Spatial Patterns of Species Diversity of Amphibians in a Nature Reserve in Eastern China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
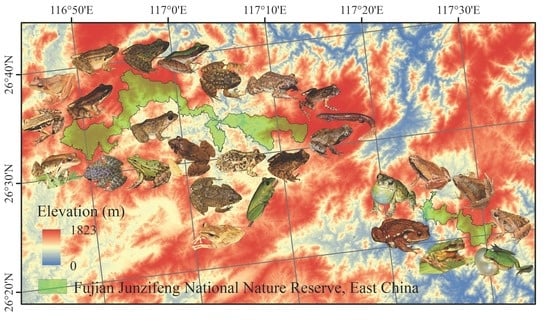
2.1. Study Area
2.2. Survey Process
2.3. DNA Sequences Collection and Phylogenetic Tree Reconstruction
2.4. Species Diversity Metrics
- S = number of species;
- C = 1−;
- H’ = ;
- E = H’/lnS;
- DMG = (S – 1)/lnN;
2.5. Testing Elevational Rapoport’s Rule
2.6. Data Analyses
3. Results
3.1. Species Composition and Their Phylogeny
3.2. Regional Taxonomic and Phylogenetic Diversities
3.3. Elevational Taxonomic and Phylogenetic Diversities
3.4. Elevational Rapoport’s Rule
4. Discussion
4.1. Regional Species Diversity
4.2. Elevational Pattern
4.3. Elevational Rapoport’s Rule Examination
4.4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Wen, Z.X.; Feijó, A.; Cheng, J.L.; Wang, Y.Q.; Li, S.; Ge, D.Y.; Xia, L.; Yang, Q.S. Elevation patterns and critical environmental drivers of the taxonomic, functional, and phylogenetic diversity of small mammals in a karst mountain area. Ecol. Evol. 2020, 10, 10899–10911. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.B.; Fan, L.Q.; Xu, Z.H.; Wen, Z.X.; Cai, T.L.; Feijo, A.; Hu, J.H.; Lei, F.M.; Yang, Q.S.; Qiao, H.J. A multi-faceted comparative perspective on elevational beta-diversity: The patterns and their causes. Proc. R. Soc. B 2021, 288, 20210343. [Google Scholar] [CrossRef] [PubMed]
- Quintero, I.; Jetz, W. Global elevational diversity and diversification of birds. Nature 2018, 555, 246–250. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, S.N.; Guo, C.P.; Tang, K.; Jiang, J.P.; Hu, J.H. Amphibian diversity and conservation along an elevational gradient on Mount Emei, southwestern China. Amphib. Reptile Conserv. 2020, 14, 46–56. [Google Scholar]
- Ramírez-Bautista, A.; Williams, J.N. The importance of productivity and seasonality for structuring small rodent diversity across a tropical elevation gradient. Oecologia 2019, 190, 275–286. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhong, M.J.; Zhang, J.; Si, X.F.; Yang, S.N.; Jiang, J.P.; Hu, J.H. Multidimensional amphibian diversity and community structure along a 2600 m elevational gradient on the eastern margin of the Qinghai-Tibetan Plateau. Zool. Res. 2022, 43, 40–51. [Google Scholar] [CrossRef]
- Chettri, B.; Acharya, B.K. Distribution of amphibians along an elevation gradient in the Eastern Himalaya, India. Basic Appl. Ecol. 2020, 47, 57–70. [Google Scholar] [CrossRef]
- Frishkoff, L.O.; Gabot, E.; Sandler, G.; Marte, C.; Mahler, D.L. Elevation shapes the reassembly of Anthropocene lizard communities. Nat. Ecol. Evol. 2019, 3, 638–646. [Google Scholar] [CrossRef]
- Herzog, S.K.; Kessler, M.; Bach, K. The elevational gradient in Andean bird species richness at the local scale: A foothill peak and a high–elevation plateau. Ecography 2005, 28, 209–222. [Google Scholar] [CrossRef]
- Hopkins, W.A. Amphibians as models for studying environmental change. ILAR J. 2007, 48, 270–277. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Haugaasen, T. Anuran species richness and abundance along an elevational gradient in Chitwan, Nepal. Zool. Ecol. 2015, 25, 110–119. [Google Scholar] [CrossRef]
- Naniwadekar, R.; Vasudevan, K. Patterns in diversity of anurans along an elevational gradient in the Western Ghats, South India. J. Biogeogr. 2007, 34, 842–853. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Zhao, T.; Chen, Y.H.; Wang, B.; Xie, F.; Cannatella, D.C.; Jiang, J.P. Amphibian community structure along elevation gradients in eastern Nepal Himalaya. BMC Ecol. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcdowall, R.M. Jordan’s and other ecogeographical rules, and the vertebral number in fishes. J. Biogeogr. 2008, 35, 501–508. [Google Scholar] [CrossRef]
