Facing Resistant Bacteria with Plant Essential Oils: Reviewing the Oregano Case
Abstract
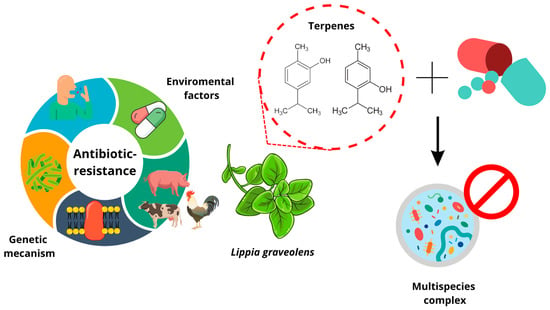
:1. Introduction
2. Antibiotic Bacterial Resistance: A Serious and Multidisciplinary Problem
2.1. Consequences of Bacterial Resistance
2.2. Factors Causing Bacterial Resistance to Antibiotics
3. Multispecies Challenge Involved in Antimicrobial Testing
4. Efficacy Loss of Conventional Antibiotics and Proposed Solutions
| Compounds | Species | Antibacterial Evaluation | Results | References |
|---|---|---|---|---|
| Carvacrol Cinnamaldehyde | A. baumannii | Gene expression | MIC Carvacrol-Cinnamaldehyde: 0.16 mg/mL | [68] |
| groES, groEL, dnaK: overexpression 3.9–5.1-fold. | ||||
| clp B, kat E: overexpression 26-fold, 20-fold | ||||
| EO T. vulgaris EO L. graveolens | S. enterica | Inhibition zone MIC/MBC | >20 mm 0.5–1 mg/mL | [7] |
| EO Lippia alba Citral | Staphylococcus aureus | Inhibition of biofilm | EO L. alba: 1 mg/mL | [69] |
| data | Citral: 0.5 mg/mL | |||
| EO L. berlandieri | P. aeruginosa | Inhibition of biofilm | 250–600 mg/L | [9] |
| Salmonella Typhimurium | ||||
| trans-Cinnamaldehyde Eugenol Antibiotics β-lactams and monobactams. | A. baumannii | Efflux pumps and resistance gene expression | MIC: 4 mM Supression of AdeABC efflux pump. Genes adeA and adeB: downregulated 3–14-fold Genes blaP, mdrp: downregulated 3-fold | [10] |
| EO Lavandula pubescens Carvacrol | Acinetobacter baumannii, Salmonella typhimurium, Shigella sonnei, Enterococcus faecalis y Staphylococcus epidermidis | MIC MBC Inhibition zone | MIC EO: 78–312 µg/mL MBC EO: 156–625 µg/mL MIC carvacrol: 250–500 µg/mL MBC carvacrol: 500–1000 µg/mL Inhibition zone 12–24 mm | [8] |
| EO Ocimum basilicum EO Thymus capitatus EO Melaleuca alternifolia EO Thymus vulgaris | A. baumannii, E. coli, K. pneumoniae, P. aeruginosa | MIC MBC | Melaleuca alternifolia: 0.12–1.50 (%v/v) Thymus capitatus y Thymus vulgaris: 0.5–>4 (%v/v) Ocimum basilicum: >4 (%v/v) | [63] |
| Capsaicin Colistin | A. baumannii | Synergism Gene regulation | FICI = 0.03–0.06 Increase 271 genes Decrease 327 genes Inhibition efflux pumps | [11] |
| Polymyxin B EO Origanum vulgare | A. baumannii | MIC Synergism | MIC = 1.75–3.50 mg/mL FICI = 0.18–0.37 | [12] |
| Carvacrol Meropenem | K. pneumoniae | MIC Synergism | MIC = 32–128 µg/mL FICI = 0.5 | [13] |
| EO Origanum vulgare | A. baumannii P. aeruginosa | MIC | 13.78–62.5 µL/mL | [64] |
| Extract of Origanum vulgare | P. aeruginosa | MIC | 6.3–25 µg/mL | [65] |
| EO L. graveolens | P. fragi Salmonella sp. | Inhibition zone | 24.82–24.95 mm 24.85–29.23 mm | [66] |
5. Antibacterial Capacity of L. graveolens and O. vulgare
