Antimicrobial Activity of Azithromycin Encapsulated into PLGA NPs: A Potential Strategy to Overcome Efflux Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of AZI-PLGA NPs
2.2.2. Characterization of Nanoparticles
Particle Size and Zeta-Potential
Drug Loading
Validation of HPLC Analysis
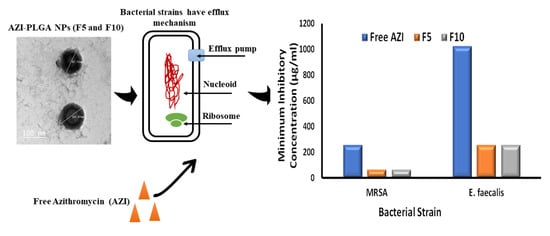
Transmission Electron Microscopy (TEM)
Differential Scanning Calorimetry (DSC)
Release Study
2.2.3. Cytotoxicity Assay
2.2.4. Antibacterial Study
Azithromycin Susceptibility Test
Determination of Minimum Inhibitory Concentration (MIC)
Investigation of Bacterial Efflux Activity
- Determination of efflux activity by cartwheel method
- Determination of AZI efflux using efflux pump inhibitor (EPI)
- Determination of MIC for AZI-PLGA NPs
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of AZI-PLGA NPs
3.1.1. Particle size and Zeta Potential
3.1.2. Drug Loading (DL%)
3.1.3. Transmission Electron Microscopy (TEM)
3.1.4. Differential Scanning Calorimetry
3.1.5. Release Study
3.2. Antibacterial Study
3.2.1. Susceptibility Test and Determination of MIC for Free AZI
3.2.2. Investigation of Bacterial Efflux Activity
Determination of Efflux Activity by Cartwheel Method
Determination of AZI Efflux via Efflux Pump Inhibitor (EPI)
3.2.3. Cytotoxicity Test
3.2.4. Antibacterial Activity of AZI-PLGA NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and Economic Impact of Antibiotic Resistance in Developing Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [Green Version]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Garg, A.; Pandit, S.; Mokkapati, V.R.S.S.; Mijakovic, I. Antimicrobial Effects of Biogenic Nanoparticles. Nanomaterials 2018, 8, 1009. [Google Scholar] [CrossRef] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. ScienceDirect Antibiotics : Past, Present and Future. Curr. Opin. Microbiol. 2020, 51, 72–80. [Google Scholar] [CrossRef]
- Upadhya, R.K.; Shenoy, L.; Venkateswaran, R. Effect of Intravenous Dexmedetomidine Administered as Bolus or as Bolus-plus-Infusion on Subarachnoid Anesthesia with Hyperbaric Bupivacaine. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 46–50. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R. The Potential Anti-Infective Applications of Metal Oxide Nanoparticles: A Systematic Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1592. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Sanchez-Lopez, E.; Espina, M.; Calpena, A.C.; Silva, A.M.; Veiga, F.J.; Garcia, M.L.; Souto, E.B. Advances in Antibiotic Nanotherapy; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780323400169. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Ebbensgaard, A.E.; Løbner-Olesen, A.; Frimodt-Møller, J. The Role of Efflux Pumps in the Transition from Low-Level to Clinical Antibiotic Resistance. Antibiotics 2020, 9, 855. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The Growing Role of Nanotechnology in Combating Infectious Disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux Pump Inhibitors of Clinically Relevant Multidrug Resistant Bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef]
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef] [Green Version]
- Ritsema, J.A.S.; Van Der Weide, H.; Te Welscher, Y.M.; Goessens, W.H.F.; Van Nostrum, C.F.; Storm, G.; Bakker-Woudenberg, I.A.J.M.; Hays, J.P. Antibiotic-Nanomedicines: Facing the Challenge of Effective Treatment of Antibiotic-Resistant Respiratory Tract Infections. Future Microbiol. 2018, 13, 1683–1692. [Google Scholar] [CrossRef]