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Böhm, M.; Kemp, R.; Williams, R.; Davidson, A.D.; Garcia, A.; Mcmillan, K.M.; Bramhall, H.R.; Collen, B. Rapoport’s rule and determinants of species range size in snakes. Divers. Distrib. 2017, 23, 1472–1481. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Pan, X.Y.; Liang, J.C.; Ding, Z.F.; Zhou, J.; Hu, H.J. Species range pattern of breeding birds in the middle part of the Himalayas with a test of Rapoport’s rule-based on data from an altitude gradient of 3600 m. Chin. J. Zool. 2021, 56, 358–366. [Google Scholar]
- Luo, Z.H.; Tang, S.H.; Li, C.W.; Chen, J.; Fang, H.X.; Jiang, Z.G. Do Rapoport’s rule, mid-domain effect or environmental factors predict latitudinal range size patterns of terrestrial mammals in China? PLoS ONE 2011, 6, e27975. [Google Scholar] [CrossRef] [Green Version]
- Letcher, A.J.; Harvey, P.H. Variation in geographical range size among mammals of the Palearctic. Am. Nat. 1994, 144, 30–42. [Google Scholar] [CrossRef]
- Zhao, T.; Khatiwada, J.R.; Zhao, C.L.; Feng, J.Y.; Sun, Z.J. Elevational patterns of amphibian functional and phylogenetic structures in eastern Nepal Himalaya. Divers. Distrib. 2022, 28, 2475–2488. [Google Scholar] [CrossRef]
- Ellenberg, H.H. Vegetation Ecology of Central Europe; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Procheş, Ş.; van der Bank, M.; et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Li, Y.M. The phylogenetic diversity measurements and their uses in biodiversity conservation. Biodivers. Sci. 1998, 6, 49–54. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, L.; Xie, Z.; Li, Y.; Li, J.; Li, M.; Wei, C.; Xing, C.; Liu, J.; He, Z. Taxonomic and phylogenetic diversity of plants in a Castanopsis kawakamii natural forest. Biodivers. Sci. 2021, 29, 439. [Google Scholar] [CrossRef]
- Pellens, R.; Grandcolas, P. Biodiversity Conservation and Phylogenetic Systematics: Preserving Our Evolutionary Heritage in an Extinction Crisis; Springer Nature: Berlin, Germany, 2016. [Google Scholar]
- Njogu, A.W. Species Richness, Functional and Phylogenetic Diversity of Plant Communities Along the Altitudinal Gradients of Mount Kenya, East Africa. Ph.D. Thesis, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China, 2021. [Google Scholar]
- Tiede, J.; Wemheuer, B.; Traugott, M.; Daniel, R.; Tscharntke, T.; Ebeling, A.; Scherber, C. Trophic and non-trophic interactions in α biodiversity experiment assessed by next-generation sequencing. PLoS ONE 2016, 11, e148781. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, A.M.; Arita, H.T. Priority setting by sites and by species using rarity, richness and phylogenetic diversity: The case of neotropical glassfrogs (Anura: Centrolenidae). Biodivers. Conserv. 2014, 23, 909–926. [Google Scholar] [CrossRef]
- Voskamp, A.; Baker, D.J.; Stephens, P.A.; Valdes, P.J.; Willis, S.G. Global patterns in the divergence between phylogenetic diversity and species richness in terrestrial birds. J. Biogeogr. 2017, 44, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Jarzyna, M.A.; Jetz, W. Detecting the multiple facets of biodiversity. Trends Ecol. Evol. 2016, 31, 527–538. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species. Version 2022-1. Available online: https://www.iucnredlist.org (accessed on 3 May 2022).