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giono-Cerezo, S.; Santos-Preciado, J.I.; Morfín-Otero, M.D.R.; Torres-López, F.J.; Alcántar-Curiel, M.D. Resistencia antimicrobiana. Importancia y esfuerzos por contenerla. Gac. Médica México 2021, 156, 172–180. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Serra-Valdés, M.Á. La resistencian MICrobiana en el contexto actual y la importancia del conocimiento y aplicación en la política antimicrobiana. Rev. Habanera Cienc. Médicas 2017, 16, 402–419. [Google Scholar]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s Not Easy Being Green: A Narrative Review on the Microbiology, Virulence and Therapeutic Prospects of Multidrug-Resistant Pseudomonas Aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- CDC. Infographic: Antibiotic Resistance the Global Threat. Available online: https://www.cdc.gov/globalhealth/infographics/antibiotic-resistance/antibiotic_resistance_global_threat.htm (accessed on 30 April 2022).
- CDC. Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services: Atlanta, GA, USA, 2019; p. 150.
- Rubio-Ortega, A.; Travieso-Novelles, M.D.C.; Riverón-Alemán, Y.; Martínez-Vasallo, A.; Peña-Rodríguez, J.; Espinosa-Castaño, I.; Pino-Pérez, O. Actividad antibacteriana de aceites esenciales de plantas cultivadas en Cuba sobre cepas de Salmonella Enterica. Rev. Salud Anim. 2018, 40, 1–10. [Google Scholar]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential Oil Analysis and Antimicrobial Evaluation of Three Aromatic Plant Species Growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Hernández-Carranza, P.; Ochoa-Velasco, C.E.; Avila-Sosa, R. Inhibitory Effect of Mexican Oregano (Lippia berlandieri Schauer) Essential Oil on Pseudomonas aeruginosa and Salmonella Thyphimurium Biofilm Formation. Front. Sustain. Food Syst. 2020, 4, 36. [Google Scholar] [CrossRef]
- Karumathil, D.P.; Nair, M.S.; Gaffney, J.; Kollanoor-Johny, A.; Venkitanarayanan, K. Trans-Cinnamaldehyde and Eugenol Increase Acinetobacter baumannii Sensitivity to Beta-Lactam Antibiotics. Front. Microbiol. 2018, 9, 1011. [Google Scholar] [CrossRef]
- Guo, T.; Li, M.; Sun, X.; Wang, Y.; Yang, L.; Jiao, H.; Li, G. Synergistic Activity of Capsaicin and Colistin Against Colistin-Resistant Acinetobacter baumannii: In Vitro/Vivo Efficacy and Mode of Action. Front. Pharmacol. 2021, 12, 744494. [Google Scholar] [CrossRef]
- Amaral, S.C.; Pruski, B.B.; de Freitas, S.B.; Allend, S.O.; Ferreira, M.R.A.; Moreira, C., Jr.; Pereira, D.I.B.; Junior, A.S.V.; Hartwig, D.D. Origanum vulgare essential oil: Antibacterial activities and synergistic effect with polymyxin B against multidrug-resistant Acinetobacter Baumannii. Mol. Biol. Rep. 2020, 47, 9615–9625. [Google Scholar] [CrossRef]
- Elif, O.-K. In vitro activity of carvacrol in combination with meropenem against carbapenem-resistant Klebsiella pneumoniae. Folia Microbiol. 2022, 67, 143–156. [Google Scholar] [CrossRef]
- Chan, A.P.; Choi, Y.; Brinkac, L.M.; Krishnakumar, R.; DePew, J.; Kim, M.; Hinkle, M.K.; Lesho, E.P.; Fouts, D.E. Multidrug resistant pathogens respond differently to the presence of co-pathogen, commensal, probiotic and host cells. Sci. Rep. 2018, 8, 8656. [Google Scholar] [CrossRef]
- Centros-Para-el-Control-y-la-Prevención-de-Enfermedades. Preguntas y Respuestas Sobre el uso de Antibióticos. Available online: https://www.cdc.gov/antibiotic-use/sp/should-know.html (accessed on 30 April 2022).
