Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges
Abstract
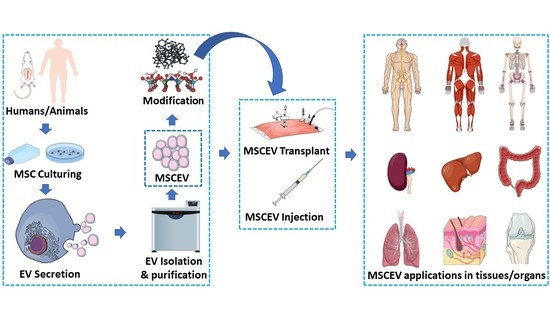
:Simple Summary
Abstract
1. Introduction
2. Biogenesis and Isolation of EVs
3. Regenerative Potential (RP) of MSCEVs
3.1. MSCEVs and Cardiac Tissue Regeneration
3.2. MSCEVs and Nervous Tissue Regeneration
3.3. MSCEVs and Bone Regeneration
3.4. MSCEVs and Liver Tissue Regeneration
3.5. MSCEVs and Kidney Regeneration
3.6. MSCEVs and Muscle Regeneration
3.7. MSCEVs and Cartilage Regeneration
3.8. MSCEVs and Wound Healing
3.9. MSCEVs and Other Tissue Regeneration
4. Apoptotic EVs or Apoptotic Bodies (ABs) and Their Regeneration Potential
5. Contents of MSCEVs
5.1. Proteins
5.2. Nucleic Acids
5.2.1. mRNA
5.2.2. microRNA (miRNA)
5.2.3. Lipids and Other Contents
6. Challenges for MSCEVs
6.1. Mass Production of MSCEVs
6.2. Scalable Methods for MSCEV Isolation
6.3. Stability
6.4. MSC-EV Biodistribution and Tissue Targeting
6.5. Heterogeneity
6.6. Safety Profile
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLS4 | Acyl-CoA synthetase long chain family member 4 |
| ADAm | A disintegrin and metalloprotease |
| ADM2 | Adrenomedullin 2 |
| AFMSCEV | Amniotic fluid-derived MSCEV |
| AGO2 | Argonaute RISC catalytic component 2 |
| AKI | Acute kidney injury |
| Akt | Serine/threonine kinase (also called as protein kinase B) |
| ALIX | ALG-2-interacting protein X |
| AMSCEV | Adipose-derived MSCEV |
| BAK1 | BCL2-antagonist/killer 1 |
| BAZ2B | Bromodomain Adjacent to Zinc Finger Domain 2B |
| BCL6B | B-cell CLL/lymphoma 6 member B protein |
| BDH2 | 3-hydroxybutyrate dehydrogenase type 2 |
| bFGF | Basic fibroblast growth factor |
| BMMSCEV | Bone marrow-derived MSCEV |
| BMP15 | Bone morphogenetic protein 15 |
| BSCB | Extracellular signal-regulated kinase |
| CACNA2D1 | Calcium voltage-gated channel auxiliary subunit alpha 2 delta 1 |
| CAMK2D | Calcium/calmodulin-dependent protein kinase type II delta |
| CAVI | Carbonic anhydrase VI |
| CCl4 | Carbon tetra chloride |
| CD | Cluster of differentiation |
| CDC42 | Cell division control protein 42 |
| CEACAM5 | Carcinoembryonic antigen-related cell adhesion molecule 5 |
| CKD | Chronic kidney damage |
| CLEC2A | C-type lectin domain family 2 member A |
| CLOCK | Circadian locomotor output cycles kaput |
| CNS | Central nervous system |
| COL1A2 | Collagen type I alpha 2 chain |
| CRLF1 | Cytokine receptor-like factor 1 |
| CTNNA1 | Catenin Alpha 1 |
| CTNNB1 | Catenin Beta 1 |
| CXCR7 | C-X-C chemokine receptor type 7 |
| 2D | 2 Dimensional |
| 3D | 3 Dimensional |
| DCF | Differential centrifugation |
| DDN | Dendrin |
| DPMSCEV | Dental pulp MSCEV |
| EAE | Experimental autoimmune Encephalomyelitis |
| ECM | Cartilage extracellular matrix |
| ECS | Extracellular space |
| EGFR | Epidermal growth factor receptor |
| ELP4 | Elongator acetyltransferase complex subunit 4 |
| EMSCEV | Embryonic-derived MSCEV |
| ENG | Endoglin |
| EPX | Eosinophil Peroxidase |
| ESCRT | Endosomal sorting complex required for |
| EV | Extra cellular vesicles |
| FABP5 | Fatty acid binding protein 5 |
| FLNA | Filamin A |
| FOXP3 | Forkhead Box P3 |
| FUT3 | Fucosyltransferase 3 |
| GAP43 | Growth Associated Protein 43 |
| GFAP | Glial fibrillary acidic protein |
| GMSCEV | Gingiva MSCEV |
| GNAI2 | Guanine nucleotide-binding protein G(i) subunit alpha-2 |
| GNG12 | G protein subunit gamma 12 |
| GRIN3A | Glutamate ionotropic receptor NMDA type subunit 3As |
| GTP | Guanosine triphosphate |
| hAMSC | Human adipose tissue-derived MSC |
| hBMSC | Human bone marrow-derived MSC |
| HES1 | Hairy and enhancer of split-1 |
| HGF | Hepatocyte growth factor |
| HK3 | Hexokinase 3 |
| HMGN4 | High mobility group nucleosomal binding domain 4 |
| HNRPH2 | Heterogeneous nuclear ribonucleoprotein H2 |
| HSP | Heat shock protein |
| hUMSC | Human umbilical-derived MSC |
| HuR | Human antigen R |
| IAP | Inhibitor of apoptosis |
| IBSP | Bone sialoprotein |
| IFN | Interferon-gamma |
| IFT57 | Intraflagellar transport 57 |
| IGF-1 | Insulin-like growth factor 1 |
| IGF2R | Insulin-like growth factor 2 receptor |
| IL | Interleukin |
| ILK | Integrin Linked Kinase |
| IL1RN | Interleukin 1 receptor antagonist |
| iPMSCEV | induced pluripotent MSCEV |
| IRF6 | Interferon regulatory factor 6 |
| ITGA1 | Integrin alpha-1 |
| JMJD1C | Jumonji domain containing 1C |
| JNK | c-Jun N-terminal Kinase |
| KCNH6 | Potassium voltage-gated channel subfamily H member 6 |
| KDM6B | Lysine-specific demethylase 6B |
| LPAR1 | Lysophosphatidic acid receptor 1 |
| LTA4H | Leukotriene A4 hydrolase |
| MAGED2 | Melanoma-associated antigen D2 |
| MAPK | Mitogen-activated protein kinase |
| MCA | Middle cerebral artery stroke |
| miR | Micro RNA |
| MMP | Matrix metallo proteinase |
| mRNA | Messenger ribose nucleic acid |
| MSC | Mesenchymal stem cells |
| MSCEV | Mesenchymal stem cell-derived extracellular vesicles |
| MSN | Moesin |
| MT1X | Metallothionein 1X |
| mTOR | Mammalian target of rapamycin |
| MYNN | Myoneurin |
| NIN | Ninein |
| NKFBIZ | NFKB Inhibitor Zeta |
| NRAS | Neuroblastoma RAS |
| O2 | Oxygen |
| OA | Osteo arthritis |
| OR11H12 | Olfactory receptor family 11 subfamily H member 12 |
| OR2M3 | Olfactory receptor 2M3 |
| pAMSC | Pig adipose tissue derived mesenchymal stem cells |
| PCP1 | Monocyte chemoattractant protein-1 |
| PDCD4 | Programmed cell death 4 |
| PDGFRB | Platelet-derived growth factor receptor beta |
| PEG | Polyethylene glycol |
| PEG3 | Paternally-expressed gene 3 |
| PI3 | Phosphatidylinositol 3 |
| PKD2L2 | Polycystic kidney disease 2-like 2 protein |
| PN | Peripheral nerve |
| POLR2E | RNA polymerase II, I and III subunit E |
| PPR2R1A | Protein phosphatase 2, regulatory subunit A |
| PRAKACA | Protein kinase A - catalytic subunit |
| PRDX2 | Peroxiredoxin-2 |
| PRKCB | Protein Kinase C Beta |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 |
| RAP | Ras-related protein |
| RAP1A | Ras-related protein Rap-1A |
| ratBMSC | Rat bone marrow-derived mesenchymal stem cells |
| RBL1 | RB transcriptional corepressor like 1 |
| RP | Regeneration potential |
| RRAS | Ras-related protein R-Ras |
| RUNX1T1 | RUNX1 partner transcriptional co-repressor 1 |
| S100A13 | S100 calcium binding protein A13 |
| SCI | Spinal cord injury |
| SCNN1G | Sodium channel epithelial 1 subunit gamma |
| SENP2 | Sentrin-specific protease 2 |
| SND | Sciatic nerve defect |
| SOD1 | Superoxide dismutase type 1 |
| STAT | Signal transducer and activator of transcription |
| Stau | Staufen double-stranded RNA binding protein |
| SUFU | Suppressor of fused protein |
| TBI | Traumatic brain injury |
| TCF4 | Transcription factor 4 |
