Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation
Abstract
:1. Introduction
2. Experiments
2.1. Materials
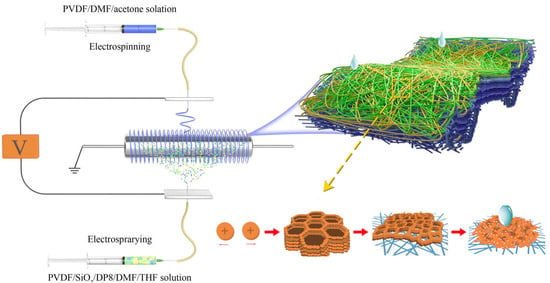
2.2. Preparation of Membranes
2.3. Characterizations
2.4. Oil–Water Separation
3. Results and Discussions
3.1. Preparation of PVDF/DP8/SiO2 Composites
3.2. Thermal Performance Analysis of PVDF/DP8/SiO2 Composite Membranes
3.3. PVDF/DP8/SiO2 Composite Membrane Water Contact Angle Test and Self–Cleaning Ability Test
3.4. Oil–Water Separation Test of PVDF/DP8/SiO2 Composite Membrane and Self–Cleaning Ability Test
3.5. Stability Test of PVDF/DP8/SiO2 Composite Film
3.6. PVDF/DP8/SiO2 Composite Film Surface Appearance and Mechanism Analysis
3.7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, J.; Hao, T.; Wang, Z.; Zhang, Y. A Scalable Method toward Superhydrophilic and Underwater Superoleophobic PVDF Membranes for Effective Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 14896–14904. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Fahmy, H.S.; Ali, G.A.M.; Abdelhamid, H.N.; Ateia, M. Membranes for Oil/Water Separation: A Review. Adv. Mater. Interfaces 2022, 9, 1–36. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic Polymer–Based Membrane for Oily Wastewater Treatment: A Review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, J.; Wang, F.; Li, Z.; Yu, J.; Ding, B. Superhydrophilic and Underwater Superoleophobic Nanofibrous Membrane with Hierarchical Structured Skin for Effective Oil–in–Water Emulsion Separation. J. Mater. Chem. A 2017, 5, 497–502. [Google Scholar] [CrossRef]
- Jernelv, A. The Threats from Oil Spills: Now, Then, and in the Future. Ambio 2010, 39, 353–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and Fabrication of Superwetting Fiber-Based Membranes for Oil/Water Separation Applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, D.; Jiang, L. PAN/PVA Composite Nanofibrous Membranes for Separating Oil-in-Water Emulsion. J. Polym. Res. 2022, 29, 108. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Jiang, L.; Jin, J. Recent Progress in Developing Advanced Membranes for Emulsified Oil/Water Separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation-Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Shen, B.; Du, C.; Wang, W.; Yu, D. Hydrophilic SPE/MPTES–PAN Electrospun Membrane Prepared via Click Chemistry for High Efficiency Oil–Water Separation. J. Mater. Sci. 2022, 57, 1474–1488. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, L.; Zheng, K.; Zhou, S. Blending Electrostatic Spinning Fabrication of Superhydrophilic/Underwater Superoleophobic Polysulfonamide/Polyvinylpyrrolidone Nanofibrous Membranes for Efficient Oil–Water Emulsion Separation. Langmuir 2022, 38, 8241–8251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Du, Z.; Tai, X.; Ma, Y. One–Step Facile Fabrication of Hydrophobic SiO2 Coated Super–Hydrophobic/Super–Oleophilic Mesh via an Improved Stöber Method to Efficient Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126404. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Superwetting Materials of Oil–Water Emulsion Separation. Chem. Lett. 2015, 44, 874–883. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Wei, X.; Liu, K.; Wang, J.; Hu, J.; Lin, J. An Integrated Strategy for Achieving Oil-in-Water Separation, Removal, and Anti–Oil/Dye/Bacteria–Fouling. Chem. Eng. J. 2021, 413, 127493. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Alotaibi, H.F.; Al Thaher, Y.; Perni, S.; Prokopovich, P. Role of Processing Parameters on Surface and Wetting Properties Controlling the Behaviour of Layer–by–Layer Coated Nanoparticles. Curr. Opin. Colloid Interface Sci. 2018, 36, 130–142. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro–Responsive Membranes for Effective Oilg-Water Separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, H.; Tang, N.; Ge, J.; Yu, J.; Ding, B. Publisher Correction: Direct Electronetting of High–Performance Membranes Based on Self-Assembled 2D Nanoarchitectured Networks. Nat. Commun. 2020, 11, 41467. [Google Scholar] [CrossRef] [Green Version]
- Nayak, K.; Tripathi, B.P. Molecularly Grafted PVDF Membranes with In–Air Superamphiphilicity and Underwater Superoleophobicity for Oil/Water Separation. Sep. Purif. Technol. 2021, 259, 118068. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.F. Electrospinning Superhydrophobic–Superoleophilic Fibrous PVDF Membranes for High–Efficiency Water–Oil Separation. Mater. Lett. 2015, 160, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Ngang, H.P.; Ahmad, A.L.; Low, S.C.; Ooi, B.S. Preparation of Thermoresponsive PVDF/SiO2–PNIPAM Mixed Matrix Membrane for Saline Oil Emulsion Separation and Its Cleaning Efficiency. Desalination 2017, 408, 1–12. [Google Scholar] [CrossRef]
- Bae, J.; Kim, H.; Kim, K.S.; Choi, H. Effect of Asymmetric Wettability in Nanofiber Membrane by Electrospinning Technique on Separation of Oil/Water Emulsion. Chemosphere 2018, 204, 235–242. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Cao, L.; Wang, Y.; Li, L.; Li, W. Electrospun PVDF–SiO2 Nanofibrous Membranes with Enhanced Surface Roughness for Oil–Water Coalescence Separation. Sep. Purif. Technol. 2021, 269, 118726. [Google Scholar] [CrossRef]
- Du, C.; Wang, Z.; Liu, G.; Wang, W.; Yu, D. One–Step Electrospinning PVDF/PVP–TiO2 Hydrophilic Nanofiber Membrane with Strong Oil–Water Separation and Anti–Fouling Property. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126790. [Google Scholar] [CrossRef]
- Jadav, G.L.; Singh, P.S. Synthesis of Novel Silica–Polyamide Nanocomposite Membrane with Enhanced Properties. J. Memb. Sci. 2009, 328, 257–267. [Google Scholar] [CrossRef]
- Myronchuk, V.G.; Dzyazko, Y.S.; Zmievskii, Y.G.; Ukrainets, A.I.; Bildukevich, A.V.; Kornienko, L.V.; Rozhdestvenskaya, L.M.; Palchik, A.V. Organic–Inorganic Membranes for Filtration of Corn Distillery. Acta Period. Technol. 2016, 47, 153–165. [Google Scholar] [CrossRef]
- Pang, R.; Li, X.; Li, J.; Lu, Z.; Sun, X.; Wang, L. Preparation and Characterization of ZrO2/PES Hybrid Ultrafiltration Membrane with Uniform ZrO2 Nanoparticles. Desalination 2014, 332, 60–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Zhang, L.; Wang, Y.; Liu, W.; Ma, C.; Liu, S. Porous SiO2 Coated AlxFeyZr1−x−yO2 Solid Superacid Nanoparticles with Negative Charge for Polyvinylidene Fluoride (PVDF) Membrane: Cleaning and Partial Desalinating Seawater. J. Hazard. Mater. 2020, 384, 121471. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Wang, L.; Huang, X.; Xue, H. Flexible Membranes with a Hierarchical Nanofiber/Microsphere Structure for Oil Adsorption and Oil/Water Separation. J. Ind. Eng. Chem. 2018, 68, 416–424. [Google Scholar] [CrossRef]