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 30 April 2022).
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Buelow, E.; Ploy, M.-C.; Dagot, C. Role of pollution on the selection of antibiotic resistance and bacterial pathogens in the environment. Curr. Opin. Microbiol. 2021, 64, 117–124. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Shortage of Innovative Antibiotics Fuels Emergence and Spread of Drug-Resistance. Available online: https://www.who.int/es/news/item/15-04-2021-global-shortage-of-innovative-antibiotics-fuels-emergence-and-spread-of-drug-resistance (accessed on 30 April 2022).
- Centros-Para-el-Control-y-la-Prevención-de-Enfermedades-(CDC). Biggest Threats and Data: 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 30 April 2022).
- World Health Organization. WHO Publishes List of Bacteria Urgently Needed for New Antibiotics. Available online: https://www.who.int/es/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 April 2022).
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef] [PubMed]
- Marturano, J.E.; Lowery, T.J. ESKAPE Pathogens in Bloodstream Infections Are Associated With Higher Cost and Mortality but Can Be Predicted Using Diagnoses Upon Admission. Open Forum Infect. Dis. 2019, 6, ofz503. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, J.; Yang, J.; Liu, S.; Ding, Z.; Hao, J.; Ding, Y.; Zeng, Z.; Liu, J. Pathogenic Characteristics and Risk Factors for ESKAPE Pathogens Infection in Burn Patients. Infect. Drug Resist. 2021, 14, 4727–4738. [Google Scholar] [CrossRef]
- Sosa-Hernández, Ó.; Matías-Téllez, B.; González-Martínez, J.; Juárez-Vargas, R.; Estrada-Hernández, A.; Sánchez-Rivas, M.P.; Cureño-Díaz, M.A. Infecciones asociadas a la atención de la salud por bacterias del grupo ESKAPE en un hospital de la Ciudad de México 2013–2017. Enferm. Infecc. Y Microbiol. 2019, 39, 59–64. [Google Scholar]
- Llaca-Díaz, J.M.; Mendoza-Olazarán, S.; Camacho-Ortiz, A.; Flores, S.; Garza-González, E. One-year surveillance of ESKAPE pathogens in an intensive care unit of Monterrey, Mexico. Chemotherapy 2012, 58, 475–481. [Google Scholar] [CrossRef]
- Enciso-Martínez, Y.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; González-Pérez, C.J.; Valencia-Rivera, D.E.; Barrios-Villa, E.; Ayala-Zavala, J.F. Relevance of tracking the diversity of Escherichia coli pathotypes to reinforce food safety. Int. J. Food Microbiol. 2022, 374, 109736. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The Mechanisms of Disease Caused by Acinetobacter Baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Hu, R. Risk factors for pneumonia caused by antimicrobial drug-resistant or drug-sensitive Acinetobacter baumannii infections: A retrospective study. Medicine 2020, 99, e21051. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Zahreddine, N.; Tayyar, R.; Sfeir, J.; Araj, G.F.; Matar, G.M.; Kanj, S.S. Multi-drug resistant Acinetobacter species: A seven-year experience from a tertiary care center in Lebanon. Antimicrob. Resist. Infect. Control 2018, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Čiginskienė, A.; Dambrauskienė, A.; Rello, J.; Adukauskienė, D. Ventilator-Associated Pneumonia due to Drug-Resistant Acinetobacter baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of In-Hospital Mortality. Medicina 2019, 55, 49. [Google Scholar] [CrossRef] [Green Version]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Naciones Unidas. La Resistencia a los Antibióticos Supone un Riesgo Para las Personas, los Animales y el medio Ambiente. Available online: https://news.un.org/es/story/2021/04/1491502 (accessed on 30 April 2022).