| TCPF2 | Transcription factor CP2 |
| TGFB1 | Transforming growth factor beta induced |
| TIA | T-cell intracellular antigen |
| TMF1 | TATA element modulatory factor |
| TNF | Tumor necrosis factor |
| TOPORS | Topoisomerase I-binding RS protein |
| TSG | Tumor susceptibility gene 101 |
| UCF | Ultracentrifugation |
| UCMSCEV | Umbilical-derived MSCEV |
| UMSCEV | Urinary MSCEV |
| UPAR | Urokinase plasminogen activator surface receptor |
| USP9X | Ubiquitin specific peptidase 9 X-linked |
| VEGF | Vascular endothelial growth factor |
| VPS | Vacuolar protein sorting-associated protein transport |
| Wnt | Wingless-related integration site |
| XHX1 | Zinc fingers and homeoboxes 1 |
| ZBTB1 | Zinc finger and BTB domain containing 1 |
| ZNF217 | Zinc finger protein 217 |
References
- Platt, J.L.; Cascalho, M. New and Old Technologies for Organ Replacement. Curr. Opin. Organ Transplant. 2013, 18, 179. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossu, G.; Birchall, M.; Brown, T.; De Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S. Lancet Commission: Stem Cells and Regenerative Medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef] [Green Version]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue Engineering: Strategies, Stem Cells and Scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, T.; Creane, M.; Windebank, A.J.; Terzic, A.; Dietz, A.B. Translating Stem Cell Research to the Clinic: A Primer on Translational Considerations for Your First Stem Cell Protocol. Stem Cell Res. Ther. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Günter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational Cardiac Stem Cell Therapy: Advancing from First-Generation to next-Generation Cell Types. NPJ Regen. Med. 2017, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, A.-K.; Giebel, B. Exosomes: Small Vesicles Participating in Intercellular Communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Grobbee, E.J.; van der Vlugt, M.; van Vuuren, A.J.; Stroobants, A.K.; Mallant-Hent, R.C.; Lansdorp-Vogelaar, I.; Bossuyt, P.M.; Kuipers, E.J.; Dekker, E.; Spaander, M.C. Diagnostic Yield of One-Time Colonoscopy vs One-Time Flexible Sigmoidoscopy vs Multiple Rounds of Mailed Fecal Immunohistochemical Tests in Colorectal Cancer Screening. Clin. Gastroenterol. Hepatol. 2020, 18, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, Paracrine Actions of Infused Mesenchymal Stem Cells Are Important to the Recovery from Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [Green Version]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal Stem Cell: An Efficient Mass Producer of Exosomes for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Park, K.-S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; O’Driscoll, L. Inhibiting Extracellular Vesicles Formation and Release: A Review of EV Inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K. Apoptotic Cell-Derived Extracellular Vesicles: More than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolar, J.; Le Blanc, K.; Keating, A.; Blazar, B.R. Concise Review: Hitting the Right Spot with Mesenchymal Stromal Cells. Stem Cells 2010, 28, 1446–1455. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobarrez, F.; Sjövik, C.; Soop, A.; Hållström, L.; Frostell, C.; Pisetsky, D.S.; Wallén, H. CD40L Expression in Plasma of Volunteers Following LPS Administration: A Comparison between Assay of CD40L on Platelet Microvesicles and Soluble CD40L. Platelets 2015, 26, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J. Extracellular Vesicles: Lipids as Key Components of Their Biogenesis and Functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [Green Version]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic Biomaterials Based on Extracellular Vesicles: Classification of Bio-Engineering and Mimetic Preparation Routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Jo, W.; Jeong, D.; Kim, J.; Jeong, H.; Park, J. Generation of Nanovesicles with Sliced Cellular Membrane Fragments for Exogenous Material Delivery. Biomaterials 2015, 59, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Beltrami, A.P.; Urbanek, K.; Kajstura, J.; Yan, S.-M.; Finato, N.; Bussani, R.; Nadal-Ginard, B.; Silvestri, F.; Leri, A.; Beltrami, C.A. Evidence That Human Cardiac Myocytes Divide after Myocardial Infarction. New Engl. J. Med. 2001, 344, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian Heart Renewal by Pre-Existing Cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M. Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Exosomes Derived from AKt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl. Med. 2017, 6, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X. Induced Pluripotent Stem Cell (IPSC)–Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair than IPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Lodder, K.; Costa, A.; Moimas, S.; Moccia, F.; van Herwaarden, T.; Rosti, V.; Campagnoli, F.; Palmeri, A.; De Biasio, P. Reactivating Endogenous Mechanisms of Cardiac Regeneration via Paracrine Boosting Using the Human Amniotic Fluid Stem Cell Secretome. Int. J. Cardiol. 2019, 287, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, L.; Li, Q.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y. Combinatorial Treatment of Acute Myocardial Infarction Using Stem Cells and Their Derived Exosomes Resulted in Improved Heart Performance. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Wu, R.; Shehadeh, L.A.; Zhou, Q.; Jiang, C.; Huang, X.; Zhang, L.; Gao, F.; Liu, X.; Yu, H. Severe Hypoxia Exerts Parallel and Cell-Specific Regulation of Gene Expression and Alternative Splicing in Human Mesenchymal Stem Cells. BMC Genom. 2014, 15, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity without Remuscularization. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.; Qiu, X.-T.; Li, C.-C. Hypoxia-Elicited Mesenchymal Stem Cell-Derived Exosomes Facilitates Cardiac Repair through MiR-125b-Mediated Prevention of Cell Death in Myocardial Infarction. Theranostics 2018, 8, 6163. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X. Myocardial Reparative Functions of Exosomes from Mesenchymal Stem Cells Are Enhanced by Hypoxia Treatment of the Cells via Transferring MicroRNA-210 in an NSMase2-Dependent Way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F. Human Umbilical Cord Mesenchymal Stem Cell Derived Exosomes Encapsulated in Functional Peptide Hydrogels Promote Cardiac Repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ge, S.; Zhang, J.; Zhou, D.; Li, L.; Wang, X.; Su, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Nerve Regeneration after Sciatic Nerve Crush Injury in Rats. Int. J. Clin. Exp. Pathol. 2017, 10, 10032. [Google Scholar]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Improve Nerve Regeneration after Sciatic Nerve Transection in Rats. J. Cell. Mol. Med. 2019, 23, 2822–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucan, V.; Vaslaitis, D.; Peck, C.-T.; Strauß, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration after Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef] [Green Version]
