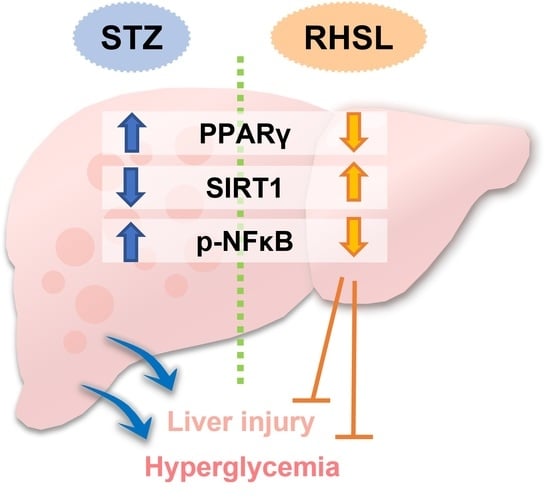
Effects of Rice-Husk Silica Liquid in Streptozotocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent Preparation
2.1.1. Rice-Husk Silica Liquid (RHSL) and Food-Grade Silica Liquid (FDSL)
2.1.2. Streptozotocin (STZ)
2.1.3. Rosiglitazone (RSG)
2.2. Establishment of Diabetic Mice and Treatment Interventions
2.3. Determination of Organ Indices
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Plasms Insulin Analysis
2.6. Assessment of Liver Pathology
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of RHSL and FDSL on Body Weight and Organ Weight in STZ-Induced Diabetic Mice
3.2. Effects of RHSL and FDSL on Water Intake, Food Intake, and Blood Glucose in STZ-Induced Diabetic Mice
3.3. Effects of RHSL and FDSL on Blood Glucose Regulation and Insulin Tolerance in STZ-Induced Diabetic Mice
3.4. Effects of RHSL and FDSL on Liver Morphology in STZ-Induced Diabetic Mice
3.5. Effects of RHSL and FDSL on Hepatic Glucose-Regulation-Related Markers in STZ-Induced Diabetic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications, Nature reviews. Endocrinology 2018, 14, 88–98. [Google Scholar]
- Forlani, G.; Giorda, C.; Manti, R.; Mazzella, N.; De Cosmo, S.; Rossi, M.C.; Nicolucci, A.; Di Bartolo, P.; Ceriello, A.; Guida, P. The Burden of NAFLD and Its Characteristics in a Nationwide Population with Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2931985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Adhikary, P.; Cheng, K. Cellular protein markers, therapeutics, and drug delivery strategies in the treatment of diabetes-associated liver fibrosis. Adv. Drug Deliv. Rev. 2021, 174, 127–139. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [Green Version]
- Goud, B.J. Streptozotocin—A Diabetogenic Agent in Animal Models. Int. J. Pharmacogn. Phytochem. Res. 2015, 3, 253–269. [Google Scholar]
- Schnedl, W.J.; Ferber, S.; Johnson, J.H.; Newgard, C.B. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 1994, 43, 1326–1333. [Google Scholar] [CrossRef]
- Leturque, A.; Brot-Laroche, E.; Le Gall, M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E985–E992. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, S.; Zhou, S.; Qiu, J.; Shi, C.; Liu, R.; Zhou, H.; Lu, L. Hyperglycemia aggravates acute liver injury by promoting liver-resident macrophage NLRP3 inflammasome activation via the inhibition of AMPK/mTOR-mediated autophagy induction. Immunol. Cell Biol. 2020, 98, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Nopparat, J.; Nualla-Ong, A.; Phongdara, A. Treatment with Pluchea indica (L.) Less. leaf ethanol extract alleviates liver injury in multiple low-dose streptozotocin-induced diabetic BALB/c mice. Exp. Ther. Med. 2020, 20, 1385–1396. [Google Scholar] [CrossRef]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Ekim Üstünel, B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv. Sci. 2021, 8, e2100275. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, L.; Wang, F.; Liu, X.; Huang, Z.; Zhao, H.; Qi, B.; Zhang, G. Exogenous silicon enhances resistance to 1,2,4-trichlorobenzene in rice. Sci. Total Environ. 2022, 845, 157248. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on the possible nutritional importance of silicon. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2014, 28, 379–382. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.; Evans, B.A.; Thompson, R.P.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chiang, Y.F.; Wang, K.L.; Huang, T.C.; Ali, M.; Shieh, T.M.; Chang, H.Y.; Hong, Y.H.; Hsia, S.M. Rice Husk Silica Liquid Protects Pancreatic β Cells from Streptozotocin-Induced Oxidative Damage. Antioxidants 2021, 10, 1080. [Google Scholar] [CrossRef] [PubMed]