- Wang, J.; Liu, Y.H.; Martin, K.; Luo, F.; Meng, L.Z. Implications of continuous amphibian diversity monitoring in Daweishan National Nature Reserve in tropical SE Yunnan, China. Glob. Ecol. Conserve. 2019, 20, e00694. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, J.; Barbosa, A.M.; Martínez-Solano, Í. Present and past climatic effects on the current distribution and genetic diversity of the Iberian spadefoot toad (Pelobates cultripes): An integrative approach. J. Biogeogr. 2017, 44, 245–258. [Google Scholar] [CrossRef]
- Lin, P.; Li, Z.J.; Zhang, J. Report on the Comprehensive Scientific Study of Fujian Junzifeng Nature Reserve; Xiamen University Press: Xiamen, China, 2005. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurvich, C.M.; Tsai, C. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Ma, K.P.; Liu, Y.M. Measurement of biotic community diversity I α diversity (Part 2). Biodivers. Sci. 1994, 2, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Shen, Z.H.; Tang, Z.R.; Wang, Z.H. The protocol for the survey plan for plant species diversity of China’s mountains. Biodivers. Sci. 2004, 12, 5–9. [Google Scholar]
- Jiang, X.Y.; Gao, S.J.; Jiang, Y.; Tian, Y.; Jia, X.; Zha, T.S. Species diversity, functional diversity, and phylogenetic diversity in plant communities at different phases of vegetation restoration in the Mu Us sandy grassland. Biodivers. Sci. 2022, 30, 21387. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Cornwell, W.K.; Webb, C.O.; Ackerly, D.D. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am. Nat. 2007, 170, 271–283. [Google Scholar] [CrossRef]
- Swenson, N.G. Functional and Phylogenetic Ecology in R; Springer: Berlin, Germany, 2014. [Google Scholar]
- Zheng, Z.; Gong, D.J.; Sun, C.X. Elevational pattern of species richness and species range size of herpetofauna in Baishuijiang Nature Reserve: A test of Rapoport’s rule. Chin. J. Ecol. 2014, 33, 537–546. [Google Scholar]
- Wei, T.Y.; Simko, V.; Levy, M.; Xie, Y.H.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Harrell, E.F., Jr. Regression Modeling Strategies; Springer: New York, NY, USA, 2016. [Google Scholar]
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. Caper: Comparative Analyses of Phylogenetics and Evolution in R (version 0.5.2). Available online: https://cran.r-project.org/web/packages/caper/index.html (accessed on 3 February 2022).