- Haertinger, M.; Weiss, T.; Mann, A.; Tabi, A.; Brandel, V.; Radtke, C. Adipose Stem Cell-Derived Extracellular Vesicles Induce Proliferation of Schwann Cells via Internalization. Cells 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.; Nguyen, P.D.; Shanti, R.M.; Shi, S.; Shakoori, P.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng. Part. A 2019, 25, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-Mediated Transfer of MiR-133b from Multipotent Mesenchymal Stromal Cells to Neural Cells Contributes to Neurite Outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetta, L.; Marcus, S.G.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of Von Willebrand Factor, an Endothelial Cell Marker, is up-Regulated by Angiogenesis Factors: A Potential Method for Objective Assessment of Tumor Angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.-G.; Aigner, L. Doublecortin Expression Levels in Adult Brain Reflect Neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of Exosomes Derived from Multipluripotent Mesenchymal Stromal Cells on Functional Recovery and Neurovascular Plasticity in Rats after Traumatic Brain Injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting Pericytes for Therapeutic Approaches to Neurological Disorders. Acta Neuropathol. 2018, 136, 507–523. [Google Scholar] [CrossRef] [Green Version]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of Vascular Disruption and Blood–Spinal Cord Barrier Permeability Following Traumatic Spinal Cord Injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-Catenin Signaling Pathway. Cell Transplant. 2019, 28, 1373–1383. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes Derived from MiR-133b-Modified Mesenchymal Stem Cells Promote Recovery after Spinal Cord Injury. Front. Neurosci. 2018, 12, 845. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.; Zhang, S.; Barthe, S.; Kumar, P.; Pivetti, C.; Kreutzberg, N.; Reed, C.; Wang, Y.; Paxton, Z.; Farmer, D. Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Myelin Regeneration in an Animal Model of Multiple Sclerosis. Cells 2019, 8, 1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lasser, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal Stem Cell-Derived Exosomes Promote Neurogenesis and Cognitive Function Recovery in a Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 1626. [Google Scholar]
- Sisa, C.; Kholia, S.; Naylor, J.; Herrera Sanchez, M.B.; Bruno, S.; Deregibus, M.C.; Camussi, G.; Inal, J.M.; Lange, S.; Hristova, M. Mesenchymal Stromal Cell Derived Extracellular Vesicles Reduce Hypoxia-Ischaemia Induced Perinatal Brain Injury. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.-K.; Radtke, S.; Peters, V.; Janssen, L. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain after Hypoxia-Ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation in Vitro and Promote Bone Regeneration in Vivo. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Liang, J.-M.; Ding, J.-N.; Xu, J.; Xu, J.-G.; Chai, Y.-M. Dimethyloxaloylglycine-Stimulated Human Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Enhance Bone Regeneration through Angiogenesis by Targeting the AKT/MTOR Pathway. Stem Cell Res. Ther. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.; Zreiqat, H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng. Part. A 2017, 23, 1212–1220. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as Biomimetic Tools for Stem Cell Differentiation: Applications in Dental Pulp Tissue Regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human Mesenchymal Stem Cells Differentiate into an Osteogenic Lineage in Presence of Strontium Containing Bioactive Glass Nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/Tricalcium Phosphate Combination Scaffolds Can Enhance Bone Regeneration by Activating the PI3K/Akt Signaling Pathway. Stem Cell Res. Ther. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Zhang, W.J.; Chen, Z. Extracellular Vesicle-Functionalized Decalcified Bone Matrix Scaffolds with Enhanced pro-Angiogenic and pro-Bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.-K.; Ho, H.K. Mesenchymal Stem Cell-Derived Exosomes Promote Hepatic Regeneration in Drug-Induced Liver Injury Models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective Effect of Exosomes from Human-Induced Pluripotent Stem Cell–Derived Mesenchymal Stromal Cells against Hepatic Ischemia-Reperfusion Injury in Rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from Human-Induced Pluripotent Stem Cell–Derived Mesenchymal Stromal Cells (HiPSC-MSCs) Protect Liver against Hepatic Ischemia/Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.L.; Yarla, N.S.; Menschikowski, M.; Sukocheva, O.A. Regulatory Role of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Signaling in Progenitor/Stem Cells. World J. Stem Cells 2018, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Rat Hepatic Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Neutrophil Inflammatory Response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef] [Green Version]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Falda, M.; Bussolati, B.; Tetta, C. Mesenchymal Stem Cell-Derived Microvesicles Protect against Acute Tubular Injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Genetic Communication by Extracellular Vesicles Is an Important Mechanism Underlying Stem Cell-Based Therapy-Mediated Protection against Acute Kidney Injury. Stem Cell Res. Ther. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R.; et al. Extracellular Vesicles: Evolving Factors In Stem Cell Biology. Stem Cells Int. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.; Zhao, M.; Wang, C.; Li, L.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Zhang, J. Injectable Extracellular Vesicle-Released Self-Assembling Peptide Nanofiber Hydrogel as an Enhanced Cell-Free Therapy for Tissue Regeneration. J. Control. Release 2019, 316, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.; Cheng, J.; Zhong, L.; Wu, S.; Zou, X.; Zhang, G.; Gu, D.; Miao, S.; Zhu, Y.; Sun, J. Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Tubular Epithelial Cell Dedifferentiation and Growth via Hepatocyte Growth Factor Induction. PLoS ONE 2015, 10, e0121534. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through MiR-30. Stem Cells Int. 2016, 2016, 2093940. [Google Scholar] [CrossRef] [Green Version]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; Dimuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The Effects of Glomerular and Tubular Renal Progenitors and Derived Extracellular Vesicles on Recovery from Acute Kidney Injury. Stem Cell Res. Ther. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccoli, G.B.; Grassi, G.; Cabiddu, G.; Nazha, M.; Roggero, S.; Capizzi, I.; De Pascale, A.; Priola, A.M.; Di Vico, C.; Maxia, S. Chapter II. Diabetic Nephropathy. Rev. Diabet Stud. 2015, 12, 87–109. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Liu, Y.; Niu, X.; Yin, J.; Hu, B.; Guo, S.; Fan, Y.; Wang, Y.; Wang, N. Exosomes Secreted by Human Urine-Derived Stem Cells Could Prevent Kidney Complications from Type I Diabetes in Rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal Stem Cell Therapy Ameliorates Diabetic Nephropathy via the Paracrine Effect of Renal Trophic Factors Including Exosomes. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem Cell-Derived Extracellular Vesicles Inhibit and Revert Fibrosis Progression in a Mouse Model of Diabetic Nephropathy. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front. Immunol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-Vesicles Derived from Bone Marrow Stem Cells Protect the Kidney Both in Vivo and in Vitro by MicroRNA-Dependent Repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef]
- Matsukura, T.; Inaba, C.; Weygant, E.A.; Kitamura, D.; Janknecht, R.; Matsumoto, H.; Hyink, D.P.; Kashiwada, S.; Obara, T. Extracellular Vesicles from Human Bone Marrow Mesenchymal Stem Cells Repair Organ Damage Caused by Cadmium Poisoning in a Medaka Model. Physiol. Rep. 2019, 7, e14172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical Cord Mesenchymal Stem Cells Derived Extracellular Vesicles Can Safely Ameliorate the Progression of Chronic Kidney Diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-Stem-Cell-Derived Exosomes Accelerate Skeletal Muscle Regeneration. Febs Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F. Protein and Molecular Characterization of a Clinically Compliant Amniotic Fluid Stem Cell-Derived Extracellular Vesicle Fraction Capable of Accelerating Muscle Regeneration through Enhancement of Angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Tomkins, J.E.; Denecke, B.; Musante, L. Secretome of Adipose-Derived Mesenchymal Stem Cells Promotes Skeletal Muscle Regeneration through Synergistic Action of Extracellular Vesicle Cargo and Soluble Proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes Isolated from Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated with Torn Rotator Cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef]
- Wu, R.; Huang, C.; Wu, Q.; Jia, X.; Liu, M.; Xue, Z.; Qiu, Y.; Niu, X.; Wang, Y. Exosomes Secreted by Urine-Derived Stem Cells Improve Stress Urinary Incontinence by Promoting Repair of Pubococcygeus Muscle Injury in Rats. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Figliolini, F.; Ranghino, A.; Grange, C.; Cedrino, M.; Tapparo, M.; Cavallari, C.; Rossi, A.; Togliatto, G.; Femminò, S.; Gugliuzza, M.V. Extracellular Vesicles from Adipose Stem Cells Prevent Muscle Damage and Inflammation in a Mouse Model of Hind Limb Ischemia: Role of Neuregulin-1. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef] [Green Version]
- Zavatti, M.; Beretti, F.; Casciaro, F.; Bertucci, E.; Maraldi, T. Comparison of the Therapeutic Effect of Amniotic Fluid Stem Cells and Their Exosomes on Monoiodoacetate-Induced Animal Model of Osteoarthritis. Biofactors 2020, 46, 106–117. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.F.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.; Lorenowicz, M.J. Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Human Cartilage Regeneration in Vitro. Theranostics 2018, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Farahabadi, S.; Geetha-Loganathan, P.; Fu, K.; Nimmagadda, S.; Yang, H.J.; Richman, J.M. Dual Functions for WNT5A during Cartilage Development and in Disease. Matrix Biol. 2013, 32, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes Derived from MiR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Xu, B.; Xu, H. TGF-Β1 Promoted Chondrocyte Proliferation by Regulating Sp1 through MSC-Exosomes Derived MiR-135b. Cell Cycle 2018, 17, 2756–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H. Exosomes from Embryonic Mesenchymal Stem Cells Alleviate Osteoarthritis through Balancing Synthesis and Degradation of Cartilage Extracellular Matrix. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Wu, X. Exosomes Produced from 3D Cultures of Umbilical Cord Mesenchymal Stem Cells in a Hollow-Fiber Bioreactor Show Improved Osteochondral Regeneration Activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of Stem Cell-Derived Exosomes with in Situ Hydrogel Glue as a Promising Tissue Patch for Articular Cartilage Regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S. Desktop-Stereolithography 3D Printing of a Radially Oriented Extracellular Matrix/Mesenchymal Stem Cell Exosome Bioink for Osteochondral Defect Regeneration. Theranostics 2019, 9, 2439. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Badiavas, E.V. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes Released from Human Induced Pluripotent Stem Cells-Derived MSCs Facilitate Cutaneous Wound Healing by Promoting Collagen Synthesis and Angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.-A.; Kim, S.Y.; Park, J.H.; Jo, D.-G.; Cho, Y.W. Functional Recovery in Photo-Damaged Human Dermal Fibroblasts by Human Adipose-Derived Stem Cell Extracellular Vesicles. J. Extracell. Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes Derived from Human Adipose Mensenchymal Stem Cells Accelerates Cutaneous Wound Healing via Optimizing the Characteristics of Fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Da Ferreira, A.F.; da Cunha, P.S.; Carregal, V.M.; de Silva, P.C.; de Miranda, M.C.; Kunrath-Lima, M.; de Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L.; Frezard, F. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem/Stromal Cells Accelerate Migration and Activate AKT Pathway in Human Keratinocytes and Fibroblasts Independently of MiR-205 Activity. Stem Cells Int. 2017, 2017, 9841035. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, G.; Avanzini, M.A.; Icaro Cornaglia, A.; De Silvestri, A.; Mantelli, M.; Travaglino, P.; Croce, S.; Romano, P.; Avolio, L.; Iacob, G. Extracellular Vesicles Derived from Mesenchymal Cells: Perspective Treatment for Cutaneous Wound Healing in Pediatrics. Regen. Med. 2018, 13, 385–394. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: Applications in skin wound healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W. Human Umbilical Cord Mesenchymal Stem Cell Exosomes Enhance Angiogenesis through the Wnt4/β-Catenin Pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal Micrornas Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway during Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joglekar, M.V.; Hardikar, A.A.; Phan, T.H.; Khanal, D.; Tharkar, P.; Limantoro, C.; Johnson, J.; Kalionis, B.; Chrzanowski, W. Placenta Stem/Stromal Cell–Derived Extracellular Vesicles for Potential Use in Lung Repair. Proteomics 2019, 19, 1800166. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Influenza Virus-Induced Acute Lung Injury in a Pig Model. Stem Cell Res. Ther. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Pulmonary Vascular Permeability and Lung Injury Induced by Hemorrhagic Shock and Trauma. J. Trauma Acute Care Surg. 2018, 84, 245. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Zhao, R.; Zhang, K.; Midgley, A.C.; Kong, D.; Li, Z.; Zhao, Q. MSC-Derived SEVs Enhance Patency and Inhibit Calcification of Synthetic Vascular Grafts by Immunomodulation in a Rat Model of Hyperlipidemia. Biomaterials 2019, 204, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in Vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Xu, H.; Wang, G.; Liu, M.; Tian, D.; Yuan, Z. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Attenuate Dextran Sodium Sulfate-Induced Ulcerative Colitis by Promoting M2 Macrophage Polarization. Int. Immunopharmacol. 2019, 72, 264–274. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Q.; Niu, X.; Zhang, G.; Ling, X.; Zhang, J.; Wang, Y.; Deng, Z. Extracellular Vesicles Secreted by Human Urine-Derived Stem Cells Promote Ischemia Repair in a Mouse Model of Hind-Limb Ischemia. Cell. Physiol. Biochem. 2018, 47, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.; Perretta, S.; Perrod, G.; Pidial, L.; Lindner, V.; Carn, F.; Lemieux, S.; Alloyeau, D.; Boucenna, I.; Menasché, P. Thermoresponsive Gel Embedded with Adipose Stem-Cell-Derived Extracellular Vesicles Promotes Esophageal Fistula Healing in a Thermo-Actuated Delivery Strategy. ACS Nano 2018, 12, 9800–9814. [Google Scholar] [CrossRef]
- Fadeel, B.; Orrenius, S. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Human Disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K. A Novel Mechanism of Generating Extracellular Vesicles during Apoptosis via a Beads-on-a-String Membrane Structure. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Depraetere, V. “Eat Me” Signals of Apoptotic Bodies. Nat. Cell Biol. 2000, 2, E104. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic Bodies from Endothelial Cells Enhance the Number and Initiate the Differentiation of Human Endothelial Progenitor Cells in Vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Paone, S.; Caruso, S.; Atkin-Smith, G.K.; Phan, T.K.; Hulett, M.D.; Poon, I.K. Determining the Contents and Cell Origins of Apoptotic Bodies by Flow Cytometry. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Brock, C.K.; Wallin, S.T.; Ruiz, O.E.; Samms, K.M.; Mandal, A.; Sumner, E.A.; Eisenhoffer, G.T. Stem Cell Proliferation Is Induced by Apoptotic Bodies from Dying Cells during Epithelial Tissue Maintenance. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Bruno, S.; Incarnato, D.; Dettori, D.; Neri, F.; Provero, P.; Pomatto, M.; Oliviero, S.; Tetta, C.; Quesenberry, P.J. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J. Am. Soc. Nephrol. 2015, 26, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Staahl, A.; Arvidsson, I. Extracellular Vesicles in Renal Disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef]
- Dieudé, M.; Bell, C.; Turgeon, J.; Beillevaire, D.; Pomerleau, L.; Yang, B.; Hamelin, K.; Qi, S.; Pallet, N.; Béland, C. The 20S Proteasome Core, Active within Apoptotic Exosome-like Vesicles, Induces Autoantibody Production and Accelerates Rejection. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunavat, T.R.; Cheng, L.; Kim, D.-K.; Bhadury, J.; Jang, S.C.; Lässer, C.; Sharples, R.A.; López, M.D.; Nilsson, J.; Gho, Y.S. Small RNA Deep Sequencing Discriminates Subsets of Extracellular Vesicles Released by Melanoma Cells–Evidence of Unique MicroRNA Cargos. RNA Biol. 2015, 12, 810–823. [Google Scholar] [CrossRef] [Green Version]
- Gebara, N.; Rossi, A.; Skovronova, R.; Aziz, J.M.; Asthana, A.; Bussolati, B. Extracellular Vesicles, Apoptotic Bodies and Mitochondria: Stem Cell Bioproducts for Organ Regeneration. Curr. Transpl. Rep. 2020, 7, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Hauser, P.; Wang, S.; Didenko, V.V. Apoptotic bodies: Selective detection in extracellular vesicles. In Signal Transduction Immunohistochemistry; Springer: Berlin, Germany, 2017; pp. 193–200. [Google Scholar]
- Caruso, S.; Atkin-Smith, G.K.; Baxter, A.A.; Tixeira, R.; Jiang, L.; Ozkocak, D.C.; Santavanond, J.P.; Hulett, M.D.; Lock, P.; Phan, T.K. Defining the Role of Cytoskeletal Components in the Formation of Apoptopodia and Apoptotic Bodies during Apoptosis. Apoptosis 2019, 24, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, H.; Zeng, M.; He, W.; Li, M.; Huang, X.; Deng, D.Y.; Wu, J. Bone Marrow Mesenchymal Stem Cells Protect Alveolar Macrophages from Lipopolysaccharide-Induced Apoptosis Partially by Inhibiting the Wnt/β-Catenin Pathway. Cell Biol. Int. 2015, 39, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Mikami, K.; Venugopal, S.; Li, Y.; Török, N.J. Apoptotic Body Engulfment by Hepatic Stellate Cells Promotes Their Survival by the JAK/STAT and Akt/NF-ΚB-Dependent Pathways. J. Hepatol. 2009, 51, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y. Circulating Apoptotic Bodies Maintain Mesenchymal Stem Cell Homeostasis and Ameliorate Osteopenia via Transferring Multiple Cellular Factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E. Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12-Dependent Vascular Protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J. Apoptotic Bodies Derived from Mesenchymal Stem Cells Promote Cutaneous Wound Healing via Regulating the Functions of Macrophages. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Lopatina, T.; Gai, C.; Deregibus, M.C.; Kholia, S.; Camussi, G. Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids. Front. Oncol. 