Characterization and Tribological Behavior of Electroless-Deposited Ni-P-PTFE Films on NBR Substrates for Dynamic Contact Applications
Abstract
:1. Introduction
2. Materials and Methods
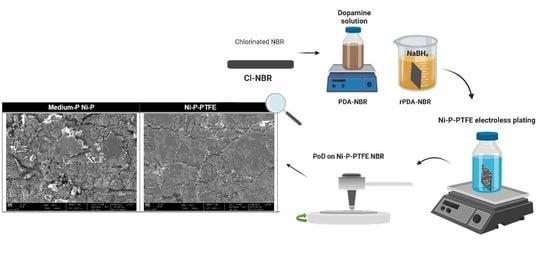
2.1. Surface Pre-Treatment and Activation
2.2. Electroless Ni-P-PTFE Alloy Plating
2.3. Characterization
2.3.1. Wettability and Chemical Composition
2.3.2. Morphology and Crystalline Structure
2.3.3. Mechanical Properties
2.4. Tribological Behavior
3. Results
3.1. Surface Morphology
3.2. Influence of Cationic Surfactant CTAB
3.3. Coating Composition and Plating Rate
3.4. Crystalline Structure and Adhesion
3.5. Roughness and Wettability
3.6. Frictional Behavior
3.7. Wear Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flitney, R.K. Seals and Sealing Handbook, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Tiwari, A.; Miyashita, N.; Espallargas, N.; Persson, B.N.J. Rubber friction: The contribution from the area of real contact. J. Chem. Phys. 2018, 148, 224701. [Google Scholar] [CrossRef] [PubMed]
- Muhr, A.H.; Roberts, A.D. Rubber abrasion and wear. Wear 1992, 158, 213–228. [Google Scholar] [CrossRef]
- Martinez-Martinez, D.; De Hosson, J.T.M. On the deposition and properties of DLC protective coatings on elastomers: A critical review. Surf. Coat. Tech. 2014, 258, 677–690. [Google Scholar] [CrossRef]
- Bai, C.; Gong, Z.; An, L.; Qiang, L.; Zhang, J.; Yushkov, G.; Nikolaev, A.; Shandrikov, M.; Zhang, B. Adhesion and friction performance of DLC/rubber: The influence of plasma pretreatment. Friction 2021, 9, 627–641. [Google Scholar] [CrossRef]
- Lubwama, M.; Corcoran, B.; McDonnell, K.A.; Dowling, D.; Kirabira, J.B.; Sebbit, A.; Sayers, K. Flexibility and frictional behaviour of DLC and Si-DLC films deposited on nitrile rubber. Surf. Coat. Tech. 2014, 239, 84–94. [Google Scholar] [CrossRef]
- Lubwama, M.; Corcoran, B.; Sayers, K. DLC films deposited on rubber substrates: A review. Surf. Eng. 2015, 31, 1–10. [Google Scholar] [CrossRef]
- Martínez, L.; Nevshupa, R.; Álvarez, L.; Huttel, Y.; Méndez, J.; Román, E.; Mozas, E.; Valdés, J.R.; Jimenez, M.A.; Gachon, Y.; et al. Application of diamond-like carbon coatings to elastomers frictional surfaces. Tribol. Int. 2009, 42, 584–590. [Google Scholar] [CrossRef]
- Pei, Y.T.; Bui, X.L.; De Hosson, J.T.M. Flexible protective diamond-like carbon film on rubber. Scr. Mater. 2010, 63, 649–652. [Google Scholar] [CrossRef]
- Pei, Y.T.; Bui, X.L.; van der Pal, J.P.; Martinez-Martinez, D.; De Hosson, J.T.M. Flexible diamond-like carbon film coated on rubber. Prog. Org. Coat. 2013, 76, 1773–1778. [Google Scholar] [CrossRef]
- Bui, X.L.; Pei, Y.T.; Mulder, E.D.G.; De Hosson, J.T.M. Adhesion improvement of hydrogenated diamond-like carbon thin films by pre-deposition plasma treatment of rubber substrate. Surf. Coat. Tech. 2009, 203, 1964–1970. [Google Scholar] [CrossRef] [Green Version]
