Free Fatty Acid Species Differentially Modulate the Inflammatory Gene Response in Primary Human Skeletal Myoblasts
Abstract
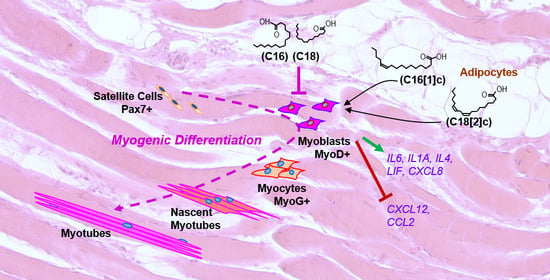
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fatty Acids (FAs)
2.2. Cell Culture
2.3. Multiplex Protein Quantification
2.4. Multiplex mRNA Quantification
2.5. Monitoring Mitochondrial Abundance and Function
2.6. Immunohistochemistry
2.7. Cellular Lipid Storage
2.8. Fluorescence Microscopy/Imaging
2.9. Protein Phosphorylation Array
2.10. Statistical Analysis
3. Results
3.1. FFAs Affect the Expression of Eight Inflammatory Proteins in Primary Human Myoblasts
3.2. FFAs Regulate Inflammatory Target Genes at the Transcriptional Level
3.3. Saturated C16 and C18 Inhibit Human Skeletal Myoblast Expansion
3.4. Desmin Expression in Human Primary Myoblasts Is Not Affected by FFAs
3.5. C18[2]c Increases Lipid Accumulation in Human Primary Myoblasts
3.6. FFAs Activate Different Receptor Tyrosine Kinases in Human Primary Myoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.Q.; Xiao, G.L.; Fan, Y.B.; He, M.; Lv, S.; Li, Y.S. Sarcopenic obesity: Research advances in pathogenesis and diagnostic criteria. Aging Clin. Exp. Res. 2021, 33, 247–252. [Google Scholar] [CrossRef]
- Sardon Puig, L.; Pillon, N.J.; Naslund, E.; Krook, A.; Zierath, J.R. Influence of obesity, weight loss, and free fatty acids on skeletal muscle clock gene expression. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Kob, R.; Fellner, C.; Bertsch, T.; Wittmann, A.; Mishura, D.; Sieber, C.C.; Fischer, B.E.; Stroszczynski, C.; Bollheimer, C.L. Gender-specific differences in the development of sarcopenia in the rodent model of the ageing high-fat rat. J. Cachexia Sarcopenia Muscle 2015, 6, 181–191. [Google Scholar] [CrossRef]
- Collins, K.H.; Paul, H.A.; Hart, D.A.; Reimer, R.A.; Smith, I.C.; Rios, J.L.; Seerattan, R.A.; Herzog, W. A High-Fat High-Sucrose Diet Rapidly Alters Muscle Integrity, Inflammation and Gut Microbiota in Male Rats. Sci. Rep. 2016, 6, 37278. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, ig.7, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Devarshi, P.P.; McNabney, S.M.; Henagan, T.M. Skeletal Muscle Nucleo-Mitochondrial Crosstalk in Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2017, 18, 831. [Google Scholar] [CrossRef] [Green Version]
- Boden, G.; Chen, X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J. Clin. Investig. 1995, 96, 1261–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, A.; Elizbaryan, K.; Tashakorinia, P.; Lam, N.H.; Sweeney, G. An adiponectin-S1P autocrine axis protects skeletal muscle cells from palmitate-induced cell death. Lipids Health Dis. 2020, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- da Paixao, A.O.; Bolin, A.P.; Silvestre, J.G.; Rodrigues, A.C. Palmitic Acid Impairs Myogenesis and Alters Temporal Expression of miR-133a and miR-206 in C2C12 Myoblasts. Int. J. Mol. Sci. 2021, 22, 2748. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Liu, H.W.; Chen, Y.T.; Chen, Y.A.; Chen, Y.J.; Chang, S.J. Resveratrol protects muscle cells against palmitate-induced cellular senescence and insulin resistance through ameliorating autophagic flux. J. Food Drug Anal. 2018, 26, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wei, X.; Hu, H.; Yang, D.; Zhang, B.; Fan, X.; Liu, J.; He, H.; Oh, Y.; Wu, Q.; et al. The saturated fatty acid palmitate induces insulin resistance through Smad3-mediated down-regulation of FNDC5 in myotubes. Biochem. Biophys. Res. Commun. 2019, 520, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Weigert, C.; Brodbeck, K.; Staiger, H.; Kausch, C.; Machicao, F.; Haring, H.U.; Schleicher, E.D. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. 2004, 279, 23942–23952. [Google Scholar] [CrossRef] [Green Version]
