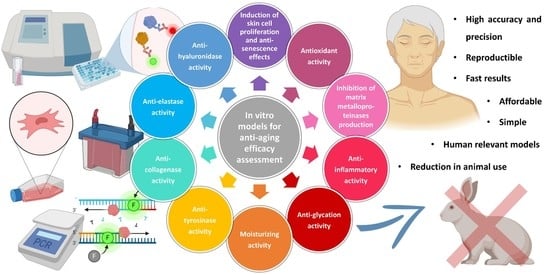
In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research
Abstract
:1. Introduction
1.1. Healthy Skin and Skin Aging
1.2. In Vitro Skin Models
2. In Vitro Models for Anti-Aging Efficacy Assessment
2.1. Induction of Skin Cell Proliferation and Anti-Senescence Effects
2.2. Anti-Collagenase Activity
2.3. Anti-Elastase Activity
2.4. Anti-Hyaluronidase Activity
2.5. Anti-Tyrosinase Activity
2.6. Antioxidant Activity
2.7. Inhibition of Matrix Metalloproteinase Production
2.8. Other Assays: Anti-Inflammatory, Anti-Glycation, and Moisturizing Activity Asessment
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nakajima, S.; Nomura, T.; Common, J.; Kabashima, K. Insights into Atopic Dermatitis Gained from Genetically Defined Mouse Models. J. Allergy Clin. Immunol. 2019, 143, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Elsevie: Amsterdam, The Netherlands, 2016; pp. 1–14. [Google Scholar]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The Dynamic Anatomy and Patterning of Skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J. Anatomy, Histology and Immunohistochemistry of Normal Human Skin. Eur. J. Dermatol. 2002, 12, 390–401. [Google Scholar]
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining Skin Aging and Its Risk Factors: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Dermatoendocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Neves, L.M.G.; Wilgus, T.A.; Bayat, A. In Vitro, Ex Vivo, and In Vivo Approaches for Investigation of Skin Scarring: Human and Animal Models. Adv. Wound Care 2023, 12, 97–116. [Google Scholar] [CrossRef]
- Stokes, W. Animals and the 3Rs in Toxicology Research and Testing. Hum. Exp. Toxicol. 2015, 34, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- John, L.M.; Dalsgaard, C.M.; Jeppesen, C.B.; Conde-Frieboes, K.W.; Baumann, K.; Knudsen, N.P.H.; Skov, P.S.; Wulff, B.S. In Vitro Prediction of in Vivo Pseudo-Allergenic Response via MRGPRX2. J. Immunotoxicol. 2021, 18, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.; Choksi, N.Y.; Ceger, P.; Daniel, A.B.; Truax, J.; Allen, D.; Kleinstreuer, N. Analysis of Variability in the Rabbit Skin Irritation Assay. Regul. Toxicol. Pharmacol. 2021, 122, 104920. [Google Scholar] [CrossRef] [PubMed]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.J.M.; van den Bogaard, E.H. 3D Skin Models for 3R Research: The Potential of 3D Reconstructed Skin Models to Study Skin Barrier Function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Chérouvrier Hansson, A.; Johansson, H.; Lemkine, G.F.; Thénot, J.-P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Singh, P.; Bhat, S.S.; Singh, N.; Venkanna, B.U.; Mohamed, R.; Rao, R.P. Cell-Based Model Systems for Validation of Various Efficacy-Based Claims for Cosmetic Ingredients. Cosmetics 2022, 9, 107. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety (SCCS). The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 11th ed; European Union: Brussels, Belgium, 2021. [Google Scholar]
- Szilágyi, Z.; Németh, Z.; Bakos, J.; Kubinyi, G.; Necz, P.P.; Szabó, E.; Thuróczy, G.; Pinto, R.; Selmaoui, B. Assessment of Inflammation in 3D Reconstructed Human Skin Exposed to Combined Exposure to Ultraviolet and Wi-Fi Radiation. Int. J. Mol. Sci. 2023, 24, 2853. [Google Scholar] [CrossRef]
- Goncalves, K.; De Los Santos Gomez, P.; Costello, L.; Smith, L.; Mead, H.; Simpson, A.; Przyborski, S. Investigation into the Effect of Skin Tone Modulators and Exogenous Stress on Skin Pigmentation Utilizing a Novel Bioengineered Skin Equivalent. Bioeng. Transl. Med. 2023, 8, e10415. [Google Scholar] [CrossRef]
- Leman, G.; Moosbrugger-Martinz, V.; Blunder, S.; Pavel, P.; Dubrac, S. 3D-Organotypic Cultures to Unravel Molecular and Cellular Abnormalities in Atopic Dermatitis and Ichthyosis Vulgaris. Cells 2019, 8, 489. [Google Scholar] [CrossRef]
