Impact of Remelting in the Microstructure and Corrosion Properties of the Ti6Al4V Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Manufacturing
2.2. Microstructural Characterization
2.3. Corrosion Tests
3. Results and Discussion
3.1. Microstructure and Defects
3.2. Microhardness
3.3. Corrosion Behavior
3.3.1. Linear Polarization Resistance (LPR)
3.3.2. Tafel Tests
3.3.3. Cyclic Polarization
3.3.4. Mass Gain
4. Conclusions
- Ti6Al4V specimens were successfully fabricated by L-PBF equipped with a CO2 laser, and a process of laser remelting in each layer was established and was applied to the manufacturing of the samples.
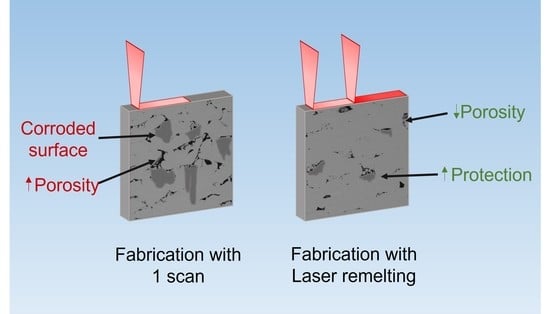
- The application of a second laser pass reduced the porosity of the samples and caused a slight reduction of the hardness, which was still greater than that of non-AM samples.
- The grain size of the microstructure of the L-PBF samples increased with the heat input and with the application of a second laser pass. Furthermore, XRD showed that a medium scanning speed and the laser remelting promoted an increment of the β phase due to the transformation of the metastable α’ into α and β.
- The corrosion behavior of the samples is improved by the application of a second laser pass in each layer, as was revealed by the OCP, Rp, and Tafel measurements shown. Furthermore, cyclic polarization tests showed that no pits were generated after one hour of immersion and that the passive layer of the parts fabricated with the laser remelting strategy was more stable than in the single laser pass manufactured samples.
- No pitting corrosion damage or corrosion products were found on the surface of the specimens after a week of immersion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammed, M.T. Mechanical Properties of SLM-Titanium Materials for Biomedical Applications: A Review. Mater. Today Proc. 2018, 5, 17906–17913. [Google Scholar] [CrossRef]
- Rahulan, N.; Sharma, S.S.; Rakesh, N.; Sambhu, R. A Short Review on Mechanical Properties of SLM Titanium Alloys Based on Recent Research Works. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Chen, X.; He, Y.; Cheng, L.; Huo, M.; Yin, J.; Hao, F.; Chen, S.; Wang, P.; et al. Additive Manufacturing of Structural Materials. Mater. Sci. Eng. R Rep. 2021, 145, 100596. [Google Scholar] [CrossRef]
- Bici, M.; Brischetto, S.; Campana, F.; Ferro, C.G.; Seclì, C.; Varetti, S.; Maggiore, P.; Mazza, A. Development of a Multifunctional Panel for Aerospace Use through SLM Additive Manufacturing. Procedia CIRP 2018, 67, 215–220. [Google Scholar] [CrossRef]
- Nouri, A.; Rohani Shirvan, A.; Li, Y.; Wen, C. Additive Manufacturing of Metallic and Polymeric Load-Bearing Biomaterials Using Laser Powder Bed Fusion: A Review. J. Mater. Sci. Technol. 2021, 94, 196–215. [Google Scholar] [CrossRef]
- Vinodh, K.; Tigga, A.K.; Barad, S. Influence of Post-Processing Techniques on Residual Stresses of SLM Processed HPNGV. J. Manuf. Process. 2021, 66, 189–197. [Google Scholar] [CrossRef]
- Dareh Baghi, A.; Nafisi, S.; Hashemi, R.; Ebendorff-Heidepriem, H.; Ghomashchi, R. Effective Post Processing of SLM Fabricated Ti-6Al-4 V Alloy: Machining vs Thermal Treatment. J. Manuf. Process. 2021, 68, 1031–1046. [Google Scholar] [CrossRef]