- Ho, D.K.; Nichols, B.L.B.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.M. Challenges and Strategies in Drug Delivery Systems for Treatment of Pulmonary Infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef]
- Mondorf, A.W.; Breier, J.; Hendus, J.; Scherberich, J.E.; Mackenrodt, G.; Shah, P.M.; Stille, W.; Schoeppe, W. The Effect of Aminoglycosides on Proximal Tubular Membranes of Human Kidneys. Infection 1979, 7, 133–142. [Google Scholar] [CrossRef]
- Prayle, A.; Watson, A.; Fortnum, H.; Smyth, A. Side Effects of Aminoglycosides on the Kidney, Ear and Balance in Cystic Fibrosis. Thorax 2010, 65, 654–658. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Jiang, G.; Gao, R.; Chen, G.; Ren, Y.; Liu, J.; van der Mei, H.C.; Busscher, H.J. Circumventing Antimicrobial-Resistance and Preventing Its Development in Novel, Bacterial Infection-Control Strategies. Expert Opin. Drug Deliv. 2020, 17, 1151–1164. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Bakkar, M.R.; Elkhouly, G.E.; Raya, N.R.; Zaafar, D. Rhamnolipid Nano-Micelles Versus Alcohol-Based Hand Sanitizer : A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns. Antibiotics 2022, 11, 605. [Google Scholar]
- Sobhy, Y.; Mady, M.; Mina, S.; Abo-zeid, Y. Phytochemical and Pharmacological Values of Two Major Constituents of Asparagus Species and Their Nano Formulations: A Review. J. Adv. Pharm. Res. 2022, 6, 94–106. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R.; Touabi, L.; Mclean, G.R. An Investigation of Rhinovirus Infection on Cellular Uptake of Poly (Glycerol-Adipate) Nanoparticles. Int. J. Pharm. 2020, 589, 119826. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Diab, R.; Sanad, R.; Skran, W. Recent Advances in Herbal-Based Nanomedicine for Anti-Inflammatory Purposes. J. Adv. Pharm. Res. 2021, 5, 387–397. [Google Scholar] [CrossRef]
- Hashim, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and Characterization of Niosomes Containing Ribavirin for Liver Targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef]
- Ali, A.M.; Hill, H.J.; Elkhouly, G.E.; Bakkar, M.R.; Raya, N.R.; Stamataki, Z.; Abo-zeid, Y. Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits. Antibiotics 2022, 11, 1556. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Urbanowicz, R.A.; Thomson, B.J.; William, L.; Tarr, A.W.; Garnett, M.C. Enhanced Nanoparticle Uptake into Virus Infected Cells: Could Nanoparticles Be Useful in Antiviral Therapy? Int. J. Pharm. 2018, 547, 572–581. [Google Scholar]
- Abo-zeid, Y.; Mantovani, G.; Irving, W.L.; Garnett, M.C. Synthesis of Nucleoside-Boronic Esters Hydrophobic pro-Drugs: A Possible Route to Improve Hydrophilic Nucleoside Drug Loading into Polymer Nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 46, 354–364. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.; McLean, G.R.; Hamdy, N.M. A Molecular Docking Study Repurposes FDA Approved Iron Oxide Nanoparticles to Treat and Control COVID-19 Infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 1–30. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Bakkar, M.R.; Faraag, A.H.I.; Soliman, E.R.S.; Fouda, M.S.; Sarguos, A.M.M.; McLean, G.R.; Hebishy, A.M.S.; Elkhouly, G.E.; Raya, N.R.; Abo-zeid, Y. Rhamnolipids Nano-Micelles as a Potential Hand Sanitizer. Antibiotics 2021, 10, 751. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Amer, A.; El-Houssieny, B.; Bakkar, M.; Sakran, W. Overview on Bacterial Resistance and Nanoparticles to Overcome Bacterial Resistance. J. Adv. Pharm. Res. 2021, 5, 312–326. [Google Scholar] [CrossRef]