- Jang, H.-J.; Lee, J.B.; Yoon, J.-K. Advanced In Vitro Three-Dimensional Skin Models of Atopic Dermatitis. Tissue Eng. Regen. Med. 2023. [Google Scholar] [CrossRef]
- Moon, S.; Kim, D.H.; Shin, J.U. In Vitro Models Mimicking Immune Response in the Skin. Yonsei Med. J. 2021, 62, 969. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The Hallmarks of Fibroblast Ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Yonezawa, Y. Changes in the Migratory Ability of Human Lung and Skin Fibroblasts during in Vitro Aging and in Vivo Cellular Senescence. Mech. Ageing Dev. 1992, 63, 223–233. [Google Scholar] [CrossRef]
- Sanchez, M.M.; Bagdasarian, I.A.; Darch, W.; Morgan, J.T. Organotypic Cultures as Aging Associated Disease Models. Aging 2022, 14, 9338–9383. [Google Scholar] [CrossRef]
- Quiles, J.; Cabrera, M.; Jones, J.; Tsapekos, M.; Caturla, N. In Vitro Determination of the Skin Anti-Aging Potential of Four-Component Plant-Based Ingredient. Molecules 2022, 27, 8101. [Google Scholar] [CrossRef]
- Panichakul, T.; Ponnikorn, S.; Tupchiangmai, W.; Haritakun, W.; Srisanga, K. Skin Anti-Aging Potential of Ipomoea Pes-Caprae Ethanolic Extracts on Promoting Cell Proliferation and Collagen Production in Human Fibroblasts (CCD-986sk Cells). Pharmaceuticals 2022, 15, 969. [Google Scholar] [CrossRef]
- Gu, Y.; Xue, F.; Xiao, H.; Chen, L.; Zhang, Y. Bamboo Leaf Flavonoids Suppress Oxidative Stress-Induced Senescence of HaCaT Cells and UVB-Induced Photoaging of Mice through P38 MAPK and Autophagy Signaling. Nutrients 2022, 14, 793. [Google Scholar] [CrossRef]
- Lee, J.-J.; Ng, S.-C.; Hsu, J.-Y.; Liu, H.; Chen, C.-J.; Huang, C.-Y.; Kuo, W.-W. Galangin Reverses H2O2-Induced Dermal Fibroblast Senescence via SIRT1-PGC-1α/Nrf2 Signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef]
- Li, W.; Mu, X.; Wu, X.; He, W.; Liu, Y.; Liu, Y.; Deng, J.; Nie, X. Dendrobium Nobile Lindl. Polysaccharides Protect Fibroblasts against UVA-Induced Photoaging via JNK/c-Jun/MMPs Pathway. J. Ethnopharmacol. 2022, 298, 115590. [Google Scholar] [CrossRef]
- Ouyang, Q.; Li, Y.; Mei, S.; Zhang, Q.; Li, X.; Luo, H.; Zhu, Y.; Wu, K. Protective Effects of GLHP from Gracilaria Lemaneiformis against UVB-Induced Photodamage in Human Immortalized Keratinocytes Cells and BALB/c Mice. Exp. Gerontol. 2021, 155, 111550. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Nanda, S. In-Vitro Evaluation of Antioxidant, Anti-Elastase, Anti-Collagenase, Anti-Hyaluronidase Activities of Safranal and Determination of Its Sun Protection Factor in Skin Photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna Odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Girsang, E.; Amalia, A.; Wibowo, S.H.B.; Kusuma, H.; Rizal, R. Anti-Aging Effects of Mangosteen Peel Extract and Its Phytochemical Compounds: Antioxidant Activity, Enzyme Inhibition and Molecular Docking Simulation. Trop. Life Sci. Res. 2020, 31, 127–144. [Google Scholar] [CrossRef]
- Abdel Shakour, Z.T.; El-Akad, R.H.; Elshamy, A.I.; El Gendy, A.E.-N.G.; Wessjohann, L.A.; Farag, M.A. Dissection of Moringa Oleifera Leaf Metabolome in Context of Its Different Extracts, Origin and in Relationship to Its Biological Effects as Analysed Using Molecular Networking and Chemometrics. Food Chem. 2023, 399, 133948. [Google Scholar] [CrossRef]
- Barak, T.H.; KURT CELEP, İ.; ŞENTÜRK, T.B.; BARDAKCI, H.; CELEP, E. Anti-Aging Potential Evaluation of Maclura Pomifera (Rafin.) Schneider 80% Methanol Extract with Quantitative HPTLC Analysis. Turk J. Pharm. Sci. 2022, 19, 400–407. [Google Scholar] [CrossRef]
- Eaknai, W.; Bunwatcharaphansakun, P.; Phungbun, C.; Jantimaporn, A.; Chaisri, S.; Boonrungsiman, S.; Nimmannit, U.; Khongkow, M. Ethanolic Fenugreek Extract: Its Molecular Mechanisms against Skin Aging and the Enhanced Functions by Nanoencapsulation. Pharmaceuticals 2022, 15, 254. [Google Scholar] [CrossRef]