- Lin, Y.F.; Chen, Z.Q.; Tang, Y.; Chen, J.Y.; Ding, G.H.; Lin, Z.H.; Ji, X. Advertisement calls of Fejervarya multistriata (Anura: Dicroglossidae), with a review of anurans in China. Anim. Biol. 2020, 70, 459–481. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Lin, Y.F.; Tang, Y.; Ding, G.H.; Wu, Y.Q.; Lin, Z.H. Acoustic divergence in advertisement calls among three sympatric Microhyla species from East China. PeerJ 2020, 8, e8708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Guo, K.; Zhang, G.Z.; Lin, L.H.; Ji, X. Evolutionary transitions in body plan and reproductive mode alter maintenance metabolism in squamates. BMC Evol. Biol. 2018, 18, 45. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.P.; Xie, F. China’s Red List of Biodiversity: Vertebrates Volume 4: Amphibians; Science Press: Beijing, China, 2021. [Google Scholar]
- Lyu, Z.T.; Zeng, Z.C.; Wang, J.; Liu, Z.Y.; Huang, Y.Q.; Li, W.Z.; Wang, Y.Y. Four new species of Panophrys (Anura, Megophryidae) from eastern China, with discussion on the recognition of Panophrys as a distinct genus. Zootaxa 2021, 4927, 9–40. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ye, C.Y. Amphibians of China Volume (I); Sichuan Science & Technology Press: Chengdu, China, 2012; pp. 327–329. [Google Scholar]
- Liu, M.H.; Chen, Y.M.; Zou, C.X.; Ding, J.H.; Lv, T.T.; Zhang, Y. Comprehensive evaluation method and empirical study of biodiversity maintenance function at regional scale. J. Ecol. Rural Environ. 2021, 37, 287–294. [Google Scholar]
- Wang, B. Geographical Distribution Pattern and Climate Change Impact on Amphibians in the Karst Aeras of Southwest China. Ph.D. Thesis, Central South University of Foresty and Technology, Changsha, China, 2020. [Google Scholar]
- Schartau, A.K.; Mariash, H.L.; Christoffersen, K.S.; Bogan, D.; Dubovskaya, O.P.; Fefilova, E.B.; Hayden, B.; Ingvason, H.R.; Ivanova, E.A.; Kononova, O.N.; et al. First circumpolar assessment of Arctic freshwater phytoplankton and zooplankton diversity: Spatial patterns and environmental factors. Freshw. Biol. 2022, 67, 141–158. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Li, J.; Dai, Q.; Wang, Y.; Yang, D. Amphibian species richness patterns in karst regions in Southwest China and its environmental associations. Biodivers. Sci. 2018, 26, 941–950. [Google Scholar] [CrossRef]
- Gaitán, J.J.; López, C.R.; Bran, D.E. Vegetation composition and its relationship with the environment in mallines of north Patagonia, Argentina. Wetl. Ecol. Manag. 2011, 19, 121–130. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Vrcibradic, D.; Almeida-Gomes, M.; Rocha, C.F.D. Assessing the importance of reproductive modes for the evaluation of altitudinal distribution patterns in tropical frogs. Biotropica 2021, 53, 786–797. [Google Scholar] [CrossRef]
- Zheng, Z.; Gong, D.J.; Sun, C.X.; Li, X.J.; Li, W.J. Elevational pattern of amphibian and reptile diversity in Qinling Range and explanation. Biodivers. Sci. 2014, 22, 596–607. [Google Scholar]
- Meza-Joya, F.L.; Torres, M. Spatial diversity patterns of Pristimantis frogs in the Tropical Andes. Ecol. Evol. 2016, 6, 1901–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwell, R.K.; Rahbek, C.; Gotelli, N.J. The mid-domain effect and species richness patterns: What have we learned so far? Am. Nat. 2004, 163, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.P.; He, S.Y.; Jiang, Y.M.; Wang, T.; Zhao, H.H.; Cui, W.H.; Zheng, Y.M.; Hai, Y.; Wan, H.W. Species range size patterns and their significance on biodiversity conservation. Sci. Sin. Vitae 2019, 49, 929–937. [Google Scholar]