- Garnett, M.C.; Kallinteri, P. Nanomedicines and Nanotoxicology: Some Physiological Principles. Occup. Med. 2006, 56, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Burgess, K.; Li, H.; Abo-Zeid, Y.; Fatimah; Williams, G.R. The Effect of Molecular Properties on Active Ingredient Release from Electrospun Eudragit Fibers. Pharmaceutics 2018, 10, 103. [Google Scholar] [CrossRef]
- Shaaban, M.I.; Shaker, M.A.; Mady, F.M. Imipenem/Cilastatin Encapsulated Polymeric Nanoparticles for Destroying Carbapenem-Resistant Bacterial Isolates. J. Nanobiotechnol. 2017, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Derbali, R.M.; Aoun, V.; Moussa, G.; Frei, G.; Tehrani, S.F.; Del’Orto, J.C.; Hildgen, P.; Roullin, V.G.; Chain, J.L. Tailored Nanocarriers for the Pulmonary Delivery of Levofloxacin against Pseudomonas Aeruginosa: A Comparative Study. Mol. Pharm. 2019, 16, 1906–1916. [Google Scholar] [CrossRef] [Green Version]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Abo-zeid, Y.; Garnett, M.C. Polymer Nanoparticle as a Delivery System for Ribavirin: Do Nanoparticle Avoid Uptake by Red Blood Cells? J. Drug Deliv. Sci. Technol. 2020, 56, 101552. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of Nanoparticles into Cells: The Importance of Nanoparticle Properties. Polymer Chemistry 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Mahmoud, Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Omid, C.; Mahmoudi, M.; et al. HHS Public Access. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2018, 46, 4218–4244. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar]
- Hasani, A.; Madhi, M.; Gholizadeh, P.; Shahbazi Mojarrad, J.; Ahangarzadeh Rezaee, M.; Zarrini, G.; Samadi Kafil, H. Metal Nanoparticles and Consequences on Multi-Drug Resistant Bacteria: Reviving Their Role. SN Appl. Sci. 2019, 1, 360. [Google Scholar]
- Sun, M.; Zhu, C.; Long, J.; Lu, C.; Pan, X.; Wu, C. PLGA Microsphere-Based Composite Hydrogel for Dual Delivery of Ciprofloxacin and Ginsenoside Rh2 to Treat Staphylococcus Aureus-Induced Skin Infections. Drug Deliv. 2020, 27, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Singh, A.; Khan, A.U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett. 2017, 12, 454. [Google Scholar]
- Salas Orozco, M.F.; Niño-Martínez, N.; Martínez-Castañón, G.A.; Méndez, F.T.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Kamato, D.; Sime, F.B.; Roberts, J.A.; Popat, A.; Falconer, J.R.; Kumeria, T. Influence of PEGylated Porous Silicon Nanoparticles on Permeation and Efflux of an Orally Administered Antibiotic. Mater. Today Adv. 2022, 13, 100210. [Google Scholar] [CrossRef]
- Moazeni, M.; Kelidari, H.R.; Saeedi, M.; Morteza-Semnani, K.; Nabili, M.; Gohar, A.A.; Akbari, J.; Lotfali, E.; Nokhodchi, A. Time to Overcome Fluconazole Resistant Candida Isolates: Solid Lipid Nanoparticles as a Novel Antifungal Drug Delivery System. Colloids Surf. B Biointerfaces 2016, 142, 400–407. [Google Scholar] [CrossRef]
- Aboutaleb, E.; Noori, M.; Gandomi, N.; Atyabi, F.; Fazeli, M.R.; Jamalifar, H.; Dinarvand, R. Improved Antimycobacterial Activity of Rifampin Using Solid Lipid Nanoparticles. Int. Nano Lett. 2012, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-Date Overview. Polymers 2021, 13, 724. [Google Scholar]