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/es/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 30 April 2022).
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Kurihara, M.N.L.; Sales, R.O.D.; Silva, K.E.D.; Maciel, W.G.; Simionatto, S. Multidrug-resistant Acinetobacter baumannii outbreaks: A global problem in healthcare settings. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200248. [Google Scholar] [CrossRef]
- Maglangit, F.; Fang, Q.; Kyeremeh, K.; Sternberg, J.M.; Ebel, R.; Deng, H. A Co-Culturing Approach Enables Discovery and Biosynthesis of a Bioactive Indole Alkaloid Metabolite. Molecules 2020, 25, 256. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.-H.; Cao, H.; Cai, P.; Sørensen, S.J. The initial inoculation ratio regulates bacterial coculture interactions and metabolic capacity. ISME J. 2021, 15, 29–40. [Google Scholar] [CrossRef]
- Khan, N.; Maezato, Y.; McClure, R.S.; Brislawn, C.J.; Mobberley, J.M.; Isern, N.; Chrisler, W.B.; Markillie, L.M.; Barney, B.M.; Song, H.-S.; et al. Phenotypic responses to interspecies competition and commensalism in a naturally-derived microbial co-culture. Sci. Rep. 2018, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Cendra, M.D.M.; Blanco-Cabra, N.; Pedraz, L.; Torrents, E. Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci. Rep. 2019, 9, 16284. [Google Scholar] [CrossRef]
- Barraza, J.P.; Whiteley, M. A Pseudomonas aeruginosa Antimicrobial Affects the Biogeography but Not Fitness of Staphylococcus aureus during Coculture. mBio 2021, 12, e00047-21. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Chandad, F.; Rébillard, A.; Cillard, J.; Bonnaure-Mallet, M. Silver-zeolite combined to polyphenol-rich extracts of Ascophyllum nodosum: Potential active role in prevention of periodontal diseases. PLoS ONE 2014, 9, e105475. [Google Scholar] [CrossRef]
- Boulanger, S.; Mitchell, G.; Bouarab, K.; Marsault, É.; Cantin, A.; Frost, E.H.; Déziel, E.; Malouin, F. Bactericidal Effect of Tomatidine-Tobramycin Combination against Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Is Enhanced by Interspecific Small-Molecule Interactions. Antimicrob. Agents Chemother. 2015, 59, 7458–7464. [Google Scholar] [CrossRef] [Green Version]
- Medell-Gago, M.; Hart-Casares, M.; Mora-Diaz, I. Acinetobacter baumannii versus Pseudomonas aeruginosa. Comportamiento en pacientes críticos con ventilación mecánica. Rev. Cuba. Med. 2012, 51, 239–246. [Google Scholar]
- Leontyev, A.E.; Pavlenko, I.V.; Kovalishena, O.V.; Saperkin, N.V.; Tulupov, A.A.; Beschastnov, V.V. Application of Phagotherapy in the Treatment of Burn Patients (Review). Sovrem. Tehnol. Med. 2021, 12, 95–103. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Wang, L.; Li, G.; Cong, C.; Li, R.; Cui, H.; Murtaza, B.; Xu, Y. The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microb. Biotechnol. 2021, 14, 403–418. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter Baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef]