- Chen, Z.Q.; Tang, Y.; Wang, Y.Y.; Li, Y.D.; Lin, L.H.; Ding, G.H. Species diversity and elevational distribution of amphibians in the Xianxialing and Wuyishan mountain ranges, southeastern China. Asian Herpetol. Res. 2020, 11, 44–55. [Google Scholar]
- Wang, T.L.; Cheng, L.; Lan, W.J.; Zou, S.C.; Cheng, S.L.; Wang, J.C. Species diversity and vertical distribution characteristics of amphibians in Wuyishan Nature Reserve, Jiangxi Province. Chin. J. Ecol. 2015, 34, 2009–2014. [Google Scholar]
- Dehling, D.M.; Fritz, S.A.; Töpfer, T.; Päckert, M.; Estler, P.; Böhning Gaese, K.; Schleuning, M. Functional and phylogenetic diversity and assemblage structure of frugivorous birds along an elevational gradient in the tropical Andes. Ecography 2014, 37, 1047–1055. [Google Scholar] [CrossRef]
- Hanz, D.M.; Böhning-Gaese, K.; Ferger, S.W.; Fritz, S.A.; Neuschulz, E.L.; Quitián, M.; Santillán, V.; Töpfer, T.; Schleuning, M. Functional and phylogenetic diversity of bird assemblages are filtered by different biotic factors on tropical mountains. J. Biogeogr. 2019, 46, 291–303. [Google Scholar] [CrossRef]
- Ding, Z.F.; Hu, H.J.; Cadotte, M.W.; Liang, J.C.; Hu, Y.M.; Si, X.F. Elevational patterns of bird functional and phylogenetic structure in the central Himalaya. Ecography 2021, 44, 1403–1417. [Google Scholar] [CrossRef]
- Montaño Centellas, F.A.; Mccain, C.; Loiselle, B.A. Using functional and phylogenetic diversity to infer avian community assembly along elevational gradients. Glob. Ecol. Biogeogr. 2020, 29, 232–245. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; Mcpeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef] [Green Version]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Choler, P.; Michalet, R.; Callaway, R.M. Facilitation and competition on gradients in alpine plant communities. Ecology 2001, 82, 3295–3308. [Google Scholar] [CrossRef]
- Xu, J.S.; Chen, Y.; Zhang, L.X.; Chai, Y.F.; Wang, M.; Guo, Y.X.; Li, T.; Yue, M. Using phylogeny and functional traits for assessing community assembly along environmental gradients: A deterministic process driven by elevation. Ecol. Evol. 2017, 7, 5056–5069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, X.; Ren, X.; Hu, J.; Onditi, K.O.; Xu, Y.; Zhang, M.; Chang, W.; Chen, Z. Human Disturbance and Geometric Con-straints Drive Small Mammal Diversity and Community Structure along an Elevational Gradient in Eastern China. Animals 2022, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Q.; Wen, Z.; Xia, L.; Zhang, Q.; Zhou, H. What drives the species richness patterns of non-volant small mammals along a subtropical elevational gradient? Ecography 2013, 36, 185–196. [Google Scholar] [CrossRef]
- Hu, X.L.; Chang-Yang, C.H.; Mi, X.C.; Du, Y.J.; Chang, Z.Y. Influence of climate, phylogeny, and functional traits on flowering phenology in a subtropical evergreen broad-leaved forest, East China. Biodivers. Sci. 2015, 23, 601–609. [Google Scholar] [CrossRef]
- Stevens, G.C. Extending Rapoport’s rule to Pacific marine fishes. J. Biogeogr. 1996, 23, 149–154. [Google Scholar] [CrossRef]
- Whitton, F.J.; Purvis, A.; Orme, C.D.L.; Olalla Tárraga, M.Á. Understanding global patterns in amphibian geographic range size: Does Rapoport rule? Glob. Ecol. Biogeogr. 2012, 21, 179–190. [Google Scholar] [CrossRef]
- Ruggiero, A.; Werenkraut, V. One-dimensional analyses of Rapoport’s rule reviewed through meta-analysis. Glob. Ecol. Biogeogr. 2007, 16, 401–414. [Google Scholar] [CrossRef]