- Murakami, M.; Cabral, H.; Matsumoto, Y.; Wu, S.; Kano, M.R.; Yamori, T.; Nishiyama, N.; Kataoka, K. Improving Drug Potency and Efficacy by Nanocarrier-Mediated Subcellular Targeting. Sci. Transl. Med. 2011, 3, 64ra2. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Hooda, Y.; Tanmoy, A.M.; Sajib, M.S.I.; Saha, S. Mass Azithromycin Administration: Considerations in an Increasingly Resistant World. BMJ Glob. Health 2020, 5, e002446. [Google Scholar] [CrossRef]
- Echeverría-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; De-Antonio Cuscó, M.; Ferrández, O.; Horcajada, J.P.; Grau, S. Azithromycin in the Treatment of COVID-19: A Review. Expert Rev. Anti. Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of Action and Their Relevance for Clinical Applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- Porco, T.C.; Gebre, T.; Ayele, B.; House, J.; Keenan, J.; Zhou, Z.; Hong, K.C.; Stoller, N.; Ray, K.J.; Emerson, P.; et al. Effect of Mass Distribution of Azithromycin for Trachoma Control on Overall Mortality in Ethiopian Children: A Randomized Trial. JAMA J. Am. Med. Assoc. 2009, 302, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.; Worden, L.; Hinterwirth, A.; Arzika, A.M.; Maliki, R.; Abdou, A.; Zhong, L.; Chen, C.; Cook, C.; Lebas, E.; et al. Macrolide and Nonmacrolide Resistance with Mass Azithromycin Distribution. N. Engl. J. Med. 2020, 383, 1941–1950. [Google Scholar] [CrossRef]
- Leclercq, R. Mechanisms of Resistance to Macrolides and Lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef]
- Vaara, M. Outer Membrane Permeability Barrier to Azithromycin, Clarithromycin, and Roxithromycin in Gram-Negative Enteric Bacteria. Antimicrob. Agents Chemother. 1993, 37, 354–356. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, H.P. Efflux as a Mechanism of Resistance to Antimicrobials in Pseudomonas Aeruginosa and Related Bacteria: Unanswered Questions. Genet. Mol. Res. 2003, 2, 48–62. [Google Scholar]
- Chevalier, M.T.; Gonzalez, J.; Alvarez, V. Biodegradable Polymeric Microparticles as Drug Delivery Devices. IFMBE Proc. 2015, 49, 187–190. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of Antibiotics with Polymeric Particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef]
- Nader, A.; El-Hosseiny, G.; Elleboudy, N.; Yassein, M. Prevalence and Antimicrobial Susceptibility Pattern of Enterococcus Sp. Isolated from Different Clinical Specimens from Some Hospitalized Patients in Egypt. In Proceedings of the 8th Annual International Ain Shams University Congress, Cairo, Egypt, 1–4 April 2019. [Google Scholar]
- Mohammadi, G.; Valizadeh, H.; Barzegar-Jalali, M.; Lotfipour, F.; Adibkia, K.; Milani, M.; Azhdarzadeh, M.; Kiafar, F.; Nokhodchi, A. Development of Azithromycin-PLGA Nanoparticles: Physicochemical Characterization and Antibacterial Effect against Salmonella Typhi. Colloids Surf. B Biointerfaces 2010, 80, 34–39. [Google Scholar] [CrossRef]
- Ghari, T.; Kobarfard, F.; Mortazavi, S.A. Development of a Simple RP-HPLC-UV Method for Determination of Azithromycin in Bulk and Pharmaceutical Dosage Forms as an Alternative to the USP Method. Iran. J. Pharm. Res. 2013, 12, 55–61. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical Delivery of Tetrahydrocurcumin Lipid Nanoparticles Effectively Inhibits Skin Inflammation: In Vitro and in Vivo Study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver : An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- CLSI. CLSI Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing Supplement M100S; CLSI: Wayne, PA, USA, 2016; ISBN 1-56238-923-8. [Google Scholar]