- Majeed, A.; Zhang, Y.; Lv, J.; Peng, T.; Atta, Z.; Ahmed, A. Investigation of T4 and T6 Heat Treatment Influences on Relative Density and Porosity of AlSi10Mg Alloy Components Manufactured by SLM. Comput. Ind. Eng. 2020, 139, 106194. [Google Scholar] [CrossRef]
- Liu, B.; Fang, G.; Lei, L. An Analytical Model for Rapid Predicting Molten Pool Geometry of Selective Laser Melting (SLM). Appl. Math. Model. 2021, 92, 505–524. [Google Scholar] [CrossRef]
- Tonelli, L.; Fortunato, A.; Ceschini, L. CoCr Alloy Processed by Selective Laser Melting (SLM): Effect of Laser Energy Density on Microstructure, Surface Morphology, and Hardness. J. Manuf. Process. 2020, 52, 106–119. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.-J. Lasers in Additive Manufacturing: A Review. Int. J. Precis. Eng. Manuf. Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Prabakaran, M.P.; Kannan, G.R. Optimization of CO2 Laser Beam Welding Process Parameters to Attain Maximum Weld Strength in Dissimilar Metals. Mater. Today Proc. 2018, 5, 6607–6616. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Bai, Q.; Xie, G. Correlation of Microstructure and Mechanical Properties of Ti2AlNb Manufactured by SLM and Heat Treatment. Intermetallics 2021, 139, 107367. [Google Scholar] [CrossRef]
- Karimi, J.; Suryanarayana, C.; Okulov, I.; Prashanth, K.G. Selective Laser Melting of Ti6Al4V: Effect of Laser Re-Melting. Mater. Sci. Eng. A 2021, 805, 140558. [Google Scholar] [CrossRef]
- Xu, Z.; Dong, Z.; Yu, Z.; Wang, W.; Zhang, J. Relationships between Microhardness, Microstructure, and Grain Orientation in Laser-Welded Joints with Different Welding Speeds for Ti6Al4V Titanium Alloy. Trans. Nonferrous Met. Soc. China 2020, 30, 1277–1289. [Google Scholar] [CrossRef]
- Shui, J.; Chen, S.L.; Xia, T.T.; Liu, H.; Yang, G.J.; Niu, J.; Gong, W.Q. Effect of Non-Equilibrium Solid State Phase Transformation on Welding Temperature Field during Keyhole Mode Laser Welding of Ti6Al4V Alloy. Opt. Laser Technol. 2022, 145, 107461. [Google Scholar] [CrossRef]
- Kumar, B.; Bag, S.; Paul, C.P.; Das, C.R.; Ravikumar, R.; Bindra, K.S. Influence of the Mode of Laser Welding Parameters on Microstructural Morphology in Thin Sheet Ti6Al4V Alloy. Opt. Laser Technol. 2020, 131, 106456. [Google Scholar] [CrossRef]
- Jia, W.; Zan, Y.; Mao, C.; Li, S.; Zhou, W.; Li, Q.; Zhang, S.; Ji, V. Microstructure Evolution and Mechanical Properties of a Lamellar Near-α Titanium Alloy Treated by Laser Shock Peening. Vacuum 2021, 184, 109906. [Google Scholar] [CrossRef]
- Kahlin, M.; Ansell, H.; Moverare, J. Fatigue Crack Growth for through and Part-through Cracks in Additively Manufactured Ti6Al4V. Int. J. Fatigue 2022, 155, 106608. [Google Scholar] [CrossRef]
- Ochonogor, O.F.; Akinlabi, E.T.; Nyembwe, D. A Review on the Effect of Creep and Microstructural Change under Elevated Temperature of Ti6Al4V Alloy for Turbine Engine Application. Mater. Today Proc. 2017, 4, 250–256. [Google Scholar] [CrossRef]