- Lovsletten, N.G.; Bakke, S.S.; Kase, E.T.; Ouwens, D.M.; Thoresen, G.H.; Rustan, A.C. Increased triacylglycerol - Fatty acid substrate cycling in human skeletal muscle cells exposed to eicosapentaenoic acid. PLoS ONE 2018, 13, e0208048. [Google Scholar] [CrossRef] [PubMed]
- Lovsletten, N.G.; Vu, H.; Skagen, C.; Lund, J.; Kase, E.T.; Thoresen, G.H.; Zammit, V.A.; Rustan, A.C. Treatment of human skeletal muscle cells with inhibitors of diacylglycerol acyltransferases 1 and 2 to explore isozyme-specific roles on lipid metabolism. Sci. Rep. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef]
- Honda, K.L.; Lamon-Fava, S.; Matthan, N.R.; Wu, D.; Lichtenstein, A.H. EPA and DHA exposure alters the inflammatory response but not the surface expression of Toll-like receptor 4 in macrophages. Lipids 2015, 50, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Frommer, K.W.; Schaffler, A.; Rehart, S.; Lehr, A.; Muller-Ladner, U.; Neumann, E. Free fatty acids: Potential proinflammatory mediators in rheumatic diseases. Ann. Rheum Dis. 2015, 74, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Schaeffler, A.; Gross, P.; Buettner, R.; Bollheimer, C.; Buechler, C.; Neumeier, M.; Kopp, A.; Schoelmerich, J.; Falk, W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009, 126, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Haversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.M.; Murphy, C.H.; Castro, E.M.; Roche, H.M. Inflammation and metabolism: The role of adiposity in sarcopenic obesity. Proc. Nutr. Soc. 2020, 79, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Hommelberg, P.P.; Plat, J.; Langen, R.C.; Schols, A.M.; Mensink, R.P. Fatty acid-induced NF-kappaB activation and insulin resistance in skeletal muscle are chain length dependent. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E114–E120. [Google Scholar] [CrossRef]
- Masuda, S.; Tanaka, M.; Inoue, T.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; Satoh-Asahara, N. Chemokine (C-X-C motif) ligand 1 is a myokine induced by palmitate and is required for myogenesis in mouse satellite cells. Acta Physiol. 2018, 222. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Min, K.H.; Lee, W. Palmitic Acid-Induced miR-429-3p Impairs Myoblast Differentiation by Downregulating CFL2. Int. J. Mol. Sci. 2021, 22, 10972. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Moreira, K.; Bain, G. Characterization of primary human skeletal muscle cells from multiple commercial sources. In Vitro Cell Dev. Biol. Anim. 2013, 49, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Alsabeeh, N.; Chausse, B.; Kakimoto, P.A.; Kowaltowski, A.J.; Shirihai, O. Cell culture models of fatty acid overload: Problems and solutions. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 143–151. [Google Scholar] [CrossRef]
- Laurentius, T.; Kob, R.; Fellner, C.; Nourbakhsh, M.; Bertsch, T.; Sieber, C.C.; Bollheimer, L.C. Long-Chain Fatty Acids and Inflammatory Markers Coaccumulate in the Skeletal Muscle of Sarcopenic Old Rats. Dis. Markers 2019, 2019, 9140789. [Google Scholar] [CrossRef] [Green Version]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Agafonov, A.V.; Astashev, M.E.; Kazakov, A.S.; Saris, N.E.; Mironova, G.D. Ca(2+)-dependent permeabilization of mitochondria and liposomes by palmitic and oleic acids: A comparative study. Biochim. Biophys. Acta 2014, 1838, 2600–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitraju, C.; Mejhert, N.; Haas, J.T.; Diaz-Ramirez, L.G.; Grueter, C.A.; Imbriglio, J.E.; Pinto, S.; Koliwad, S.K.; Walther, T.C.; Farese, R.V., Jr. Triglyceride Synthesis by DGAT1 Protects Adipocytes from Lipid-Induced ER Stress during Lipolysis. Cell Metab. 2017, 26, 407–418.e3. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Saltin, B.; Olsen, D.B.; van Hall, G. High triacylglycerol turnover rate in human skeletal muscle. J. Physiol. 2004, 561, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.D.; Romeo, O.M.; Higo, K.; Cohen, A.; Rafaat, K.; Olefsky, J.M. TNF-alpha-induced insulin resistance in vivo and its prevention by troglitazone. Diabetes 1997, 46, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.D.; Flores-Riveros, J.R.; Hresko, R.C.; Kaestner, K.H.; Liao, K.; Janicot, M.; Hoffman, R.D.; McLenithan, J.C.; Kastelic, T.; Christy, R.J. Insulin-receptor tyrosine kinase and glucose transport. Diabetes Care 1990, 13, 565–575. [Google Scholar] [CrossRef]