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindoso, R.S.; Collino, F.; Bruno, S.; Araujo, D.S.; Sant’Anna, J.F.; Tetta, C.; Provero, P.; Quesenberry, P.J.; Vieyra, A.; Einicker-Lamas, M. Extracellular Vesicles Released from Mesenchymal Stromal Cells Modulate MiRNA in Renal Tubular Cells and Inhibit ATP Depletion Injury. Stem Cells Dev. 2014, 23, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tu, H.; Yang, Y.; Fang, L.; Wu, Q.; Li, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Roles In Tumor Growth, Progression, and Drug Resistance. Stem Cells Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, K.C.; Penfornis, P.; Dhule, S.; Guillonneau, F.; Adams, K.V.; Mo, Y.Y.; Xu, R.; Liu, Y.; Watabe, K.; Vemuri, M.C. Extracellular Vesicles from Bone Marrow Mesenchymal Stem/Stromal Cells Transport Tumor Regulatory MicroRNA, Proteins, and Metabolites. Oncotarget 2015, 6, 4953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C. MSC Surface Markers (CD44, CD73, and CD90) Can Identify Human MSC-Derived Extracellular Vesicles by Conventional Flow Cytometry. Cell Commun. Signal. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human Adipose Tissue-Derived Mesenchymal Stem Cells Secrete Functional Neprilysin-Bound Exosomes. Sci. Rep. 2013, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; De Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Z.; Hong, M.M.; Zhang, H.; Chen, C.; Xiao, M.; Wang, J.; Yao, F.; Ba, M.; Liu, J. Proangiogenic Compositions of Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells. PLoS ONE 2014, 9, e115316. [Google Scholar] [CrossRef] [PubMed]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of MiRNAs. PLoS ONE 2010, 5, e11803. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of Growth Factor Receptor MRNA via Exosomes Unravels the Regenerative Effect of Mesenchymal Stem Cells. Stem Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirin, A.; Riester, S.M.; Zhu, X.-Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and MRNA Cargo of Extracellular Vesicles from Porcine Adipose Tissue-Derived Mesenchymal Stem Cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Choi, D.-Y.; Yun, S.J.; Choi, S.-M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.-W. Proteomic Analysis of Microvesicles Derived from Human Mesenchymal Stem Cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Ochiya, T. Molecular Signatures of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Mediated Tissue Repair. Stem Cell Res. Ther. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-F.; Yang, G.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y. Altered MicroRNA Expression Profile in Exosomes during Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E. BM Mesenchymal Stromal Cell–Derived Exosomes Facilitate Multiple Myeloma Progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from Bone Marrow Mesenchymal Stem Cells Contain a MicroRNA That Promotes Dormancy in Metastatic Breast Cancer Cells. Sci. Signal. 2014, 7, ra63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W. Exosomes Derived from Mesenchymal Stem Cells Suppress Angiogenesis by Down-Regulating VEGF Expression in Breast Cancer Cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Lanzón, M.P.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N. Human Bone Marrow-and Adipose-Mesenchymal Stem Cells Secrete Exosomes Enriched in Distinctive MiRNA and TRNA Species. Stem Cell Res. Ther. 2015, 6, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Dong, J.; Zhang, Z.; Zhang, D.-C.; You, X.-Y.; Zhong, Y.; Chen, M.-S.; Liu, S.-M. MiR-10a Restores Human Mesenchymal Stem Cell Differentiation by Repressing KLF4. J. Cell. Physiol. 2013, 228, 2324–2336. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal Stem Cell Secretes Microparticles Enriched in Pre-MicroRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b Promotes Neural Plasticity and Functional Recovery After Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats Via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic Preconditioning Potentiates the Protective Effect of Stem Cells through Secretion of Exosomes by Targeting Mecp2 via MiR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Chaudhry, G.R. Advances and Challenges in Stem Cell Culture. Colloids Surf. B: Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and Scale-up of Wharton’s Jelly-Derived Mesenchymal Stem Cells for Clinical Applications. Stem Cell Res. 2010, 5, 244–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver-Vila, I.; Coca, M.I.; Grau-Vorster, M.; Pujals-Fonts, N.; Caminal, M.; Casamayor-Genescà, A.; Ortega, I.; Reales, L.; Pla, A.; Blanco, M. Evaluation of a Cell-Banking Strategy for the Production of Clinical Grade Mesenchymal Stromal Cells from Wharton’s Jelly. Cytotherapy 2016, 18, 25–35. [Google Scholar] [CrossRef]
- Whitford, W.; Guterstam, P. Exosome Manufacturing Status. Future Med. Chem. 2019, 11, 1225–1236. [Google Scholar] [CrossRef] [Green Version]
- Colao, I.L.; Corteling, R.; Bracewell, D.; Wall, I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol. Med. 2018, 24, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Whitford, W.; Ludlow, J.W.; Cadwell, J.J. Continuous Production of Exosomes: Utilizing the Technical Advantages of Hollow-Fiber Bioreactor Technology. Genet. Eng. Biotechnol. News 2015, 35, 34. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A. del Applying Extracellular Vesicles Based Therapeutics in Clinical Trials–an ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Shelke, G.V.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of Exosome Depletion Protocols to Eliminate Functional and RNA-Containing Extracellular Vesicles from Fetal Bovine Serum. J. Extracell. Vesicles 2014, 3, 24783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lee, Y.; Johansson, H.J.; Mäger, I.; Vader, P.; Nordin, J.Z.; Wiklander, O.P.; Lehtiö, J.; Wood, M.J.; Andaloussi, S.E. Serum-Free Culture Alters the Quantity and Protein Composition of Neuroblastoma-Derived Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 26883. [Google Scholar] [CrossRef] [PubMed]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice–Grade Standard Protocol for Exclusively Human Mesenchymal Stromal Cell–Derived Extracellular Vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering Mesenchymal Stem Cells to Improve Their Exosome Efficacy and Yield for Cell-Free Therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014, 3. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [Green Version]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.; Grauls, G.; Mariman, E.C.; Wouters, E.F.