- Martins, M.; Viveiros, M.; Couto, I.; Costa, S.S.; Pacheco, T.; Fanning, S.; Pagès, J.M.; Amaral, L. Identification of Efflux Pump-Mediated Multidrug-Resistant Bacteria by the Ethidium Bromide-Agar Cartwheel Method. In Vivo 2011, 25, 171–178. [Google Scholar]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar]
- Christena, L.R.; Mangalagowri, V.; Pradheeba, P.; Ahmed, K.B.A.; Shalini, B.I.S.; Vidyalakshmi, M.; Anbazhagan, V.; Subramanian, N.S. Copper Nanoparticles as an Efflux Pump Inhibitor to Tackle Drug Resistant Bacteria. RSC Adv. 2015, 5, 12899–12909. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic Resistance Breakers: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [Green Version]
- Laudy, A.E.; Kulińska, E.; Tyski, S. The Impact of Efflux Pump Inhibitors on the Activity of Selected Non-Antibiotic Medicinal Products against Gram-Negative Bacteria. Molecules 2017, 22, 114. [Google Scholar] [CrossRef]
- Putri, D.C.A.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of Mixing Temperature and Sonication Duration in Liposomes Preparation. J. Pharm. Sci. Community 2017, 14, 79–85. [Google Scholar]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Yang, X.P.; Ma, L.Z. Analysis of Biosurfactants from Industrially Viable Pseudomonas Strain Isolated from Crude Oil Suggests How Rhamnolipids Congeners Affect Emulsification Property and Antimicrobial Activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Aucamp, M.; Odendaal, R.; Liebenberg, W.; Hamman, J.; Odendaal, R.; Liebenberg, W.; Hamman, J. Amorphous Azithromycin with Improved Aqueous Solubility and Intestinal Membrane Permeability. Drug Dev. Ind. Pharm. 2014, 41, 1100–1108. [Google Scholar] [CrossRef]
- Joshi, A.S.; Gahane, A.; Thakur, A.K. Deciphering the Mechanism and Structural Features of Polysorbate 80 during Adsorption on PLGA Nanoparticles by Attenuated Total Re Fl Ectance—Fourier Transform Infrared Spectroscopy †. R. Soc. Chem. 2016, 6, 108545–108557. [Google Scholar] [CrossRef] [Green Version]
- Graca, M.; Bongaerts, J.H.H.; Stokes, J.R.; Granick, S. Friction and Adsorption of Aqueous Polyoxyethylene (Tween) Surfactants at Hydrophobic Surfaces. J. Colloid Interface Sci. 2007, 315, 662–670. [Google Scholar] [CrossRef]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St. Pourçain, C.B.; Garnett, M.C. Novel Functionalized Biodegradable Polymers for Nanoparticle Drug Delivery Systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of Process and Formulation Variables on the Preparation of Parenteral Paclitaxel-Loaded Biodegradable Polymeric Nanoparticles : A Co-Surfactant Study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; Available online: https://www.aoac.org/aoac_prod_imis/AOAC_Docs/StandardsDevelopment/SLV_Guidelines_Dietary_Supplements.pdf (accessed on 10 November 2022).
- European Association for The Study of The Liver. EASL Recommendations on Treatment of Hepatitis C. J. Hepatol. 2016, 73, 1170–1218. [Google Scholar]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The Mechanisms of Drug Release in Poly (Lactic-Co-Glycolic Acid)-Based Drug Delivery Systems—A Review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S. PLGA / TPGS Nanoparticles for Controlled Release of Paclitaxel : Effects of the Emulsifier and Drug Loading Ratio. Pharma. Res. 2003, 20, 1864–1872. [Google Scholar]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of Sustained Release Apremilast-Loaded PLGAlga Nanoparticles: In Vitro Characterization and in Vivo Pharmacokinetic Study in Rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Langendonk, R.F.; Neill, D.R.; Fothergill, J.L. The Building Blocks of Antimicrobial Resistance in Pseudomonas Aeruginosa: Implications for Current Resistance-Breaking Therapies. Front. Cell. Infect. Microbiol. 2021, 11, 665759. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas Aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar]