- Cárdenas, J.; Castillo, O.; De-Cámara, C.; González, V. Combatiendo la resistencia bacteriana: Una revisión sobre las terapias alternas a los antibióticos convencionales. Bol. Venez. Infectol. 2018, 29, 9. [Google Scholar]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Basavegowda, N.; Patra, J.K.; Baek, K.-H. Essential Oils and Mono/bi/tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef] [Green Version]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. -Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Roy, R.; Tiwari, M. Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Front. Microbiol. 2015, 6, 618. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A.A.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Synergistic mode of action of catechin, vanillic and protocatechuic acids to inhibit the adhesion of uropathogenic Escherichia coli on silicone surfaces. J. Appl. Microbiol. 2020, 128, 387–400. [Google Scholar] [CrossRef]
- Cruz-Valenzuela, M.R.; Ayala-Soto, R.E.; Ayala-Zavala, J.F.; Espinoza-Silva, B.A.; González-Aguilar, G.A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Nazzaro, F.; Fratianni, F.; Tapia-Rodríguez, M.R.; et al. Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts. Foods 2022, 11, 2588. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Diaz-Arenas, G.L.; Agudelo, L.P.A.; Stashenko, E.; Contreras-Castillo, C.J.; da Gloria, E.M. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules 2021, 26, 2888. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Siddique, H.; Pendry, B.; Rahman, M.M. Terpenes from Zingiber montanum and Their Screening against Multi-Drug Resistant and Methicillin Resistant Staphylococcus Aureus. Molecules 2019, 24, 385. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, H.; Gousia, P.; Economou, V.; Sakkas, V.; Petsios, S.; Papadopoulou, C. In vitro antimicrobial activity of five essential oils on multidrug resistant Gram-negative clinical isolates. J. Intercult. Ethnopharmacol. 2016, 5, 212–218. [Google Scholar] [CrossRef]
- Jan, S.; Rashid, M.; Abd Allah, E.F.; Ahmad, P. Biological Efficacy of Essential Oils and Plant Extracts of Cultivated and Wild Ecotypes of Origanum vulgare L. BioMed Res. Int. 2020, 2020, 8751718. [Google Scholar] [CrossRef]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum vulgare Extract and Its Major Constituents. Oxidative Med. Cell. Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican (Lippia graveolens H.B.K.) and European (Origanum vulgare L.) oregano essential oils. Sci. World J. 2014, 2014, 641814. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Montagu, A.; Joly-Guillou, M.-L.; Rossines, E.; Cayon, J.; Kempf, M.; Saulnier, P. Stress Conditions Induced by Carvacrol and Cinnamaldehyde on Acinetobacter Baumannii. Front. Microbiol. 2016, 7, 1133. [Google Scholar] [CrossRef] [Green Version]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; de Carvalho, M.G.; Costa, R.A.; Júnior, F.E.A.C. In Vitro Antibacterial and Antibiofilm Activity of Lippia alba Essential Oil, Citral, and Carvone against Staphylococcus Aureus. Sci. World J. 2017, 2017, 4962707. [Google Scholar] [CrossRef] [Green Version]
- Moo, C.L.; Osman, M.A.; Yang, S.K.; Yap, W.S.; Ismail, S.; Lim, S.H.; Chong, C.M.; Lai, K.S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef] [PubMed]
- Díaz-De León, C.I.; González-Álvarez, M.; Guzmán-Lucio, M.A.; Núñez-Guzmán, G.R.; Moreno-Limón, S. El orégano de los géneros Lippia (Verbenaceae) y Poliomintha (Lamiaceae) en el Estado de Nuevo León, México. Polibotánica 2020, 50, 1–18. [Google Scholar] [CrossRef]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran. J. Pharm. Res. IJPR 2021, 20, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Didry, N.; Dubreuil, L.; Pinkas, M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm. Acta Helv. 1994, 69, 25–28. [Google Scholar] [CrossRef]