- Lubwama, M.; Corcoran, B.; Sayers, K.; Kirabira, J.B.; Sebbit, A.; McDonnell, K.A.; Dowling, D. Adhesion and composite micro-hardness of DLC and Si-DLC films deposited on nitrile rubber. Surf. Coat. Tech. 2012, 206, 4881–4886. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of electroless nickel coatings—A review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Vasconcelos, B.; Serra, R.; Oliveira, J.C.; Fonseca, C. Durable electroless deposited Ni-P films on NBR for dynamic contacts. Characterization and tribological performance. Surf. Coat. Tech. 2021, 423, 127579. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, L.; Ren, L.; Liu, J. Electroless Plating of Ni-P and Ni-P-PTFE on Micro-Arc Oxidation Coatings for Improved Tribological Performance. Mat. Res. 2022, 25, e20220096. [Google Scholar] [CrossRef]
- Yanhai, C.; Lu, R.; Xianliang, M.; Jinyong, Y.; Xianhua, T. The effect of PTFE addition on mechanical and anti-corrosion properties of coating of heat exchangers. Mater. Res. Express 2019, 6, 085207. [Google Scholar] [CrossRef]
- Unal, H.; Mimaroglu, A.; Kadıoglu, U.; Ekiz, H. Sliding friction and wear behaviour of polytetrafluoroethylene and its composites under dry conditions. Mater. Des. 2004, 25, 239–245. [Google Scholar] [CrossRef]
- Pancrecious, J.K.; Ulaeto, S.B.; Ramya, R.; Rajan, T.P.D.; Pai, B. Metallic composite coatings by electroless technique—A critical review. Int. Mater. Rev. 2018, 63, 488–512. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Song, L.; Liang, L. Simultaneous friction and wear reduction of Ni-P/PTFE composites under dry sliding condition. Ind. Lub. Tribol. 2021, 73, 581–587. [Google Scholar] [CrossRef]
- Ankita, S.; Singh, A.K. Corrosion and wear resistance study of Ni-P and Ni-P-PTFE nanocomposite coatings. Cent. Eur. J. Eng. 2011, 1, 234–243. [Google Scholar] [CrossRef]
- Yanhai, C.; Hengyang, C.; Dongtai, H.; Yong, Z.; Zhencai, Z. Effect of PTFE addition on the properties of electroless Ni-Cu-P-PTFE deposits. Rare Met. Mater. Eng. 2014, 43, 1025–1030. [Google Scholar] [CrossRef]
- Hadipour, A.; Rahsepar, M.; Hayatdavoudi, H. Fabrication and characterisation of functionally graded Ni-P coatings with improved wear and corrosion resistance. Surf. Eng. 2019, 35, 883–890. [Google Scholar] [CrossRef]
- Mafi, I.R.; Dehghanian, C. Comparison of the coating properties and corrosion rates in electroless Ni–P/PTFE composites prepared by different types of surfactants. Appl. Surf. Sci. 2011, 257, 8653–8658. [Google Scholar] [CrossRef]
- Ger, M.D.; Hwang, B.J. Effect of surfactants on codeposition of PTFE particles with electroless Ni-P coating. Mater. Chem. Phys. 2002, 76, 38–45. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Müller-Steinhagen, H.; Liu, G. Graded Ni–P–PTFE coatings and their potential applications. Surf. Coat. Tech. 2002, 155, 279–284. [Google Scholar] [CrossRef]
- Elansezhian, R.; Ramamoorthy, B.; Nair, P.K. The influence of SDS and CTAB surfactants on the surface morphology and surface topography of electroless Ni–P deposits. J. Mater. Process. Technol. 2009, 209, 233–240. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.S.; Jiang, Q.; Jiang, Z.H.; Li, G.Y.; Elansezhian, R. The performance of surfactant on the surface characteristics of electroless nickel coating on magnesium alloy. Prog. Org. Coat. 2012, 74, 788–793. [Google Scholar] [CrossRef]
- ASTM International Web Site. Available online: https://www.astm.org/d4541-22.html (accessed on 24 August 2022).