- Slama, T.G. Gram-Negative Antibiotic Resistance: There Is a Price to Pay. Crit. Care 2008, 12, S4. [Google Scholar]
- Portillo, A.; Ruiz-Larrea, F.; Zarazaga, M.; Alonso, A.; Martinez, J.L.; Torres, C. Macrolide Resistance Genes in Enterococcus spp. Antimicrob. Agents Chemother. 2000, 44, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Suresh, M.; Nithya, N.; Jayasree, P.R.; Manish Kumar, P.R. Detection and Prevalence of Efflux Pump-Mediated Drug Resistance in Clinical Isolates of Multidrug-Resistant Gram-Negative Bacteria from North Kerala, India. Asian J. Pharm. Clin. Res. 2016, 9, 9–12. [Google Scholar]
- Seral, C.; Carryn, S.; Tulkens, P.M.; Van Bambeke, F. Influence of P-Glycoprotein and MRP Effux Pump Inhibitors on the Intracellular Activity of Azithromycin and Ciprofloxacin in Macrophages Infected by Listeria Monocytogenes or Staphylococcus Aureus. J. Antimicrob. Chemother. 2003, 51, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Mullin, S.; Mani, N.; Grossman, T.H. Inhibition of Antibiotic Efflux in Bacteria by the Novel Multidrug Resistance Inhibitors Biricodar (VX-710) and Timcodar (VX-853). Antimicrob. Agents Chemother. 2004, 48, 4171–4176. [Google Scholar] [CrossRef] [Green Version]
- Pule, C.M.; Sampson, S.L.; Warren, R.M.; Black, P.A.; van Helden, P.D.; Victor, T.C.; Louw, G.E. Efflux Pump Inhibitors: Targeting Mycobacterial Efflux Systems to Enhance TB Therapy. J. Antimicrob. Chemother. 2016, 71, 17–26. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Demitto, F.; Do Amaral, R.C.R.; Maltempe, F.G.; Siqueira, V.L.D.; De Lima Scodro, R.B.; Lopes, M.A.; Caleffi-Ferracioli, K.R.; Canezin, P.H.; Cardoso, R.F. In Vitro Activity of Rifampicin and Verapamil Combination in Multidrug-Resistant Mycobacterium Tuberculosis. PLoS ONE 2015, 10, e0116545. [Google Scholar] [CrossRef]
- Zhang, Q.; Plummer, P.J. Mechanisms of Antibiotic Resistance in Campylobacter; Wiley: Hoboken, NJ, USA, 2014; pp. 263–276. [Google Scholar] [CrossRef]
- De Rossi, E.; Aínsa, J.A.; Riccardi, G. Role of Mycobacterial Efflux Transporters in Drug Resistance: An Unresolved Question. FEMS Microbiol. Rev. 2006, 30, 36–52. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are Nanostructured Lipid Carriers (NLCs) Better than Solid Lipid Nanoparticles (SLNs): Development, Characterizations and Comparative Evaluations of Clotrimazole-Loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Gao, M.; Long, X.; Du, J.; Teng, M.; Zhang, W.; Wang, Y.; Wang, X.; Wang, Z.; Zhang, P.; Li, J. Enhanced Curcumin Solubility and Antibacterial Activity by Encapsulation in PLGA Oily Core Nanocapsules. Food Funct. 2020, 11, 448–455. [Google Scholar] [CrossRef]
- Cabeen, M.T.; Jacobs-Wagner, C. Bacterial Cell Shape. Nat. Rev. Microbiol. 2005, 3, 601–610. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Beveridge, T.J. Sructure of Fram-Negative Cell Walls and Their Derived Mebrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.J.; Chorover, J. ATR-FTIR Spectroscopy Reveals Bond Formation During Bacterial Adhesion to Iron Oxide. Langmuir 2006, 8492–8500. [Google Scholar]