- Shankar, S.; Nithyaprakash, R.; Abbas, G.; Naveen Kumar, R.; Pramanik, A.; Kumar Basak, A.; Prakash, C. Tribological Behavior of Zirconia-Toughened Alumina (ZTA) against Ti6Al4V under Different Bio-Lubricants in Hip Prosthesis Using Experimental and Finite Element Concepts. Mater. Lett. 2022, 307, 131107. [Google Scholar] [CrossRef]
- Hamza, H.M.; Deen, K.M.; Haider, W. Microstructural Examination and Corrosion Behavior of Selective Laser Melted and Conventionally Manufactured Ti6Al4V for Dental Applications. Mater. Sci. Eng. C 2020, 113, 110980. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaie, K.S.; Melotti, S.; Rabelo, A.; Paiva, J.M.; Elbestawi, M.A.; Veldhuis, S.C. Machinability of SLM-Produced Ti6Al4V Titanium Alloy Parts. J. Manuf. Process. 2020, 57, 768–786. [Google Scholar] [CrossRef]
- Ni, J.; Liu, F.; Yang, G.; Lee, G.-H.; Chung, S.-M.; Lee, I.-S.; Chen, C. 3D-Printed Ti6Al4V Femoral Component of Knee: Improvements in Wear and Biological Properties by AIP TiN and TiCrN Coating. J. Mater. Res. Technol. 2021, 14, 2322–2332. [Google Scholar] [CrossRef]
- Yan, X.; Shi, C.; Liu, T.; Ye, Y.; Chang, C.; Ma, W.; Deng, C.; Yin, S.; Liao, H.; Liu, M. Effect of Heat Treatment on the Corrosion Resistance Behavior of Selective Laser Melted Ti6Al4V ELI. Surf. Coat. Technol. 2020, 396, 125955. [Google Scholar] [CrossRef]
- Bedmar, J.; Riquelme, A.; Rodrigo, P.; Torres, B.; Rams, J. Comparison of Different Additive Manufacturing Methods for 316L Stainless Steel. Materials 2021, 14, 6504. [Google Scholar] [CrossRef]
- Valente, E.H.; Nadimpalli, V.K.; Christiansen, T.L.; Pedersen, D.B.; Somers, M.A.J. In-Situ Interstitial Alloying during Laser Powder Bed Fusion of AISI 316 for Superior Corrosion Resistance. Addit. Manuf. Lett. 2021, 1, 100006. [Google Scholar] [CrossRef]
- Fashanu, O.; Buchely, M.F.; Spratt, M.; Newkirk, J.; Chandrashekhara, K.; Misak, H.; Walker, M. Effect of SLM Build Parameters on the Compressive Properties of 304L Stainless Steel. J. Manuf. Mater. Process. 2019, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.H.; Ashton, I.R.T.; Jones, E.; Fox, P.; Sutcliffe, C. The effect of hatch angle rotation on parts manufactured using selective laser melting. Rapid Prototyp. J. 2019, 25, 289–298. [Google Scholar] [CrossRef]
- Dareh Baghi, A.; Nafisi, S.; Hashemi, R.; Ebendorff-Heidepriem, H.; Ghomashchi, R. Experimental Realisation of Build Orientation Effects on the Mechanical Properties of Truly As-Built Ti-6Al-4V SLM Parts. J. Manuf. Process. 2021, 64, 140–152. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.-P. A Study of the Microstructural Evolution during Selective Laser Melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Rautio, T.; Hamada, A.; Mäkikangas, J.; Jaskari, M.; Järvenpää, A. Laser Welding of Selective Laser Melted Ti6Al4V: Microstructure and Mechanical Properties. Mater. Today Proc. 2020, 28, 907–911. [Google Scholar] [CrossRef]
- Toptan, F.; Alves, A.C.; Carvalho, Ó.; Bartolomeu, F.; Pinto, A.M.P.; Silva, F.; Miranda, G. Corrosion and Tribocorrosion Behaviour of Ti6Al4V Produced by Selective Laser Melting and Hot Pressing in Comparison with the Commercial Alloy. J. Mater. Process. Technol. 2019, 266, 239–245. [Google Scholar] [CrossRef]