- Castejón, N.; Thorarinsdottir, K.A.; Einarsdóttir, R.; Kristbergsson, K.; Marteinsdóttir, G. Exploring the Potential of Icelandic Seaweeds Extracts Produced by Aqueous Pulsed Electric Fields-Assisted Extraction for Cosmetic Applications. Mar. Drugs 2021, 19, 662. [Google Scholar] [CrossRef]
- An, J.Y.; Kim, C.; Park, N.R.; Jung, H.S.; Koo, T.; Yuk, S.H.; Lee, E.H.; Cho, S.H. Clinical Anti-aging Efficacy of Propolis Polymeric Nanoparticles Prepared by a Temperature-induced Phase Transition Method. J. Cosmet. Dermatol. 2022, 21, 4060–4071. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus Globulus Leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; El-labbad, E.M.; Al-Azzawi, M.A.; Ashmawy, N.S. A New Xanthone Glycoside from Mangifera indica L.: Physicochemical Properties and In Vitro Anti-Skin Aging Activities. Molecules 2022, 27, 2609. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal Extracts as Potential Antioxidant, Anti-Aging, Anti-Inflammatory, and Whitening Cosmeceutical Ingredients. Chem. Biodivers 2021, 18, e2100245. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.Y.; Mady, M.S.; Atya, H.B.; Ali, S.A.; Elsayed, H.E.; Moharram, F.A. Melaleuca Rugulosa (Link) Craven Tannins: Appraisal of Anti-Inflammatory, Radical Scavenging Activities, and Molecular Modeling Studies. J. Ethnopharmacol. 2022, 298, 115596. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago Officinalis Extracts on Skin Cells. Molecules 2023, 28, 868. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, P.; Haure, M.-J.; Ceruti, I.; Bessou-Touya, S.; Castex-Rizzi, N. Results from in Vitro and Ex Vivo Skin Aging Models Assessing the Antiglycation and Anti-Elastase MMP-12 Potential of Glycylglycine Oleamide. Clin. Cosmet. Investig. Dermatol. 2016, 9, 143–150. [Google Scholar] [CrossRef]
- Chaikhong, K.; Chumpolphant, S.; Rangsinth, P.; Sillapachaiyaporn, C.; Chuchawankul, S.; Tencomnao, T.; Prasansuklab, A. Antioxidant and Anti-Skin Aging Potential of Selected Thai Plants: In Vitro Evaluation and In Silico Target Prediction. Plants 2022, 12, 65. [Google Scholar] [CrossRef]
- Ibrahim, N.; Abbas, H.; El-Sayed, N.S.; Gad, H.A. Rosmarinus officinalis L. Hexane Extract: Phytochemical Analysis, Nanoencapsulation, and in Silico, in Vitro, and in Vivo Anti-Photoaging Potential Evaluation. Sci. Rep. 2022, 12, 13102. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Boonlum, N.; Chaiyana, W.; Tima, S.; Anuchapreeda, S.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Nimitkeatkai, H.; et al. Mushroom β-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma Lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications. Polymers 2022, 14, 4202. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In Vitro Determination of the Anti-Aging Potential of Four Southern African Medicinal Plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef]
- McCook, J.; Dorogi, P.; Vasily, D.; Cefalo, D. In Vitro Inhibition of Hyaluronidase by Sodium Copper Chlorophyllin Complex and Chlorophyllin Analogs. Clin. Cosmet. Investig. Dermatol. 2015, 8, 443–448. [Google Scholar] [CrossRef]
- Murthy, R.; Roos, J.C.P.; Goldberg, R.A. Periocular Hyaluronic Acid Fillers. Curr. Opin. Ophthalmol. 2019, 30, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Kim, D.S.; Park, S.H.; Shin, C.Y.; Woo, J.J.; Kim, J.W.; An, R.-B.; Lee, C.; Cho, J.Y. Antioxidant Capacity of Potentilla Paradoxa Nutt. and Its Beneficial Effects Related to Anti-Aging in HaCaT and B16F10 Cells. Plants 2022, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Mkadmini Hammi, K.; Dauvergne, X.; Magné, C.; Ksouri, R. Anti-Aging Activities of Extracts from Tunisian Medicinal Halophytes and Their Aromatic Constituents. EXCLI J. 2017, 16, 755–769. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.; Lad, P.S.; Neupane, P. An Open-Label Single-Arm, Monocentric Study Assessing the Efficacy and Safety of Natural Pterostilbene (Pterocarpus Marsupium) for Skin Brightening and Antiaging Effects. Clin. Cosmet. Investig. Dermatol. 2020, 13, 105–116. [Google Scholar] [CrossRef]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus Persica (L.) Var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef]