- Chen, P.C.; Xu, Z.K. Mineral–Coated Polymer Membranes with Superhydrophilicity and Underwater Superoleophobicity for Effective Oil/Water Separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire–Haired Inorganic Membranes with Superhydrophilicity and Underwater Ultralow Adhesive Superoleophobicity for High–Efficiency Oil/Water Separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water–in–Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, X.; Yu, J.; Ding, B. Super Hygroscopic Nanofibrous Membrane–Based Moisture Pump for Solar–Driven Indoor Dehumidification. Nat. Commun. 2020, 11, 3302. [Google Scholar] [CrossRef]
- Tropmann, A.; Tanguy, L.; Koltay, P.; Zengerle, R.; Riegger, L. Completely Superhydrophobic PDMS Surfaces for Microfluidics. Langmuir 2012, 28, 8292–8295. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Cho, Y.K.; Kim, D.H.; Jeong, M.G.; Kim, Y.H.; Kim, Y.D. Hydrophobic Polydimethylsiloxane (PDMS) Coating of Mesoporous Silica and Its Use as a Preconcentrating Agent of Gas Analytes. Langmuir 2014, 30, 10256–10262. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun Fibers for Oil–Water Separation. RSC Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Lee, E.J.; Deka, B.J.; Guo, J.; Woo, Y.C.; Shon, H.K.; An, A.K. Engineering the Re–Entrant Hierarchy and Surface Energy of PDMS–PVDF Membrane for Membrane Distillation Using a Facile and Benign Microsphere Coating. Environ. Sci. Technol. 2017, 51, 10117–10126. [Google Scholar] [CrossRef]
- Jia, W.; Kharraz, J.A.; Choi, P.J.; Guo, J.; Deka, B.J.; An, A.K. Superhydrophobic Membrane by Hierarchically Structured PDMS–POSS Electrospray Coating with Cauliflower–Shaped Beads for Enhanced MD Performance. J. Memb. Sci. 2020, 597, 117638. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Wu, P.; Zhang, S.; Geng, B. Facile Preparation of Superhydrophobic and Superoleophilic Porous Polymer Membranes for Oil/Water Separation from a Polyarylester Polydimethylsiloxane Block Copolymer. J. Mater. Sci. 2016, 51, 3211–3218. [Google Scholar] [CrossRef]
- Luo, S.; Dai, X.; Sui, Y.; Li, P.; Zhang, C. Preparation of Biomimetic Membrane with Hierarchical Structure and Honeycombed Through–Hole for Enhanced Oil–Water Separation Performance. Polymer 2021, 218, 123522. [Google Scholar] [CrossRef]
- Wu, J.; Ding, Y.; Wang, J.; Li, T.; Lin, H.; Wang, J.; Liu, F. Facile Fabrication of Nanofiber- and Micro/Nanosphere-Coordinated PVDF Membrane with Ultrahigh Permeability of Viscous Water-in-Oil Emulsions. J. Mater. Chem. A 2018, 6, 7014–7020. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Xue, H.; Tang, L.; Li, R.K.Y. Facile Preparation of Hybrid Microspheres for Super–Hydrophobic Coating and Oil–Water Separation. Chem. Eng. J. 2017, 326, 443–453. [Google Scholar] [CrossRef]
- Yuan, X.T.; Xu, C.X.; Geng, H.Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO Mixed Matrix Membranes for Ultrafiltration and Fouling Detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef]
- Meringolo, C.; Mastropietro, T.F.; Poerio, T.; Fontananova, E.; De Filpo, G.; Curcio, E.; Di Profio, G. Tailoring PVDF Membranes Surface Topography and Hydrophobicity by a Sustainable Two–Steps Phase Separation Process. ACS Sustain. Chem. Eng. 2018, 6, 10069–10077. [Google Scholar] [CrossRef]
- Abdel–Hakim, A.; El–Basheer, T.M.; Abdelkhalik, A. Mechanical, Acoustical and Flammability Properties of SBR and SBR–PU Foam Layered Structure. Polym. Test. 2020, 88, 106536. [Google Scholar] [CrossRef]