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R. Ultrafiltration Combined with Size Exclusion Chromatography Efficiently Isolates Extracellular Vesicles from Cell Culture Media for Compositional and Functional Studies. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.; Vader, P. Higher Functionality of Extracellular Vesicles Isolated Using Size-Exclusion Chromatography Compared to Ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of Membrane Affinity-Based Method with Size-Exclusion Chromatography for Isolation of Exosome-like Vesicles from Human Plasma. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Karimi, N.; Cvjetkovic, A.; Jang, S.C.; Crescitelli, R.; Feizi, M.A.H.; Nieuwland, R.; Lötvall, J.; Lässer, C. Detailed Analysis of the Plasma Extracellular Vesicle Proteome after Separation from Lipoproteins. Cell. Mol. Life Sci. 2018, 75, 2873–2886. [Google Scholar] [CrossRef] [Green Version]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2018, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Li, Y.J.; Hu, X.B.; Huang, S.; Xiang, D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Wistrom, C.A. Stabilization of Dry Phospholipid Bilayers and Proteins by Sugars. Biochem. J. 1987, 242, 1. [Google Scholar] [CrossRef] [Green Version]
- Bosch, S.; De Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.-M. Trehalose Prevents Aggregation of Exosomes and Cryodamage. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Erratum to ‘Preservation of Exosomes at Room Temperature Using Lyophilization’ (International Journal of Pharmaceutics (2018) 553 (1–2)(1–7),(S0378517318307671)(10.1016/j. Ijpharm. 2018.10. 032)). Int. J. Pharm. 2019, 559, 427–428. [Google Scholar] [CrossRef]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence Imaging in Vivo: Recent Advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Bruno, S.; Chatterjee, D.; Quesenberry, P.J.; Tetta, C.; Camussi, G. Biodistribution of Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Model of Acute Kidney Injury Monitored by Optical Imaging. Int. J. Mol. Med. 2014, 33, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Dooner, M.; Papa, E.; Del Tatto, M.; Pereira, M.; Borgovan, T.; Cheng, Y.; Goldberg, L.; Liang, O.; Camussi, G. Biodistribution of Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Radiation Injury Bone Marrow Murine Model. Int. J. Mol. Sci. 2019, 20, 5468. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.B.; Bussolati, B.; Bruno, S.; Morando, L.; Mauriello-Romanazzi, G.; Sanavio, F.; Stamenkovic, I.; Biancone, L.; Camussi, G. Exogenous Mesenchymal Stem Cells Localize to the Kidney by Means of CD44 Following Acute Tubular Injury. Kidney Int. 2007, 72, 430–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaoz, E.; Sun, E.; Demir, C.S. Mesenchymal Stem Cell-Derived Exosomes Do Not Promote the Proliferation of Cancer Cells in Vitro. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 177. [Google Scholar] [PubMed]
- Kuang, M.; Huang, Y.; Zhao, X.; Zhang, R.; Ma, J.; Wang, D.; Ma, X. Exosomes Derived from Wharton’s Jelly of Human Umbilical Cord Mesenchymal Stem Cells Reduce Osteocyte Apoptosis in Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Rats via the MiR-21-PTEN-AKT Signalling Pathway. Int. J. Biol. Sci. 2019, 15, 1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, B.; Yue, J.; Liu, F.; Liu, Y.; Fu, W.; Si, Y. Exosomes from Mesenchymal Stem Cells Expressing MiR-125b Inhibit Neointimal Hyperplasia via Myosin IE. J. Cell. Mol. Med. 2019, 23, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.J.; Sung, J.H.; Kim, D.H.; Kim, E.H.; Cho, Y.H.; Son, J.P.; Cha, J.M.; Bang, O.Y. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl. Stroke Res. 2019, 10, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The Dynamic in Vivo Distribution of Bone Marrow-Derived Mesenchymal Stem Cells after Infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [Green Version]
- Nouraee, N.; Mowla, S.J. MiRNA Therapeutics in Cardiovascular Diseases: Promises and Problems. Front. Genet. 2015, 6, 232. [Google Scholar] [CrossRef] [Green Version]
- Kotmakçı, M.; Çetintaş, V.B. Extracellular Vesicles as Natural Nanosized Delivery Systems for Small-Molecule Drugs and Genetic Material: Steps towards the Future Nanomedicines. J. Pharm. Pharm. Sci. 2015, 18, 396–413. [Google Scholar] [CrossRef] [Green Version]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X. Generation and Testing of Clinical-Grade Exosomes for Pancreatic Cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, G.J.; Cho, Y.H.; Kang, H.Y.; Hyung, N.K.; Kim, D.; Lee, J.H.; Nam, J.Y.; Bang, O.Y. Circulating Mesenchymal Stem Cells Microparticles in Patients with Cerebrovascular Disease. PLoS ONE 2012, 7, e37036. [Google Scholar] [CrossRef]
- Wang, J.-H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A.C. Anti-HER2 ScFv-Directed Extracellular Vesicle-Mediated MRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol. Cancer Ther. 2018, 17, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting Extracellular Vesicles to Injured Tissue Using Membrane Cloaking and Surface Display. J. Nanobiotechnol. 2018, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Strategy For Liver Diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from Mesenchymal Stem/Stromal Cells: A New Therapeutic Paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, J.; Yaddanapudi, S.C.; Pigati, L.; Havens, M.A.; Jeong, S.; Weiner, G.A.; Weimer, K.M.E.; Stern, B.; Hastings, M.L.; Duelli, D.M. MicroRNAs Are Exported from Malignant Cells in Customized Particles. Nucleic Acids Res. 2012, 40, 9125–9138. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Hannafon, B.N.; Trigoso, Y.D.; Calloway, C.L.; Zhao, Y.D.; Lum, D.H.; Welm, A.L.; Zhao, Z.J.; Blick, K.E.; Dooley, W.C.; Ding, W.Q. Plasma Exosome MicroRNAs Are Indicative of Breast Cancer. Breast Cancer Res. 2016, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigati, L.; Yaddanapudi, S.C.; Iyengar, R.; Kim, D.-J.; Hearn, S.A.; Danforth, D.; Hastings, M.L.; Duelli, D.M. Selective Release of MicroRNA Species from Normal and Malignant Mammary Epithelial Cells. PLoS ONE 2010, 5, e13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.E.; Lehtiö, J.; Andaloussi, S.E. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Chin, A.R.; Yan, W.; Cao, M.; Liu, X.; Wang, S.E. Polarized Secretion of Extracellular Vesicles by Mammary Epithelia. J. Mammary Gland Biol. Neoplasia 2018, 23, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Théry, C. Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1, 18397. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Saleh, A.F.; Lázaro-Ibáñez, E.; Forsgard, M.A.-M.; Shatnyeva, O.; Osteikoetxea, X.; Karlsson, F.; Heath, N.; Ingelsten, M.; Rose, J.; Harris, J. Extracellular Vesicles Induce Minimal Hepatotoxicity and Immunogenicity. Nanoscale 2019, 11, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.L. Comprehensive Toxicity and Immunogenicity Studies Reveal Minimal Effects in Mice Following Sustained Dosing of Extracellular Vesicles Derived from HEK293T Cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Heng, B.C.; Cao, T.; Lee, E.H. Directing Stem Cell Differentiation into the Chondrogenic Lineage in Vitro. Stem Cells 2004, 22, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.-C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic Cell–Derived Exosomes for Cancer Therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef] [PubMed]


