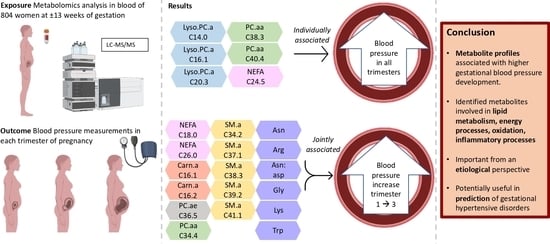
Associations of Early Pregnancy Metabolite Profiles with Gestational Blood Pressure Development
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Metabolomics Analysis
2.3. Blood Pressure and Gestational Hypertensive Disorders
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Maternal Early-Pregnancy Metabolites and Gestational Blood Pressure
3.3. Maternal Early-Pregnancy Metabolites and Prediction of Higher Blood Pressure
4. Discussion
4.1. Interpretation of Main Findings
4.2. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino-acids |
| Carn | carnitines |
| Carn.a | acyl-carnitines |
| NEFA | non-esterified fatty acids |
| Lyso.PC.a | acyl-lysophosphatidylcholines |
| Lyso.PC.e | alkyl-lysophosphatidylcholines |
| PC | phosphatidylcholines |
| PC.aa | diacyl-phosphatidylcholines |
| PC.ae | acyl-alkyl-phosphatidylcholines |
| PL | phospholipids |
| SM | sphingomyelins |
References
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F.A. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V. Blood Pressure in Different Gestational Trimesters, Fetal Growth, and the Risk of Adverse Birth Outcomes: The Generation R Study. Am. J. Epidemiol. 2011, 174, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Ebbels, T.M.; Valdes, A.; Elliott, P.; Ioannidis, J.P. Design and analysis of metabolomics studies in epidemiologic research: A primer on -omic technologies. Am. J. Epidemiol. 2014, 180, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R.; Bakker, R.; Willemsen, S.P.; Hofman, A.; Steegers, E.A.; Jaddoe, V.W. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: The Generation R Study. Eur. Heart J. 2011, 32, 3088–3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catov, J.M.; Ness, R.B.; Kip, K.E.; Olsen, J. Risk of early or severe preeclampsia related to pre-existing conditions. Int. J. Epidemiol. 2007, 36, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spracklen, C.N.; Smith, C.J.; Saftlas, A.F.; Robinson, J.G.; Ryckman, K.K. Maternal Hyperlipidemia and the Risk of Preeclampsia: A Meta-Analysis. Am. J. Epidemiol. 2014, 180, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Austdal, M.; Tangerås, L.H.; Skråstad, R.B.; Salvesen, K.; Austgulen, R.; Iversen, A.-C.; Bathen, T.F. First Trimester Urine and Serum Metabolomics for Prediction of Preeclampsia and Gestational Hypertension: A Prospective Screening Study. Int. J. Mol. Sci. 2015, 16, 21520–21538. [Google Scholar] [CrossRef] [Green Version]
- Bahado-Singh, R.; Poon, L.C.; Yilmaz, A.; Syngelaki, A.; Turkoglu, O.; Kumar, P.; Kirma, J.; Allos, M.; Accurti, V.; Li, J.; et al. Integrated Proteomic and Metabolomic prediction of Term Preeclampsia. Sci. Rep. 2017, 7, 16189. [Google Scholar] [CrossRef] [Green Version]
- Bahado-Singh, R.O.; Akolekar, R.; Mandal, R.; Dong, E.; Xia, J.; Kruger, M.; Wishart, D.S.; Nicolaides, K. Metabolomics and first-trimester prediction of early-onset preeclampsia. J. Matern.-Fetal Neonatal Med. 2012, 25, 1840–1847. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Akolekar, R.; Mandal, R.; Dong, E.; Xia, J.; Kruger, M.; Wishart, D.S.; Nicolaides, K. First-trimester metabolomic detection of late-onset preeclampsia. Am. J. Obstet. Gynecol. 2013, 208, e51–e58. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Syngelaki, A.; Mandal, R.; Graham, S.F.; Akolekar, R.; Han, B.; Bjondahl, T.C.; Dong, E.; Bauer, S.; Alpay-Savasan, Z.; et al. Metabolomic determination of pathogenesis of late-onset preeclampsia. J. Matern.-Fetal Neonatal Med. 2017, 30, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Croteau-Chonka, D.C.; Dahlin, A.; Mirzakhani, H.; Wu, A.C.; Wan, E.S.; McGeachie, M.J.; Qiu, W.; Sordillo, J.E.; Al-Garawi, A.; et al. Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics 2017, 13, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, L.C.; Broadhurst, D.I.; Dunn, W.; Brown, M.; North, R.A.; McCowan, L.; Roberts, C.; Cooper, G.J.S.; Kell, D.B.; Baker, P.N. Robust Early Pregnancy Prediction of Later Preeclampsia Using Metabolomic Biomarkers. Hypertension 2010, 56, 741–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Huang, C.; Su, J.; Pan, C.-W.; Ke, C. Identification of biomarkers for essential hypertension based on metabolomics. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Salihovic, S.; Fall, T.; Hammar, U.; Ingelsson, E.; Ärnlöv, J.; Lind, L.; Sundström, J. Global Plasma Metabolomics to Identify Potential Biomarkers of Blood Pressure Progression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e227–e237. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Li, C.; Mi, X.; Shi, M.; Gu, X.; Bazzano, L.A.; Razavi, A.C.; Nierenberg, J.L.; Dorans, K.; He, H.; et al. An untargeted metabolomics study of blood pressure: Findings from the Bogalusa Heart Study. J. Hypertens. 2020, 38, 1302. [Google Scholar] [CrossRef]
- Monni, G.; Atzori, L.; Corda, V.; Dessolis, F.; Iuculano, A.; Hurt, K.J.; Murgia, F. Metabolomics in Prenatal Medicine: A Review. Front. Med. 2021, 8, 645118. [Google Scholar] [CrossRef]
- Kooijman, M.N.; Kruithof, C.J.; van Duijn, C.M.; Duijts, L.; Franco, O.H.; van Ijzendoorn, M.H.; de Jongste, J.C.; Klaver, C.C.W.; van der Lugt, A.; Mackenbach, J.P.; et al. The Generation R Study: Design and cohort update 2017. Eur. J. Epidemiol. 2016, 31, 1243–1264. [Google Scholar] [CrossRef] [Green Version]
- Voerman, E.; Jaddoe, V.W.V.; Uhl, O.; Shokry, E.; Horak, J.; Felix, J.F.; Koletzko, B.; Gaillard, R. A population-based resource for intergenerational metabolomics analyses in pregnant women and their children: The Generation R Study. Metabolomics 2020, 16, 43. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, C.; Uhl, O.; Standl, M.; Demmelmair, H.; Heinrich, J.; Koletzko, B.; Thiering, E. Cord Blood Metabolome Is Highly Associated with Birth Weight, but Less Predictive for Later Weight Development. Obes. Facts 2017, 10, 85–100. [Google Scholar] [CrossRef]
- Hellmuth, C.; Weber, M.; Koletzko, B.; Peissner, W. Nonesterified Fatty Acid Determination for Functional Lipidomics: Comprehensive Ultrahigh Performance Liquid Chromatography–Tandem Mass Spectrometry Quantitation, Qualification, and Parameter Prediction. Anal. Chem. 2012, 84, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Uhl, O.; Fleddermann, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Phospholipid Species in Newborn and 4 Month Old Infants after Consumption of Different Formulas or Breast Milk. PLoS ONE 2016, 11, e0162040. [Google Scholar] [CrossRef] [Green Version]
- Molnos, S.; Wahl, S.; Haid, M.; Eekhoff, E.M.W.; Pool, R.; Floegel, A.; Deelen, J.; Much, D.; Prehn, C.; Breier, M.; et al. Metabolite ratios as potential biomarkers for type 2 diabetes: A DIRECT study. Diabetologia 2018, 61, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squeri, A. Coronary Artery Disease—New Insights and Novel Approaches; IntechOpen: London, UK, 2012; 272p, Available online: https://www.intechopen.com/books/660 (accessed on 15 May 2022). [CrossRef] [Green Version]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickens, C.A.; Vazquez, A.I.; Jones, A.D.; Fenton, J.I. Obesity, adipokines, and C-peptide are associated with distinct plasma phospholipid profiles in adult males, an untargeted lipidomic approach. Sci. Rep. 2017, 7, 6335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchberg, F.F.; Brandt, S.; Moß, A.; Peissner, W.; Koenig, W.; Rothenbacher, D.; Brenner, H.; Koletzko, B.; Hellmuth, C.; Wabitsch, M. Metabolomics reveals an entanglement of fasting leptin concentrations with fatty acid oxidation and gluconeogenesis in healthy children. PLoS ONE 2017, 12, e0183185. [Google Scholar] [CrossRef] [Green Version]