- Ger, M.D.; Hou, K.H.; Hwang, B.J. Transient phenomena of the codeposition of PTFE with electroless Ni–P coating at the early stage. Mater. Chem. Phys. 2004, 87, 102–108. [Google Scholar] [CrossRef]
- Fransaer, J.; Celis, J.P.; Roos, J.R. Analysis of the electrolytic codeposition of non-brownian particles with metals. J. Electrochem. Soc. 1992, 139, 413. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, J.; Shen, B.; Liu, L.; Hu, W.; Ding, W. Process and properties of electroless Ni-P-PTFE composite coatings. Plat. Surf. Finish. 2005, 92, 38–42. [Google Scholar]
- Lee, H.B.; Chen, K.-L.; Su, J.W.; Lee, C.-Y. The use of surfactants and supercritical CO2 assisted processes in the electroless nickel plating of printed circuit board with blind via. Mater. Chem. Phys. 2020, 241, 122418. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V. Electroless alloy/composite coatings: A review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Wang, J.; Tian, J.; Liu, X.; Yin, Y.; Wang, X. Effect of polytetrafluoroethylene content on electrochemical anticorrosion behaviors of electroless deposited Ni–P and Ni–P–polytetrafluoroethylene coatings in seawater. Thin Solid Film. 2011, 519, 5905–5911. [Google Scholar] [CrossRef]
- Suiyuan, C.; Ying, S.; Hong, F.; Jing, L.; Changsheng, L.; Kai, S. Synthesis of Ni-P-PTFE-nano-Al2O3 composite plating coating on 45 steel by electroless plating. J. Compos. Mater. 2012, 46, 1405–1416. [Google Scholar] [CrossRef]
- Rahmati, H.; Mahboobi, F. Studying the effect of the concentration of PTFE nanoparticles on the tribological behavior of Ni-P-PTFE composite coatings. Iran. J. Oil Gas Sci. Technol. 2015, 4, 67–75. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yao, M. Effect of electroless nickel plating on fatigue strength of 30CrMoA steel. Heat Treat. 1995, 540, 39. [Google Scholar]
- Mallory, G.O.; Altura, D. The Effect of Stress on the Properties of Electroless Nickel—Phosphorus Deposits. SAE Trans. 1983, 92, 1–9. [Google Scholar]
- Wang, W.; Zhang, W.; Wang, Y.; Mitsuzak, N.; Chen, Z. Ductile electroless Ni–P coating onto flexible printed circuit board. Appl. Surf. Sci. 2016, 367, 528–532. [Google Scholar] [CrossRef]
- Veronesi, P.; Sola, R.; Poli, G. Electroless Ni coatings for the improvement of wear resistance of bearings for lightweight rotary gear pumps. Int. J. Surf. Sci. Eng. 2008, 2, 190–201. [Google Scholar] [CrossRef]
- Gao, P.P.; Gao, M.L.; Wu, A.R.; Wu, X.B.; Liu, C.X.; Zhang, Y.; Zhou, H.K.; Peng, X.M.; Xie, Z.Y. Electrochemical characteristics of electroplating and impregnation Ni-P/SiC/PTFE composite coating on 316L stainless steel. J. Cent. South Univ. 2020, 27, 3615–3624. [Google Scholar] [CrossRef]
- You, Y.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Electrochemical preparation and characterization of Ni-PTFE composite coatings from a non-aqueous solution without additives. Int. J. Electrochem. Sci. 2012, 7, 12440–12455. [Google Scholar]
- Wu, Y.; Liu, H.; Shen, B.; Liu, L.; Hu, W. The friction and wear of electroless Ni–P matrix with PTFE and/or SiC particles composite. Tribol. Int. 2006, 39, 553–559. [Google Scholar] [CrossRef]