- TM, M.W.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica Nanoparticles in Transmucosal Drug Delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Tseng, C.-C.; Setyoningrum, D.; Yang, Z.-P.; Maftuch; Hu, S.-Y. Rice Husk Silica Enhances Innate Immune in Zebrafish (Danio rerio) and Improves Resistance to Aeromonas hydrophila and Streptococcus iniae Infection. Sustainability 2019, 11, 6504. [Google Scholar] [CrossRef] [Green Version]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S.; Funahashi, K.; Tamagawa, N.; Ning, M.; Ito, T. Taurine Ameliorates Streptozotocin-Induced Diabetes by Modulating Hepatic Glucose Metabolism and Oxidative Stress in Mice. Metabolites 2022, 12, 524. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldahmash, B.A.; El-Nagar, D.M.; Ibrahim, K.E. Attenuation of hepatotoxicity and oxidative stress in diabetes STZ-induced type 1 by biotin in Swiss albino mice. Saudi J. Biol. Sci. 2016, 23, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Farid, A.; Moussa, P.; Youssef, M.; Haytham, M.; Shamy, A.; Safwat, G. Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Saudi J. Biol. Sci. 2022, 29, 103313. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Sblano, S.; Cerchia, C.; Laghezza, A.; Piemontese, L.; Brunetti, L.; Leuci, R.; Gilardi, F.; Thomas, A.; Genovese, M.; Santi, A.; et al. A chemoinformatics search for peroxisome proliferator-activated receptors ligands revealed a new pan-agonist able to reduce lipid accumulation and improve insulin sensitivity. Eur. J. Med. Chem. 2022, 235, 114240. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.; Arul Nambi Rajan, K.; Li, P.; Parast, M.M. The role of Sirtuin1-PPARγ axis in placental development and function. J. Mol. Endocrinol. 2018, 60, R201–R212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.L.; Lu, C.; Fan, Z.F.; Zhao, T.R.; Cheng, G.G.; Wang, Y.D.; Cao, J.X.; Liu, Y.P. Hypoglycemic and hypolipidemic effects of Epigynum auritum in high fat diet and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2022, 288, 114986. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, P.; Pang, K.; Ma, Y.; Huang, H.; Zhou, T.; Yang, X. Antidiabetic effect of a flavonoid-rich extract from Sophora alopecuroides L. in HFD- and STZ- induced diabetic mice through PKC/GLUT4 pathway and regulating PPARα and PPARγ expression. J. Ethnopharmacol. 2021, 268, 113654. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tang, Z.; Zheng, Z.; Cao, P.; Shui, W.; Li, Q.; Zhang, Y. Protective effects of Angelica sinensis polysaccharide against hyperglycemia and liver injury in multiple low-dose streptozotocin-induced type 2 diabetic BALB/c mice. Food Funct. 2016, 7, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Tobita, T.; Guzman-Lepe, J.; Takeishi, K.; Nakao, T.; Wang, Y.; Meng, F.; Deng, C.X.; Collin de l’Hortet, A.; Soto-Gutierrez, A. SIRT1 Disruption in Human Fetal Hepatocytes Leads to Increased Accumulation of Glucose and Lipids. PLoS ONE 2016, 11, e0149344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Shang, Y.; Zhang, R.; Gao, X.; Zeng, Q. A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and anti-inflammatory mechanisms. Mol. Med. Rep. 2019, 19, 1040–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chen, Y.; Liu, C.; Li, L.; Li, P. N(1)-Methylnicotinamide Improves Hepatic Insulin Sensitivity via Activation of SIRT1 and Inhibition of FOXO1 Acetylation. J. Diabetes Res. 2020, 2020, 1080152. [Google Scholar] [CrossRef] [PubMed]