- Chaikul, P.; Kanlayavattanakul, M.; Somkumnerd, J.; Lourith, N. Phyllanthus emblica L. (Amla) Branch: A Safe and Effective Ingredient against Skin Aging. J. Tradit. Complement. Med. 2021, 11, 390–399. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic Acid Applications in Cosmetic and Pharmaceutical Preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Aqueous Leaf Extract of Aquilegia Pubiflora: Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other In Vitro Properties. Oxid. Med. Cell Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Devel. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Merghoub, N.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects. Int. J. Mol. Sci. 2023, 24, 1397. [Google Scholar] [CrossRef]
- Dina, E.; Sklirou, A.D.; Chatzigeorgiou, S.; Manola, M.S.; Cheilari, A.; Louka, X.P.; Argyropoulou, A.; Xynos, N.; Skaltsounis, A.-L.; Aligiannis, N.; et al. An Enriched Polyphenolic Extract Obtained from the By-Product of Rosa Damascena Hydrodistillation Activates Antioxidant and Proteostatic Modules. Phytomedicine 2021, 93, 153757. [Google Scholar] [CrossRef]
- Vaz, C.; Oliveira, A.; Silva, A.; Cortes, L.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.; et al. Protective Role of Portuguese Natural Mineral Waters on Skin Aging: In Vitro Evaluation of Anti-Senescence and Anti-Oxidant Properties. Int. J. Biometeorol. 2022, 66, 2117–2131. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; He, J.; Liu, M. Insight into Seasonal Change of Phytochemicals, Antioxidant, and Anti-Aging Activities of Root Bark of Paeonia Suffruticosa (Cortex Moutan) Combined with Multivariate Statistical Analysis. Molecules 2021, 26, 6102. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, P.; Guo, H.; Guo, M.S.S.; Xia, Y.; Xia, Y.; Gao, X.; Wang, X.; Wu, J.; Dong, T.T.X.; et al. Capsaicin, a Phytochemical From Chili Pepper, Alleviates the Ultraviolet Irradiation-Induced Decline of Collagen in Dermal Fibroblast via Blocking the Generation of Reactive Oxygen Species. Front. Pharmacol. 2022, 13, 872912. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.-H.; Lee, D.H.; Lee, S.-H.; Chung, J.H. Inhibition of Matrix Metalloproteinase Expression by Selective Clearing of Senescent Dermal Fibroblasts Attenuates Ultraviolet-Induced Photoaging. Biomed. Pharmacother. 2022, 150, 113034. [Google Scholar] [CrossRef]
- Ellistasari, E.Y.; Kariosentono, H.; Purwanto, B.; Wasita, B.; Riswiyant, R.C.A.; Pamungkasari, E.P.; Soetrisno, S. Exosomes Derived from Secretome Human Umbilical Vein Endothelial Cells (Exo-HUVEC) Ameliorate the Photo-Aging of Skin Fibroblast. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.; Belluzzi, E.; Mattiuzzo, E.; Venerando, R.; Cadamuro, M.; Ruggieri, P.; Vindigni, V.; Brun, P. Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective. Polymers 2022, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Ji, K.-Y.; Choi, Y.J.; Heo, J.B.; Youn, U.J.; Kim, S.; Shim, K.-S.; Lee, J.Y.; Kim, T.S.; Seo, Y.K.; et al. The Safety and Efficacy of 1-Monoeicosapentaenoin Isolated from the Trebouxiophyceae Micractinium on Anti-Wrinkle: A Split-Face Randomized, Double-Blind Placebo-Controlled Clinical Study. J. Clin. Med. 2023, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Vashisth, M.K.; Ge, Y.; Dai, Q.; He, F.; Wang, X. Anti-Inflammation and Anti-Aging Mechanisms of Mercaptopurine in Vivo and in Vitro. Biochem. Biophys. Res. Commun. 2023, 638, 103–111. [Google Scholar] [CrossRef]