- Straffelini, G.; Colombo, D.; Molinari, A. Surface durability of electroless Ni–P composite deposits. Wear 1999, 236, 179–188. [Google Scholar] [CrossRef]
- Yang, Z.D.; Wu, D.; Liu, M.F. Electroless Ni-P-PTFE Composite Coatings on Titanium Alloy and Their Tribological Properties. Adv. Mater.Res. 2011, 291, 12–17. [Google Scholar] [CrossRef]
- Yuan, X.D.; Yang, X.J. A study on friction and wear properties of PTFE coatings under vacuum conditions. Wear 2010, 269, 291–297. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.; Shen, B.; Hu, W. Study of self-lubricant Ni-P-PTFE-SiC composite coating. J. Mater. Sci. 2005, 40, 5057–5059. [Google Scholar] [CrossRef]
- Ramalho, A.; Miranda, J.C. Friction and wear of electroless NiP and NiP+PTFE coatings. Wear 2005, 259, 828–834. [Google Scholar] [CrossRef]
- Xu, D.; Li, N.; Ma, J.J.; Cheng, G.M.; Wang, L.L. Tribological properties and wear mechanism of self-lubricating Ni-P/PTFE. Appl. Mech. Mater. 2012, 229, 134–137. [Google Scholar] [CrossRef]
- Jappes, J.T.W.; Ramamoorthy, B.; Nair, P.K. A study on the influence of process parameters on efficiency and crystallinity of electroless Ni–P deposits. J. Mater. Process. Technol. 2005, 169, 308–313. [Google Scholar] [CrossRef]
- Wang, L.Y.; Tu, J.P.; Chen, W.X.; Wang, Y.C.; Liu, X.K.; Olk, C.; Cheng, D.H.; Zhang, X.B. Friction and wear behavior of electroless Ni-based CNT composite coatings. Wear 2003, 254, 1289–1293. [Google Scholar] [CrossRef]
- Kato, K. Wear in relation to friction—A review. Wear 2000, 241, 151–157. [Google Scholar] [CrossRef]
- Der Ger, M.; Hou, K.H.; Wang, L.M.; Hwang, B.J. The friction and wear of Ni–P–PTFE composite deposits under water lubrication. Mater. Chem. Phys. 2003, 77, 755–764. [Google Scholar] [CrossRef]







| Composition and Operating Conditions | |
|---|---|
| NiSO4·6H2O (g/L) | 40 |
| C6H5Na3O7·2H2O (g/L) | 20 |
| NaH2PO2·H2O (g/L) | 40 |
| C2H6BN (DMAB, g/L) PTFE (mL/L) CTAB (mg/L) | 2 0, 5, 7, 10 0, 200, 500, 800 |
| Temperature (°C) | 60 ± 3 |
| pH (adjusted with 3% H2SO4) | 5.0 ± 0.2 |
| Duration (min) | 60 |
| NiSO4·6H2O (g/L) | 40 |
| C6H5Na3O7·2H2O (g/L) | 20 |
| Parameter | Ni-P | Ni-P-PTFE (5_2) | Ni-P-PTFE (7_8) |
|---|---|---|---|
| Adhesion (MPa) | 1.3 ± 0.1 | 1.6 ± 0.2 | 1.5 ± 0.1 |
| Parameter | Ni-P | Ni-P-PTFE (5_2) | Ni-P-PTFE (7_8) |
|---|---|---|---|
| Ra (µm) | 3.15 | 2.41 | 4.20 |
| C.A. (°) | 70 ± 6 | 136 ± 4 | 131 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, B.; Serra, R.; Oliveira, J.; Fonseca, C. Characterization and Tribological Behavior of Electroless-Deposited Ni-P-PTFE Films on NBR Substrates for Dynamic Contact Applications. Coatings 2022, 12, 1410. https://doi.org/10.3390/coatings12101410
Vasconcelos B, Serra R, Oliveira J, Fonseca C. Characterization and Tribological Behavior of Electroless-Deposited Ni-P-PTFE Films on NBR Substrates for Dynamic Contact Applications. Coatings. 2022; 12(10):1410. https://doi.org/10.3390/coatings12101410
Chicago/Turabian StyleVasconcelos, Beatriz, Ricardo Serra, João Oliveira, and Carlos Fonseca. 2022. "Characterization and Tribological Behavior of Electroless-Deposited Ni-P-PTFE Films on NBR Substrates for Dynamic Contact Applications" Coatings 12, no. 10: 1410. https://doi.org/10.3390/coatings12101410