10 | 0.02 | 0.04 | 0.29 | 0.02 | 0.23 | 0.05 |
| ID | Identity (Derivative) 2 | m/z of Charged Ion | Formula of Original Molecule | Database | p-Values | ADG (r Value) 1 | ||
|---|---|---|---|---|---|---|---|---|
| AB 3 | Fiber 3 | AB × Fiber | ||||||
| I | Oleic acid Oleic acid (HQ) | 281.2478− 424.3321+ | C18H34O2 | HMDB00207 | 0.04 (↑) | <0.01 (↑) | 0.42 | −0.01 |
| II II’ | Linoleic acid Linoleic acid (HQ) | 279.2322− 422.3164+ | C18H32O2 | HMDB00673 | 0.08 | 0.01 (↑) | 0.26 | 0.10 |
| III | LysoPC(16:0) | 496.3418+ | C24H50NO7P | HMDB10382 | 0.03 (↑) | <0.01 (↑) | 0.53 | −0.03 |
| IV | LysoPC(18:2) | 520.3417+ | C26H50NO7P | HMDB10386 | <0.01 (↑) | <0.01 (↑) | 0.05 | −0.06 |
| V | LysoPC(18:1) | 522.3568+ | C26H52NO7P | HMDB10385 | <0.01 (↑) | <0.01 (↑) | 0.32 | −0.05 |
| VI | a steroid 3 | 379.2965+ | C27H38O | HMDB60512 | 0.03 (↓) | <0.01 (↑) | 0.01 | −0.13 |
| VII | N-Lauroylglycine | 258.2066+ | C14H27NO3 | HMDB13272 | 0.44 | <0.01 (↑) | 0.70 | −0.31 |
| VIII | ND 4 | 304.3005+ | ND | <0.01 (↓) | <0.01 (↑) | 0.16 | −0.25 | |
| IX | LysoPE(15:0) | 440.2784 | C20H42NO7P | HMDB11502 | 0.43 | <0.01 (↑) | 0.35 | −0.11 |
| X | LysoPE(16:0) | 454.294+ | C21H44NO7P | HMDB11503 | 0.58 | <0.01 (↑) | 0.76 | −0.08 |
| XI | Oxo-octadecanoic acid | 297.2425− | C18H34O3 | HMDB10736 | 0.90 | <0.01 (↑) | 0.13 | −0.16 |
| XII | Methylene disalicylate | 287.0553− | C15H10O6 | <0.01 (↑) | 0.01 (↓) | 0.01 | −0.01 | |
| XIII | Lithocholic acid | 375.2897− | C24H40O3 | HMDB00761 | 0.01 (↓) | 0.07 | 0.30 | −0.14 |
| XIV | p-Cresol (DC) | 342.1154+ | C7H8O | HMDB01858 | 0.18 | 0.04 (↓) | 0.26 | −0.07 |
| XV | ND | 325.1766+ | C16H24N2O5 | 0.77 | <0.01 (↓) | 0.27 | 0.18 | |
| XVI | ND | 226.1079+ | C11H15NO4 | 0.21 | <0.01 (↓) | 0.05 | 0.13 | |
| XVII | Alanine (DC) | 323.1056+ | C3H7NO2 | HMDB00161 | 0.85 | 0.01 (↓) | 0.49 | 0.12 |
| XVIII | Stercobilin | 595.3514+ | C33H46N4O6 | HMDB0240259 | 0.97 | 0.02 (↓) | 0.50 | −0.02 |
| XIX XIX’ | Pentadecanoic acid Pentadecanoic acid (HQ) | 241.2164− 384.3009+ | C15H30O2 | HMDB00826 | 0.09 | <0.01 (↓) | 0.07 | −0.05 |
| XX XX’ | Dihydroxyquinoline | 162.0552+ 160.0394− | C9H7NO2 | HMDB04077 | 0.71 | <0.01 (↓) | 0.20 | −0.02 |
| XXI | 2-Aminooctanoic acid | 160.135+ | C8H17NO2 | HMDB00991 | 0.09 | <0.01 (↓) | 0.86 | 0.20 |
| XXII | Hydroxy-hexadecenoic acid | 271.2269− | C16H32O3 | HMDB10734 | 0.03 (↓) | 0.03 (↓) | 0.17 | −0.04 |
| XXIII | Deoxycholic acid (HQ) | 534.3691+ | C24H40O4 | HMDB00626 | 0.06 | 0.01 (↓) | 0.56 | 0.10 |
| XXIV | Phenylacetic acid | 278.129+ | C8H8O2 | HMDB00209 | 0.47 | <0.01 (↓) | 0.69 | −0.05 |
| XXV | Heptadecanoic acid | 269.2476− | C17H34O2 | HMDB02259 | 0.15 | <0.01 (↓) | 0.22 | −0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-T.; Song, Y.; Hu, Q.; Faris, R.J.; Guo, J.; Ma, Y.; Saqui-Salces, M.; Urriola, P.E.; Shurson, G.C.; Chen, C. Identification of Independent and Shared Metabolic Responses to High-Fiber and Antibiotic Treatments in Fecal Metabolome of Grow–Finish Pigs. Metabolites 2022, 12, 686. https://doi.org/10.3390/metabo12080686
Hung Y-T, Song Y, Hu Q, Faris RJ, Guo J, Ma Y, Saqui-Salces M, Urriola PE, Shurson GC, Chen C. Identification of Independent and Shared Metabolic Responses to High-Fiber and Antibiotic Treatments in Fecal Metabolome of Grow–Finish Pigs. Metabolites. 2022; 12(8):686. https://doi.org/10.3390/metabo12080686
Chicago/Turabian StyleHung, Yuan-Tai, Yajian Song, Qiong Hu, Richard J. Faris, Juanjuan Guo, Yiwei Ma, Milena Saqui-Salces, Pedro E. Urriola, Gerald C. Shurson, and Chi Chen. 2022. "Identification of Independent and Shared Metabolic Responses to High-Fiber and Antibiotic Treatments in Fecal Metabolome of Grow–Finish Pigs" Metabolites 12, no. 8: 686. https://doi.org/10.3390/metabo12080686