| Condition | Injured/Diseased Tissue | Treatment Approach | Trial Phase |
|---|---|---|---|
| Healthy | Injured lungs | Aerosol-based inhalation of AMSCEV (1 or 2 or 3 × 108 particles/3 mL) | I [64] |
| Osteoarthritis | Injured cartilage | Osteochondral explant from arthroplasty treatment with AMSCEVs | |
| Bronchopulmonary dysplasia | Chronic lung disease | Intravenous infusion of BMMSCEVs (20 or 60 or 200 pmol phospholid/kg body weight) | |
| COVID-19 | Lungs (pneumonia) | Aerosol inhalation of AMSCEVs (2 × 108 particles/3 mL) for 5 days | |
| Dystrophic epidermolysis bullosa | Skin | Local administration of allogenic BMMSCEVs | II [64] |
| Acute ischemic stroke | Injured brain | Stereotaxis-based administration of miR-124 (200 µg total EV protein) enriched MSCEVs | |
| Kidney | CKD | Injection of allogenic UMSCEVs (100 µg total EV protein/kg/dose) | II/III [96] |
| MSCEV Components | Source | Biochemical Factors/Genes | Functions | References |
|---|---|---|---|---|
| Proteins | hAMSC | Neprilysin | Degradation of β-amyloid peptide (intracellular and extracellular) in neuroblastoma cell lines | [158] |
| hBMSC | EGFR, PDGFRB, IGF2R and TGFBI | Self-renewal of MSCs | [165] | |
| hBMSC | PRKACA, CTNNB1, PPP2R1A, CHP, PRKCB, CAMK2D and 2G, and RAC1 and 2 | Wnt signaling, self-renewal and differentiation of MSCs | [165] | |
| hBMSC | CD-9, 13, 29, 44, 63, 73, 81, 90, and 105 | Surface antigen | [157,165] | |
| hBMSC | PPP2R1A, CD105, ENG, USP9X, COL1A2, and MAPK1 | TGFβ signaling and differentiation of MSCs | [165] | |
| hBMSC | CDC42 and 81; RAC1 and 2; FLNA, B, and C; HSPAB, Bl, and A1A; RAP1A and B; PRKCB and ACA; CACNA2D1; CHP; PDGFRB; RRAS2; MAP4K4; NRAS; CAVI; PPP2RIA; EGFR; RRAS; GNG12; MAPKl; RAP1A; GNAI2; PRDX2; LPAR1; ITGA1; and SOD1 | PPAR signaling and differentiation of MSCs | [165] | |
| hBMSC | ILK, ACSL4, and FABP5 | PPAR signaling and differentiation of MSCs | [165] | |
| hBMSC | ENG, USP9X | BMP signaling and differentiation of MSCs | [165] | |
| hBMSC | HuR, TIA, and TIAR | T cell internal antigen | [162] | |
| hBMSC | Stau1 and Stau2 | mRNA transportation and stability | [162] | |
| hBMSC | Ago2 | Assists in transportation and processing of miRNAs | [162] | |
| hUMSC | IL6, MCP1, IGFI, UPAR, bFGF, VEGF, VEGFR2, and angiogenin | Promotes angiogenesis | [123,161] | |
| hUMSC | Wnt4 | Enhances proliferation and migration | [162] | |
| mRNA | hBMSC | IGF-1R | Improves proliferation of cells | [82] |
| hBMSC | OR11H12, RAX2, OR2M3, GRIN3A, DDN, NIN, IBSP, BMP15, MAGED2, HK3, EPX, COL4A2, PKD2L2, CEACAM5, and SCNN1G | Mediates cell differentiation | [80] | |
| hBMSC | IRF6, CLOCK, RAX2, BCL6B, and TCFP2 | Mediates transcription | [80] | |
| hBMSC | TOPORS, HMGN4, ELP4, ESF1, HNRPH2, and POLR2E | DNA/RNA binding | [80] | |
| hBMSC | RBL1, SENP2, S100A13, and CDC14B | Cell cycle | [80] | |
| hBMSC | CXCR7, CEACAM5, and CLEC2A | Receptors | [80] | |
| hBMSC | FUT3, ADAM15, ADM2, BDH2, RAB5A, and LTA4H | Mediates metabolism | [80] | |
| hBMSC | MT1X, CRLF1, and IL1RN | Immune regulation | [80] | |
| hBMSC | CTNNA1, DDN and MSN | Cytoskeleton | [80] | |
| hBMSC | IBSP and COL4A2 | Extracellular matrix | [80] | |
| pAMSC | KDM6B, JMJD1C, and FOXP3 | Encodes transcription factors of chromosome organization | [164] | |
| pAMSC | IFT57, MDM4, PDCD4 and PEG3 | Encodes transcription factors of apoptosis | [164] | |
| pAMSC | HES1, TCF4 and HGF | Encodes transcription factors of proangiogenic pathways | [164] | |
| pAMSC | ZHX1; ZBTB1; and ZNF217, 238, 568, 461, and 667 | Encodes zinc-finger transcription factors | [164] | |
| pAMSC | BAZ2B, TMF1, JMJD1C, NFKBIZ, PEG3, MYNN, KCNH6, SUFU, and RUNX1T1 | Encodes transcription factors related to alternative splicing | [164] | |
| miRNA | ratBMSC | miRNA-133b | Contributes to neurite outgrowth | [166] |
| hBMSC | miRNA-148a, 135b, 199b, 218, and 221 | Regulates differentiation of osteoblasts | [167] | |
| hBMSC | miRNA-15a | Inhibits multiple myeloma cell growth | [168] | |
| pAMSC | miRNA-148a and 378, let-7f, and miR532-5p | Regulates apoptosis, proteolysis angiogenesis, and cellular transport | [164] | |
| hBMSC | miRNA-21 and 34a | Regulates cell survival and proliferation | [156] | |
| hBMSC | miRNA-23b | Induces dormant phenotypes | [169] | |
| hBMSC | miRNA-16 | Targets VEGF and suppresses angiogenesis | [170] | |
| hAMSC | miRNA-10a-5p, 10b-5p, 21-5p, 22-3p, 26a-5p, 51a-3p, 92a-3p, 92b-3p, 99b-5p, 100-5p, 127-3p, 143-3p, 146a-5p, 146b-5p, 191-5p, 222-3p, 486-5p, 4485; and let-7a-5p, and 7f-5p | Mediates replicative senescence and immunomodulation; regulates cell cycle progression and proliferation; modulates angiogenesis; promotes migration | [171] | |
| hBMSC | miRNA-10a-5p, 10b-5p, 21-5p, 22-3p, 27b-3p, 28-3p, 92a-3p, 92b-3p, 99b-5p, 100-5p, 125b-5p, 127-3p, 143-3p, 191-5p, 222-3p, 423-5p, 486-5p; let-7a-5p, 7f-5p, and 7i-5p | Assists ASC replicative senescence, immune modulatory function; promotes migration; regulates cell cycle progression and proliferation; modulates angiogenesis | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuloria, S.; Subramaniyan, V.; Dahiya, R.; Dahiya, S.; Sudhakar, K.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Sekar, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology 2021, 10, 172. https://doi.org/10.3390/biology10030172
Fuloria S, Subramaniyan V, Dahiya R, Dahiya S, Sudhakar K, Kumari U, Sathasivam K, Meenakshi DU, Wu YS, Sekar M, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology. 2021; 10(3):172. https://doi.org/10.3390/biology10030172
Chicago/Turabian StyleFuloria, Shivkanya, Vetriselvan Subramaniyan, Rajiv Dahiya, Sunita Dahiya, Kalvatala Sudhakar, Usha Kumari, Kathiresan Sathasivam, Dhanalekshmi Unnikrishnan Meenakshi, Yuan Seng Wu, Mahendran Sekar, and et al. 2021. "Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges" Biology 10, no. 3: 172. https://doi.org/10.3390/biology10030172