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of Rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef]





| Family | Species | Species Abbreviation | Conservation Status | Elevation Range (m) | Management District | ||
|---|---|---|---|---|---|---|---|
| XF | WN | ZY | |||||
| Salamandridae | Pachytriton brevipes | PB | LC | 937−1042 | ● | ||
| Megophryidae | Leptobrachella liui | LL | LC | 485−740 | ● | ||
| Boulenophrysboettgeri | BOB | LC | 615−883 | ● | |||
| Boulenophryssanmingensis | BOS | NE | 906−1041 | ● | |||
| Bufonidae | Bufo gargarizans | BUG | LC | 401−767 | ● | ● | ● |
| Duttaphrynus melanostictus | DM | LC | 288−809 | ● | ● | ● | |
| Hylidae | Hyla chinensis | HC | LC | 210−901 | ● | ● | ● |
| Hyla sanchiangensis | HS | LC | 339−385 | ● | |||
| Microhylidae | Microhyla butleri | MB | LC | 373−407 | ● | ● | |
| Microhyla fissipes | MF | LC | 210−916 | ● | ● | ● | |
| Microhyla heymonsi | MH | LC | 210−858 | ● | ● | ● | |
| Dicroglossinae | Fejervarya multistriata | FM | LC | 210−902 | ● | ● | ● |
| Hoplobatrachus chinensis | HOC | EN | 338−824 | ● | ● | ● | |
| Limnonectes fujianensis | LF | NT | 329−1250 | ● | ● | ● | |
| Quasipaa exilispinosa | QE | VU | 420−1042 | ● | ● | ● | |
| Quasipaa spinosa | QS | VU | 329−933 | ● | ● | ● | |
| Ranidae | Amolops ricketti | AR | LC | 346−883 | ● | ● | ● |
| Amolops wuyiensis | AW | LC | 355−1027 | ● | ● | ● | |
| Sylvirana guentheri | SG | LC | 340−773 | ● | ● | ● | |
| Hylarana latouchii | HYL | LC | 358−822 | ● | ● | ● | |
| Nidirana adenopleura | NIA | LC | 210−914 | ● | ● | ● | |
| Odorrana exiliversabilis | OE | LC | 374−1053 | ● | ● | ||
| Odorrana huanggangensis | OH | LC | 343−1009 | ● | ● | ● | |
| Odorrana graminea | OG | NT | 336−900 | ● | ● | ● | |
| Pelophylax nigromaculatus | PEN | NT | 353−837 | ● | ● | ||
| Rana longicrus | RL | LC | 347−851 | ● | ● | ● | |
| Rhacophoridae | Polypedates braueri | POB | LC | 211−900 | ● | ● | ● |
| Zhangixalus dennysi | ZD | LC | 211−841 | ● | ● | ● | |
| Data of All the Species | Removal of the Species Observed Only in One Transect Line | |||
|---|---|---|---|---|
| OLS Model | PGLS Model | OLS Model | PGLS Model | |
| N | 28 | 28 | 26 | 26 |
| Slope | −0.06 ± 0.32 | 0.45 ± 0.33 | 1.17 ± 0.37 | 1.64 ± 0.37 |
| Intercept | 542.76 ± 206.56 | −128.84 ± 292.98 | −151.10 ± 223.31 | −626.43 ± 245.72 |
| r2 | 0.001 | 0.067 | 0.293 | 0.452 |
| Ln likelihood | −190.986 | −187.074 * | −170.408 | −167.876 * |
| AIC | 387.972 | 382.147 | 346.816 | 343.752 |
| λ | – | 0.707 (0.194–0.983) | – | 0.775 (0.070-NA) |
| Statistical results | F1, 26 = 0.04, p = 0.851 | F1, 26 = 1.88, p = 0.182 | F1, 24 = 9.93, p < 0.01 | F1, 24 = 197.77, p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, H.; Feng, L.; Chen, J.; Zhong, J.; Seah, R.W.X.; Ding, G. Spatial Patterns of Species Diversity of Amphibians in a Nature Reserve in Eastern China. Biology 2023, 12, 461. https://doi.org/10.3390/biology12030461
Wang Y, Hu H, Feng L, Chen J, Zhong J, Seah RWX, Ding G. Spatial Patterns of Species Diversity of Amphibians in a Nature Reserve in Eastern China. Biology. 2023; 12(3):461. https://doi.org/10.3390/biology12030461
Chicago/Turabian StyleWang, Yanmei, Huali Hu, Lei Feng, Jingyi Chen, Junjie Zhong, Rachel Wan Xin Seah, and Guohua Ding. 2023. "Spatial Patterns of Species Diversity of Amphibians in a Nature Reserve in Eastern China" Biology 12, no. 3: 461. https://doi.org/10.3390/biology12030461