- Sampath, H.; Ntambi, J. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidol. 2008, 3, 163–173. [Google Scholar] [CrossRef]
- El Assaad, M.A.; Topouchian, J.A.; Darné, B.M.; Asmar, R.G. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press. Monit. 2002, 7, 87A. [Google Scholar] [CrossRef]
- Macdonald-Wallis, C.; Lawlor, D.A.; Fraser, A.; May, M.; Nelson, S.M.; Tilling, K. Blood Pressure Change in Normotensive, Gestational Hypertensive, Preeclamptic, and Essential Hypertensive Pregnancies. Hypertension 2012, 59, 1241–1248. [Google Scholar] [CrossRef]
- Coolman, M.; de Groot, C.J.M.; Jaddoe, V.W.; Hofman, A.; Raat, H.; Steegers, E.A.P. Medical record validation of maternally reported history of preeclampsia. J. Clin. Epidemiol. 2010, 63, 932–937. [Google Scholar] [CrossRef]
- Brown, M.A.; Lindheimer, M.D.; de Swiet, M.; Assche, A.V.; Moutquin, J.-M. The Classification and Diagnosis of the Hypertensive Disorders of Pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens. Pregnancy 2001, 20, ix–xiv. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, M.; Valvi, D.; Luque, N.; Ballesteros-Gomez, A.; Carsin, A.-E.; Fernandez, M.F.; Koch, H.M.; Mendez, M.A.; Sunyer, J.; Rubio, S.; et al. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ. Int. 2013, 56, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Philips, E.M.; Jaddoe, V.W.V.; Asimakopoulos, A.G.; Kannan, K.; Steegers, E.A.P.; Santos, S.; Trasande, L. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004-5. Environ. Res. 2018, 161, 562–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umesawa, M.; Kobashi, G. Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis. Hypertens. Res. 2017, 40, 213–220. [Google Scholar] [CrossRef]
- Bouthoorn, S.H.; Gaillard, R.; Steegers, E.A.; Hofman, A.; Jaddoe, V.W.; van Lenthe, F.J.; Raat, H. Ethnic differences in blood pressure and hypertensive complications during pregnancy: The Generation R study. Hypertension 2012, 60, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J. Hypertens. 2011, 29, 937–944. [Google Scholar] [CrossRef]
- Rurangirwa, A.A.; Gaillard, R.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V. Hemodynamic Adaptations in Different Trimesters Among Nulliparous and Multiparous Pregnant Women; The Generation R Study. Am. J. Hypertens. 2012, 25, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Bakker, R.; Steegers, E.A.; Mackenbach, J.P.; Hofman, A.; Jaddoe, V.W. Maternal smoking and blood pressure in different trimesters of pregnancy: The Generation R study. J. Hypertens. 2010, 28, 2210–2218. [Google Scholar] [CrossRef]
- Kay, V.R.; Wedel, N.; Smith, G.N. Family History of Hypertension, Cardiovascular Disease, or Diabetes and Risk of Developing Preeclampsia: A Systematic Review. J. Obs. Gynaecol. Can. 2021, 43, 227–236 e219. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Erkamp, J.S.; Jaddoe, V.W.V.; Duijts, L.; Reiss, I.K.M.; Mulders, A.G.M.G.J.; Steegers, E.A.P.; Gaillard, R. Population screening for gestational hypertensive disorders using maternal, fetal and placental characteristics: A population-based prospective cohort study. Prenat. Diagn. 2020, 40, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Buuren, S.V. Flexible Imputation of Missing Data, 2nd ed.; Chapman and Hall/CRC: London, UK, 2018. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G.L. Non-esterified fatty acids and blood pressure elevation: A mechanism for hypertension in subjects with obesity/insulin resistance? J. Hum. Hypertens. 2007, 21, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Katajamäki, T.T.; Koivula, M.-K.; Hilvo, M.; Lääperi, M.T.A.; Salminen, M.J.; Viljanen, A.M.; Heikkilä, E.T.M.; Löppönen, M.K.; Isoaho, R.E.; Kivelä, S.-L.; et al. Ceramides and Phosphatidylcholines Associate with Cardiovascular Diseases in the Elderly. Clin. Chem. 2022, hvac158, 1–7. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Haupt, R.; Alms, S.; Holzmann, G.; König, T.; Kern, H.; Kox, W.; Rüstow, B.; Schlame, M. Increase in fragmented phosphatidylcholine in blood plasma by oxidative stress. J. Lipid Res. 2000, 41, 1145–1153. [Google Scholar] [CrossRef]
- Bachlechner, U.; Floegel, A.; Steffen, A.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H. Associations of anthropometric markers with serum metabolites using a targeted metabolomics approach: Results of the EPIC-potsdam study. Nutr. Diabetes 2016, 6, e215. [Google Scholar] [CrossRef] [Green Version]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Kirchberg, F.; Mori, T.A.; Huang, R.-C.; Beilin, L.J.; Hellmuth, C.; Oddy, W.H. Lipidomics Reveals Associations of Phospholipids With Obesity and Insulin Resistance in Young Adults. J. Clin. Endocrinol. Metab. 2016, 101, 871–879. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.G.; Fritsche, A.; Häring, H.U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Floegel, A.; Kühn, T.; Sookthai, D.; Johnson, T.; Prehn, C.; Rolle-Kampczyk, U.; Otto, W.; Weikert, C.; Illig, T.; von Bergen, M.; et al. Serum metabolites and risk of myocardial infarction and ischemic stroke: A targeted metabolomic approach in two German prospective cohorts. Eur. J. Epidemiol. 2018, 33, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, C.; Uhl, O.; Demmelmair, H.; Grunewald, M.; Auricchio, R.; Castillejo, G.; Korponay-Szabo, I.R.; Polanco, I.; Roca, M.; Vriezinga, S.L.; et al. The impact of human breast milk components on the infant metabolism. PLoS ONE 2018, 13, e0197713. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Hellmuth, C.; Uhl, O.; Kirchberg, F.F.; Peissner, W.; Harder, U.; Grote, V.; Weber, M.; Xhonneux, A.; Langhendries, J.P.; et al. Rapid Growth and Childhood Obesity Are Strongly Associated with LysoPC(14:0). Ann. Nutr. Metab. 2014, 64, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, M.; Okazaki, T. Ceramide/Sphingomyelin Rheostat Regulated by Sphingomyelin Synthases and Chronic Diseases in Murine Models. J. Lipid Atheroscler. 2020, 9, 380–405. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Li, F.; Sharpe, M.R.; Williams, C.D.; Curry, S.C.; Ma, X.; Jaeschke, H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 2014, 88, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]




| Characteristic | Total Sample n = 803 |
|---|---|
| Age at enrolment, mean (±SD), years | 31.4 (4.1) |
| Parity, n (%) | |
| Nulliparous | 490 (61.0) |
| Multiparous | 313 (39.0) |
| Ethnicity, n (%) | |
| Dutch | 803 (100) |
| Other | 0 (0) |
| Education, n (%) | |
| Primary | 15 (1.9) |
| Secondary | 278 (34.8) |
| Higher | 506 (63.3) |
| Pre-pregnancy body mass index, median (95% range), kg/m2 | 22.6 (20.9, 25.2) |
| Smoking, n (%) | |
| Never smoked during pregnancy | 553 (75.9) |
| Smoked until pregnancy was known | 73 (10.0) |
| Continued smoking in pregnancy | 103 (14.1) |
| Systolic blood pressure, mean (SD), mmHg | |
| Early pregnancy | 118.9 (13.0) |