- de Souza, I.C.; Martins, L.A.; de Vasconcelos, M.; de Oliveira, C.M.; Barbé-Tuana, F.; Andrade, C.B.; Pettenuzzo, L.F.; Borojevic, R.; Margis, R.; Guaragna, R.; et al. Resveratrol Regulates the Quiescence-Like Induction of Activated Stellate Cells by Modulating the PPARγ/SIRT1 Ratio. J. Cell. Biochem. 2015, 116, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, H.; Kim, S.Y.; Lim, Y. Effects of Lespedeza Bicolor Extract on Regulation of AMPK Associated Hepatic Lipid Metabolism in Type 2 Diabetic Mice. Antioxidants 2019, 8, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- El-Demerdash, F.M.; Talaat, Y.; El-Sayed, R.A.; Kang, W.; Ghanem, N.F. Hepatoprotective Effect of Actinidia deliciosa against Streptozotocin-Induced Oxidative Stress, Apoptosis, and Inflammations in Rats. Oxidative Med. Cell. Longev. 2022, 2022, 1499510. [Google Scholar] [CrossRef]
- Hamouda, H.A.; Mansour, S.M.; Elyamany, M.F. Vitamin D Combined with Pioglitazone Mitigates Type-2 Diabetes-induced Hepatic Injury Through Targeting Inflammation, Apoptosis, and Oxidative Stress. Inflammation 2022, 45, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedimanesh, N.; Asghari, S.; Mohammadnejad, K.; Daneshvar, Z.; Rahmani, S.; Shokoohi, S.; Farzaneh, A.H.; Hosseini, S.H.; Jafari Anarkooli, I.; Noubarani, M.; et al. The anti-diabetic effects of betanin in streptozotocin-induced diabetic rats through modulating AMPK/SIRT1/NF-κB signaling pathway. Nutr. Metab. 2021, 18, 92. [Google Scholar] [CrossRef] [PubMed]







| Lung | Liver | Spleen | Kidney | Testis | |
|---|---|---|---|---|---|
| Group | Relative Organ Weight (w/w, %) | ||||
| Control | 0.61 ± 0.14 a | 4.14 ± 0.43 a | 0.20 ± 0.08 a | 1.34 ± 0.12 a | 0.70 ± 0.13 a |
| STZ | 0.78 ± 0.15 a | 5.66 ± 0.44 b | 0.23 ± 0.04 a | 1.33 ± 0.11 a | 0.79 ± 0.12 a |
| RHSL 1/100 | 0.85 ± 0.16 a | 5.35 ± 0.22 b | 0.19 ± 0.03 a | 1.46 ± 0.18 a | 0.78 ± 0.14 a |
| RHSL 1/400 | 0.69 ± 0.11 a | 5.54 ± 1.12 b | 0.19 ± 0.01 a | 1.57 ± 0.14 a | 0.74 ± 0.17 a |
| FDSL 1/100 | 0.79 ± 0.08 a | 5.91 ± 0.93 b | 0.20 ± 0.03 a | 1.53 ± 0.33 a | 0.82 ± 0.07 a |
| FDSL 1/400 | 0.78 ± 0.17 a | 5.47 ± 0.35 b | 0.23 ± 0.03 a | 1.32 ± 0.27 a | 0.80 ± 0.12 a |
| RSG 5 mg/kg | 0.78 ± 0.14 a | 5.57 ± 0.57 b | 0.22 ± 0.06 a | 1.40 ± 0.22 a | 0.79 ± 0.12 a |
| Group | Water Intake (mL/Day) | Food Intake (g/Day) |
|---|---|---|
| Control | 6.03 ± 1.65 a | 3.36 ± 0.36 a |
| STZ | 13.94 ± 4.02 b | 4.18 ± 0.38 a |
| RHSL 1/100 | 9.21 ± 1.49 c | 3.97 ± 0.63 a |
| RHSL 1/400 | 11.61 ± 1.95 b | 3.88 ± 0.48 a |
| FDSL 1/100 | 11.40 ± 2.44 b | 3.78 ± 0.35 a |
| FDSL 1/400 | 10.99 ± 1.86 b | 4.05 ± 0.84 a |
| RSG 5 mg/kg | 8.40 ± 1.51 c | 4.30 ± 1.11 a |
| Group | AUC of Blood Glucose in the OGTT Assay |
|---|---|
| Control | 12,616.25 ± 239.97 a |
| STZ | 49,509.87 ± 386.14 b |
| RHSL 1/100 | 42,681.25 ± 665.69 c |
| RHSL 1/400 | 47,890.31 ± 430.31 b |
| FDSL 1/100 | 45,690.75 ± 615.60 b |
| FDSL 1/400 | 43,638.00 ± 546.32 b |
| RSG 5 mg/kg | 37,191.00 ± 1076.89 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Hong, Y.-H.; Chiang, Y.-F.; Wang, K.-L.; Huang, T.-C.; Ali, M.; Shieh, T.-M.; Chang, H.-Y.; Hsia, S.-M. Effects of Rice-Husk Silica Liquid in Streptozotocin-Induced Diabetic Mice. Metabolites 2022, 12, 964. https://doi.org/10.3390/metabo12100964
Chen H-Y, Hong Y-H, Chiang Y-F, Wang K-L, Huang T-C, Ali M, Shieh T-M, Chang H-Y, Hsia S-M. Effects of Rice-Husk Silica Liquid in Streptozotocin-Induced Diabetic Mice. Metabolites. 2022; 12(10):964. https://doi.org/10.3390/metabo12100964
Chicago/Turabian StyleChen, Hsin-Yuan, Yong-Han Hong, Yi-Fen Chiang, Kai-Lee Wang, Tsui-Chin Huang, Mohamed Ali, Tzong-Ming Shieh, Hsin-Yi Chang, and Shih-Min Hsia. 2022. "Effects of Rice-Husk Silica Liquid in Streptozotocin-Induced Diabetic Mice" Metabolites 12, no. 10: 964. https://doi.org/10.3390/metabo12100964