- Grande, R.; Carradori, S.; Puca, V.; Vitale, I.; Angeli, A.; Nocentini, A.; Bonardi, A.; Gratteri, P.; Lanuti, P.; Bologna, G.; et al. Selective Inhibition of Helicobacter pylori Carbonic Anhydrases by Carvacrol and Thymol Could Impair Biofilm Production and the Release of Outer Membrane Vesicles. Int. J. Mol. Sci. 2021, 22, 11583. [Google Scholar] [CrossRef]
- Caballero Gómez, N.; Manetsberger, J.; Benomar, N.; Castillo Gutiérrez, S.; Abriouel, H. Antibacterial and antibiofilm effects of essential oil components, EDTA and HLE disinfectant solution on Enterococcus, Pseudomonas and Staphylococcus sp. multiresistant strains isolated along the meat production chain. Front. Microbiol. 2022, 13, 1014169. [Google Scholar] [CrossRef]
- Cortés-Chitala, M.D.C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, Á.; Estarrón-Espinosa, M.; López-Muraira, I. Identification and Quantification of Phenolic Compounds from Mexican Oregano (Lippia graveolens HBK) Hydroethanolic Extracts and Evaluation of Its Antioxidant Capacity. Molecules 2021, 26, 702. [Google Scholar] [CrossRef]
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil Against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Trujano, M.E.; Hernández-Sánchez, L.Y.; Muñoz Ocotero, V.; Dorazco-González, A.; Guevara Fefer, P.; Aguirre-Hernández, E. Pharmacological evaluation of the anxiolytic-like effects of Lippia graveolens and bioactive compounds. Pharm. Biol. 2017, 55, 1569–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrazin, S.L.F.; da Silva, L.A.; de Assunção, A.P.F.; Oliveira, R.B.; Calao, V.Y.P.; da Silva, R.; Stashenko, E.E.; Maia, J.G.S.; Mourão, R.H.V. Antimicrobial and seasonal evaluation of the carvacrol-chemotype oil from Lippia origanoides kunth. Molecules 2015, 20, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
| Priority | Pathogens |
|---|---|
| Critical | Carbapenem-resistant A. baumannii Carbapenem-resistant P. aeruginosa Carbapenem-resistant, BLEE-producing Enterobacteriaceae |
| High | Vancomycin-resistant E. faecium Methicillin-resistant S. aureus with intermediate sensitivity and resistance to vancomycin Helicobacter pylori resistant to clarithromycin Campylobacter spp. resistant to fluoroquinolones Salmonellae resistant to fluoroquinolones Neisseria gonorrhoeae resistant to cephalosporins and fluoroquinolones |
| Middle | Streptococcus pneumoniae without penicillin sensitivity Ampicillin-resistant Haemophilus influenzae Shigella spp. resistant to fluoroquinolones |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fimbres-García, J.O.; Flores-Sauceda, M.; Othon-Díaz, E.D.; García-Galaz, A.; Tapia-Rodríguez, M.R.; Silva-Espinoza, B.A.; Ayala-Zavala, J.F. Facing Resistant Bacteria with Plant Essential Oils: Reviewing the Oregano Case. Antibiotics 2022, 11, 1777. https://doi.org/10.3390/antibiotics11121777
Fimbres-García JO, Flores-Sauceda M, Othon-Díaz ED, García-Galaz A, Tapia-Rodríguez MR, Silva-Espinoza BA, Ayala-Zavala JF. Facing Resistant Bacteria with Plant Essential Oils: Reviewing the Oregano Case. Antibiotics. 2022; 11(12):1777. https://doi.org/10.3390/antibiotics11121777
Chicago/Turabian StyleFimbres-García, Jorge O., Marcela Flores-Sauceda, Elsa Daniela Othon-Díaz, Alfonso García-Galaz, Melvin R. Tapia-Rodríguez, Brenda A. Silva-Espinoza, and Jesus F. Ayala-Zavala. 2022. "Facing Resistant Bacteria with Plant Essential Oils: Reviewing the Oregano Case" Antibiotics 11, no. 12: 1777. https://doi.org/10.3390/antibiotics11121777