- Zheng, Z.; Jin, X.; Bai, Y.; Yang, Y.; Ni, C.; Lu, W.F.; Wang, H. Microstructure and Anisotropic Mechanical Properties of Selective Laser Melted Ti6Al4V Alloy under Different Scanning Strategies. Mater. Sci. Eng. A 2022, 831, 142236. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alvés, N.; Silva, F.S.; Carvalho, O.; Miranda, G. Wear Behavior of Ti6Al4V Biomedical Alloys Processed by Selective Laser Melting, Hot Pressing and Conventional Casting. Trans. Nonferrous Met. Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Gibson, I.; Awan, U.S.; Ghaderi, A. The Effect of SLM Process Parameters on Density, Hardness, Tensile Strength and Surface Quality of Ti-6Al-4V. Addit. Manuf. 2019, 25, 176–186. [Google Scholar] [CrossRef]
- Karimi, J.; Antonov, M.; Kollo, L.; Prashanth, K.G. Role of Laser Remelting and Heat Treatment in Mechanical and Tribological Properties of Selective Laser Melted Ti6Al4V Alloy. J. Alloys Compd. 2022, 897, 163207. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion Behavior of Selective Laser Melted Ti-6Al-4 V Alloy in NaCl Solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Qian, C.; Xu, H.; Zhong, Q. The Influence of Process Parameters on Corrosion Behavior of Ti6Al4V Alloy Processed by Selective Laser Melting. J. Laser Appl. 2020, 32, 032010. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.Á.; Gay-Escoda, C.; Valmaseda-Castellón, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical Properties and Corrosion Behavior of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting Behavior of SLM 316L Stainless Steel Exposed to Chloride Environments with Different Aggressiveness: Pitting Mechanism Induced by Gas Pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Shukla, A.K.; Balasubramaniam, R.; Bhargava, S. Properties of Passive Film Formed on CP Titanium, Ti–6Al–4V and Ti–13.4Al–29Nb Alloys in Simulated Human Body Conditions. Intermetallics 2005, 13, 631–637. [Google Scholar] [CrossRef]














| Sample | SLM Scanning Speed (mm/s) | Heat Input (W/mm) | Remelting |
|---|---|---|---|
| Ti-20 × 1 | 20 | 15.0 | No |
| Ti-33 × 1 | 33 | 9.1 | No |
| Ti-33 × 2 | 33 | 9.1 per scan | Yes |
| Ti-50 × 1 | 50 | 6.0 | No |
| Ti-50 × 2 | 50 | 6.0 per scan | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedmar, J.; de la Pezuela, J.; Riquelme, A.; Torres, B.; Rams, J. Impact of Remelting in the Microstructure and Corrosion Properties of the Ti6Al4V Fabricated by Selective Laser Melting. Coatings 2022, 12, 284. https://doi.org/10.3390/coatings12020284
Bedmar J, de la Pezuela J, Riquelme A, Torres B, Rams J. Impact of Remelting in the Microstructure and Corrosion Properties of the Ti6Al4V Fabricated by Selective Laser Melting. Coatings. 2022; 12(2):284. https://doi.org/10.3390/coatings12020284
Chicago/Turabian StyleBedmar, Javier, Jorge de la Pezuela, Ainhoa Riquelme, Belén Torres, and Joaquín Rams. 2022. "Impact of Remelting in the Microstructure and Corrosion Properties of the Ti6Al4V Fabricated by Selective Laser Melting" Coatings 12, no. 2: 284. https://doi.org/10.3390/coatings12020284