- Villegas-Comonfort, S.; Guzman-Silva, A.; Romero-Avila, M.T.; Takei, Y.; Tsujimoto, G.; Hirasawa, A.; Garcia-Sainz, J.A. Receptor tyrosine kinase activation induces free fatty acid 4 receptor phosphorylation, beta-arrestin interaction, and internalization. Eur. J. Pharm. 2019, 855, 267–275. [Google Scholar] [CrossRef]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.U.; Renz, H.; Dufour, J.F.; Potaczek, D.P.; Garn, H. Effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018, 128, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Meier, R.P.; Muller, Y.D.; Morel, P.; Gonelle-Gispert, C.; Buhler, L.H. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013, 11, 1348–1364. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef]
- Horsley, V.; Jansen, K.M.; Mills, S.T.; Pavlath, G.K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003, 113, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Nylen, C.; Aoi, W.; Abdelmoez, A.M.; Lassiter, D.G.; Lundell, L.S.; Wallberg-Henriksson, H.; Naslund, E.; Pillon, N.J.; Krook, A. IL6 and LIF mRNA expression in skeletal muscle is regulated by AMPK and the transcription factors NFYC, ZBTB14, and SP1. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E995–E1004. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.; Bragg, R.; Elmansi, A.M.; McGee-Lawrence, M.E.; Isales, C.M.; Hamrick, M.W.; Hill, W.D.; Fulzele, S. Stromal cell-derived factor-1 (CXCL12) and its role in bone and muscle biology. Cytokine 2019, 123, 154783. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Scuderi, F.; Provenzano, C.; Bartoccioni, E. Skeletal muscle cells: From local inflammatory response to active immunity. Gene 2011, 18, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Tachibana, H.; Morinaga, Y.; Fujimura, Y.; Yamada, K. Modulation of proliferation and differentiation of C2C12 skeletal muscle cells by fatty acids. Life Sci. 2009, 84, 415–420. [Google Scholar] [CrossRef]
- Strozen, T.G.; Sharpe, J.C.; Harris, E.D.; Uppalapati, M.; Toosi, B.M. The EphB6 Receptor: Kinase-Dead but Very Much Alive. Int. J. Mol. Sci. 2021, 22, 8211. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K.; Mahajan, N.P. ACK1/TNK2 tyrosine kinase: Molecular signaling and evolving role in cancers. Oncogene 2015, 34, 4162–4167. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Wu, Z.; Tremblay, J.; Thorin, E.; Peng, J.; Lavoie, J.L.; Hu, B.; Stoyanova, E.; Cloutier, G.; Qi, S.; et al. Receptor tyrosine kinase Ephb6 regulates vascular smooth muscle contractility and modulates blood pressure in concert with sex hormones. J. Biol. Chem. 2012, 287, 6819–6829. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauen, M.; Hao, D.; Müller, A.; Mückter, E.; Bollheimer, L.C.; Nourbakhsh, M. Free Fatty Acid Species Differentially Modulate the Inflammatory Gene Response in Primary Human Skeletal Myoblasts. Biology 2021, 10, 1318. https://doi.org/10.3390/biology10121318
Rauen M, Hao D, Müller A, Mückter E, Bollheimer LC, Nourbakhsh M. Free Fatty Acid Species Differentially Modulate the Inflammatory Gene Response in Primary Human Skeletal Myoblasts. Biology. 2021; 10(12):1318. https://doi.org/10.3390/biology10121318
Chicago/Turabian StyleRauen, Melanie, Dandan Hao, Aline Müller, Eva Mückter, Leo Cornelius Bollheimer, and Mahtab Nourbakhsh. 2021. "Free Fatty Acid Species Differentially Modulate the Inflammatory Gene Response in Primary Human Skeletal Myoblasts" Biology 10, no. 12: 1318. https://doi.org/10.3390/biology10121318