- Abdel–Hakim, A.; El–Mogy, S.A.; EL–Zayat, M.M. Radiation Crosslinking of Acrylic Rubber/Styrene Butadiene Rubber Blends Containing Polyfunctional Monomers. Radiat. Phys. Chem. 2019, 157, 91–96. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Abdel–Hakim, A.; Makhlouf, G.; El–Gamal, A.A. Effect of Iron Poly(Acrylic Acid–Co–Acrylamide) and Melamine Polyphosphate on the Flammability Properties of Linear Low–Density Polyethylene. J. Therm. Anal. Calorim. 2019, 138, 1021–1031. [Google Scholar] [CrossRef]
- Chen, L.; Si, Y.; Zhu, H.; Jiang, T.; Guo, Z. A Study on the Fabrication of Porous PVDF Membranes by In-Situ Elimination and Their Applications in Separating Oil/Water Mixtures and Nano–Emulsions. J. Memb. Sci. 2016, 520, 760–768. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Xu, Z.; Li, J.; Pan, Z.; Du, Z.; Cheng, F. Preparation of Omniphobic PVDF Membranes with Silica Nanoparticles for Treating Coking Wastewater Using Direct Contact Membrane Distillation: Electrostatic Adsorption vs. Chemical Bonding. J. Memb. Sci. 2019, 574, 349–357. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Y.; Shen, L.; Li, R.; Lin, H. Preparation of Nickel@polyvinyl Alcohol (PVA) Conductive Membranes to Couple a Novel Electrocoagulation–Membrane Separation System for Efficient Oil–Water Separation. J. Memb. Sci. 2022, 653, 120541. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Gan, Z.Q.; Bao, R.Y.; Ke, K.; Liu, Z.Y.; Yang, M.B.; Yang, W. Green and Robust Superhydrophilic Electrospun Stereocomplex Polylactide Membranes: Multifunctional Oil/Water Separation and Self–Cleaning. J. Memb. Sci. 2020, 593, 117420. [Google Scholar] [CrossRef]
- Jabbarnia, A.; Khan, W.S.; Ghazinezami, A.; Asmatulu, R. Investigating the Thermal, Mechanical, and Electrochemical Properties of PVdF/PVP Nanofibrous Membranes for Supercapacitor Applications. J. Appl. Polym. Sci. 2016, 133, 43707. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Zhang, F.; Xing, T.; Jin, J. Superhydrophilic In–Situ–Cross–Linked Zwitterionic Polyelectrolyte/PVDF–Blend Membrane for Highly Efficient Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, X.; Wang, D.; Li, J.; Jiang, L.; Jin, J. Salt–Induced Fabrication of Superhydrophilic and Underwater Superoleophobic PAA–g–PVDF Membranes for Effective Separation of Oil–in–Water Emulsions. Angew. Chem. Int. Ed. 2014, 53, 856–860. [Google Scholar] [CrossRef]
- Xiang, Y.; Shen, J.; Wang, Y.; Liu, F.; Xue, L. A PH–Responsive PVDF Membrane with Superwetting Properties for the Separation of Oil and Water. RSC Adv. 2015, 5, 23530–23539. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yang, Y.; Luo, S.; Cheng, C.; Wang, S.; Liu, B. Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation. Coatings 2022, 12, 1698. https://doi.org/10.3390/coatings12111698
Zhang C, Yang Y, Luo S, Cheng C, Wang S, Liu B. Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation. Coatings. 2022; 12(11):1698. https://doi.org/10.3390/coatings12111698
Chicago/Turabian StyleZhang, Chunling, Yichen Yang, Shuai Luo, Chunzu Cheng, Shuli Wang, and Bo Liu. 2022. "Fabrication of Superhydrophobic Composite Membranes with Honeycomb Porous Structure for Oil/Water Separation" Coatings 12, no. 11: 1698. https://doi.org/10.3390/coatings12111698