- Guimarães, G.R.; Almeida, P.P.; de Oliveira Santos, L.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lyga, J. Inflammaging in Skin and Other Tissues—The Roles of Complement System and Macrophage. Inflamm. Allergy-Drug Targets 2014, 13, 153–161. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Pageon, H. Reaction of Glycation and Human Skin: The Effects on the Skin and Its Components, Reconstructed Skin as a Model. Pathol. Biol. 2010, 58, 226–231. [Google Scholar] [CrossRef]
- Pageon, H.; Zucchi, H.; Rousset, F.; Monnier, V.M.; Asselineau, D. Skin Aging by Glycation: Lessons from the Reconstructed Skin Model. Clin. Chem. Lab. Med. 2014, 52, 169–174. [Google Scholar] [CrossRef]
- Pageon, H.; Bakala, H.; Monnier, V.M.; Asselineau, D. Collagen Glycation Triggers the Formation of Aged Skin in Vitro. Eur. J. Dermatol. 2007, 17, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Técher, M.-P.; Asselineau, D. Reconstructed Skin Modified by Glycation of the Dermal Equivalent as a Model for Skin Aging and Its Potential Use to Evaluate Anti-Glycation Molecules. Exp. Gerontol. 2008, 43, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H.; Asselineau, D. An in Vitro Approach to the Chronological Aging of Skin by Glycation of the Collagen: The Biological Effect of Glycation on the Reconstructed Skin Model. Ann. N. Y. Acad. Sci. 2005, 1043, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.; Yoon, S.H.; Park, D.; Hwang, J.S.; Jung, E. Anti-glycation Activities of Methyl Gallate in Vitro and in Human Explants. J. Cosmet. Dermatol. 2022, 21, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Falanga, D.; Di Lorenzo, R.; Tito, A.; Carotenuto, G.; Zappelli, C.; Grumetto, L.; Sacchi, A.; Laneri, S.; Apone, F. An Extract from Ficus Carica Cell Cultures Works as an Anti-Stress Ingredient for the Skin. Antioxidants 2021, 10, 515. [Google Scholar] [CrossRef]
- Leo, T.K.; Tan, E.S.S.; Amini, F.; Rehman, N.; Ng, E.S.C.; Tan, C.K. Effect of Rice (Oryza sativa L.) Ceramides Supplementation on Improving Skin Barrier Functions and Depigmentation: An Open-Label Prospective Study. Nutrients 2022, 14, 2737. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Khan, T.; Mannan, A.; Ali, A.; Ni, J. In Vitro and Ex Vivo Evaluation of Mangifera indica L. Extract-Loaded Green Nanoparticles in Topical Emulsion against Oxidative Stress and Aging. Biomedicines 2022, 10, 2266. [Google Scholar] [CrossRef]
- Kappler, K.; Grothe, T.; Srivastava, S.; Jagtap, M. Evaluation of the Efficacy and Safety of Blue Fenugreek Kale Extract on Skin Health and Aging: In-Vitro and Clinical Evidences. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, F.; Hu, X.; Xie, Y.; Du, L.; Ye, R. The Synergistic Effect of Retinyl Propionate and Hydroxypinacolone Retinoate on Skin Aging. J. Cosmet. Dermatol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Han, W.; Sun, Y.; Qian, L. Valorization of Polysaccharides From Benincasa Hispida: Physicochemical, Moisturizing, and Antioxidant Skincare Properties. Front. Pharmacol. 2022, 13, 912382. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. https://doi.org/10.3390/cosmetics10020066
Cruz AM, Gonçalves MC, Marques MS, Veiga F, Paiva-Santos AC, Pires PC. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics. 2023; 10(2):66. https://doi.org/10.3390/cosmetics10020066
Chicago/Turabian StyleCruz, Ana M., Margarida C. Gonçalves, Matilde S. Marques, Francisco Veiga, Ana Cláudia Paiva-Santos, and Patrícia C. Pires. 2023. "In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research" Cosmetics 10, no. 2: 66. https://doi.org/10.3390/cosmetics10020066