| Mid pregnancy | 119.4 (12.3) |
| Late pregnancy | 120.4 (11.0) |
| Diastolic blood pressure, mean (SD), mmHg | |
| Early pregnancy | 67.0 (10.0) |
| Mid pregnancy | 68.1 (9.7) |
| Late pregnancy | 69.9 (9.3) |
| Gestational hypertensive disorders, n (%) | |
| Gestational hypertension | 37 (4.8) |
| Preeclampsia | 12 (1.6) |
| History of hypertensive disorders, n(%) | |
| Pre-existing hypertension | 9 (1.1) |
| Gestational hypertensive disorders | 38 (4.7) |
| Model | Difference in Blood Pressure (95% CI) | Adjusted R2 (%) | SD Residuals |
|---|---|---|---|
| Clinical model systolic blood pressure | 2.6 | 11.9 | |
| Maternal age | 0.22 (−0.02, 0.46) | ||
| Pre-pregnancy BMI | −0.52 (−0.76, −0.27) | ||
| Parity | −0.41 (−1.80, 0.98) | ||
| Smoking | −0.39 (−3.09, 2.31) | ||
| Metabolite model systolic blood pressure | 9.2 | 11.3 | |
| Maternal age | 0.24 (−1.76, 0.48) | ||
| Pre-pregnancy BMI | −0.54 (−7.92, −0.28) | ||
| Parity | −0.62 (−1.99, 0.76) | ||
| Smoking | 0.19 (−2.47, 2.84) | ||
| Arginine | −0.03 (−0.25, 0.02) | ||
| Asparagine | 0.03 (−0.07, 0.13) | ||
| Glycine | 0.01 (−0.01, 0.03) | ||
| Lysine | 0.06 (0.03, 0.09) | ||
| Tryptophan | −0.14 (−0.23, −0.05) | ||
| NEFA_18_0 | −0.02 (−0.13, 0.09) | ||
| NEFA_26_0 | −7.80 (−17.80, 2.20) | ||
| PC.aa.C34.4 | −1.17 (−2.42, 0.08) | ||
| PC.ae.C36.5 | −0.18 (−0.57, 0.21) | ||
| SM.a.C37.1 | 1.73 (−0.16, 3.62) | ||
| SM.a.C38.2 | 0.14 (−0.08, 0.37) | ||
| SM.a.C39.2 | 3.31 (0.53, 6.08) | ||
| SM.a.C41.1 | −0.26 (−0.64, 0.11) | ||
| SM.a.C43.2 | −1.12 (−2.58, 0.33) | ||
| Carn.a.C16.1 | 29.34 (−12.25, 70.96) | ||
| Carn.a.C16.2 | 26.95 (−59.61, 113.52) | ||
| Asn/asp | 0.27 (−1.14, 1.68) | ||
| Clinical model diastolic blood pressure | 0.5 | 8.7 | |
| Maternal age | 0.12 (−0.06, 0.29) | ||
| Pre-pregnancy BMI | −0.08 (−0.26, 0.09) | ||
| Parity | −1.05 (−2.08, −0.03) | ||
| Smoking | 1.78 (−0.21, 3.76) | ||
| Metabolite model diastolic blood pressure | 7.5 | 8.3 | |
| Maternal age | 0.11 (−0.07, 0.28) | ||
| Pre-pregnancy BMI | −0.06 (−0.25, 1.26) | ||
| Parity | −1.18 (−2.10, −0.17) | ||
| Smoking | 2.20 (0.25, 4.15) | ||
| Arginine | −0.04 (−0.09, 0.00) | ||
| Asparagine | 0.04 (−0.04, 0.11) | ||
| Glycine | 0.01 (−0.01, 0.03) | ||
| Lysine | 0.04 (0.02, 0.06) | ||
| Tryptophan | −0.06 (−0.13, 0.00) | ||
| NEFA_18_0 | −0.03 (−0.12, 0.05) | ||
| NEFA_26_0 | −3.93 (−11.3, 3.42) | ||
| PC.aa.C34.4 | −1.04 (−1.96, −0.12) | ||
| PC.ae.C36.5 | −0.31 (−0.59, −0.02) | ||
| SM.a.C37.1 | 1.53 (0.14, 2.92) | ||
| SM.a.C38.2 | 0.14 (−0.02, 0.31) | ||
| SM.a.C39.2 | 1.27 (−0.77, 3.31) | ||
| SM.a.C41.1 | 0.13 (−0.14, 0.40) | ||
| SM.a.C43.2 | −1.20 (−2.27, −0.13) | ||
| Carn.a.C16.1 | 8.24 (−22.35, 38.83) | ||
| Carn.a.C16.2 | 43.3 (−20.39, 106.91) | ||
| Asn/asp | 0.24 (−0.79, 1.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaauwendraad, S.M.; Wahab, R.J.; van Rijn, B.B.; Koletzko, B.; Jaddoe, V.W.V.; Gaillard, R. Associations of Early Pregnancy Metabolite Profiles with Gestational Blood Pressure Development. Metabolites 2022, 12, 1169. https://doi.org/10.3390/metabo12121169
Blaauwendraad SM, Wahab RJ, van Rijn BB, Koletzko B, Jaddoe VWV, Gaillard R. Associations of Early Pregnancy Metabolite Profiles with Gestational Blood Pressure Development. Metabolites. 2022; 12(12):1169. https://doi.org/10.3390/metabo12121169
Chicago/Turabian StyleBlaauwendraad, Sophia M., Rama J. Wahab, Bas B. van Rijn, Berthold Koletzko, Vincent W. V. Jaddoe, and Romy Gaillard. 2022. "Associations of Early Pregnancy Metabolite Profiles with Gestational Blood Pressure Development" Metabolites 12, no. 12: 1169. https://doi.org/10.3390/metabo12121169