- Makin, S.A.; Beveridge, T.J. The Influence of A-Band and B-Band Lipopolysaccharide on the Surface Characteristics and Adhesion of Pseudomonas Aeruginosa to Surfaces. Microbiology 1996, 142, 299–307. [Google Scholar]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-Dependent Cellular Uptake and Localization Profiles of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the Antibacterial Mechanism of CuO Nanoparticles: Revealing the Route of Induced Oxidative Stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Pareek, V.; Gupta, R.; Panwar, J. Do Physico-Chemical Properties of Silver Nanoparticles Decide Their Interaction with Biological Media and Bactericidal Action? A Review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef]
- Hwang, J.; Mros, S.; Gamble, A.B.; Tyndall, J.D.A.; McDowell, A. Improving Antibacterial Activity of a HtrA Protease Inhibitor JO146 against Helicobacter Pylori: A Novel Approach Using Microfluidics-Engineered PLGA Nanoparticles. Pharmaceutics 2022, 14, 348. [Google Scholar] [CrossRef]




| AZI Initial Weight | ||||
|---|---|---|---|---|
| (mg)/Formula Name | Aqueous Phase | ** %DL ± SD | ** PS (D nm) ± SD (PDI) | ** ZP (mV) ± SD |
| Blank | Water | ------ | 188.5 ± 51.92 (0.193) | −24.83 ± 6.85 |
| 10/F1 | Water | 3.88 ± 0.07 | 225.5 ± 128.7 (0.276) | −27.9 ± 6.61 |
| 15/F2 | Water | 3.43 ± 0.11 | 184.5 ± 115 (0.253) | −30.1 ± 6.61 |
| 20/F3 | Water | 3.47 ± 0.24 | 101 ± 25.01 (0.181) | −34.8 ± 6.61 |
| 50/F4 | Water | 3.52 ± 0.23 | 593.7 ± 244.4 (0.651) | −29.5 ± 6.61 |
| 10/F5 | Water/Tween 80 (0.1%) | 5.74 ± 0.28 | 134 ± 57.53 (0.198) | −32.5.2 ± 4.87 |
| 15/F6 | Water/Tween 80 (0.1%) | 3.43 ± 0.14 | 114.3 ± 52.76 (0.266) | −35.7 ± 6.61 |
| 20/F7 | Water/Tween 80 (0.1%) | 4 ± 0.16 | 112.3 ± 42.08 (0.279) | −34.2 ± 6.61 |
| 50/F8 | Water/Tween 80 (0.1%) | 2.88 ± 0.34 | 154.9 ± 78.03 (0.269) | −30.2 ± 6.61 |
| 10/F9 | Phosphate buffer (10 mM PH 6) | 3.52 ± 0.23 | 118.4 ± 56.27 (0.204) | −32.7 ± 6.45 |
| 15/F10 | Phosphate buffer (10 mM PH 6) | 4.78 ± 0.13 | 107.5 ± 57.97 (0.307) | −33.3 ± 6.62 |
| 20/F11 | Phosphate buffer (10 mM PH 6) | 3.78 ± 0.28 | 105.8 ± 42.6 (0.367) | −30.9 ± 5.34 |
| 50/F12 | Phosphate buffer (10 mM, PH 6) | 3.35 ± 0.16 | 521.6 ± 158.4 (0.598) | −31.6 ± 6.3 |
| 10/F13 | Phosphate buffer (10 mM PH7.4) | 4.59 ± 0.27 | 111.4 ± 42.95 (0.301) | −41.8 ± 5.79 |
| 15/F14 | Phosphate buffer (10 mM PH7.4) | 4.35 ± 0.12 | 142.2 ± 58.73 (0.224) | −35.2.1 ± 5.87 |
| 20/F15 | Phosphate buffer (10 mM PH7.4) | 4.55 ± 0.26 | 154.3 ± 98.39 (0.316) | −34.7 ± 8.08 |
| 50/F16 | Phosphate buffer (10 mM PH7.4) | 4.32 ± 0.45 | 416 ± 50 (0.595) | −43.6 ± 6.65 |
| 10/F17 | Phosphate buffer (10 mM PH 8) | 3.19 ± 0.18 | 142.6 ± 67.46 (0.272) | −52.5 ± 7.8 |
| 15/F18 | Phosphate buffer (10 mM PH 8) | 3.49 ± 0.25 | 348.8 ± 104.1 (0.584) | −41.1 ± 4.99 |
| 20/F19 | Phosphate buffer (10 mM PH 8) | 4 ± 0.67 | 534.3 ± 246.7 (0.626) | −40.9 ± 5.06 |
| 50/F20 | Phosphate buffer (10 mM PH 8) | 3.13 ± 0.07 | 673 ± 151 (0.437) | −56.1 ± 8.06 |
| Tested Sample | P. aeruginosa | MRSA | E. faecalis |
|---|---|---|---|
| Free AZI | 256 | 256 | >1000 |
| F5 | 256 | 64 a | 256 a |
| F10 | 256 | 64 a | 256 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-zeid, Y.; Amer, A.; Bakkar, M.R.; El-Houssieny, B.; Sakran, W. Antimicrobial Activity of Azithromycin Encapsulated into PLGA NPs: A Potential Strategy to Overcome Efflux Resistance. Antibiotics 2022, 11, 1623. https://doi.org/10.3390/antibiotics11111623
Abo-zeid Y, Amer A, Bakkar MR, El-Houssieny B, Sakran W. Antimicrobial Activity of Azithromycin Encapsulated into PLGA NPs: A Potential Strategy to Overcome Efflux Resistance. Antibiotics. 2022; 11(11):1623. https://doi.org/10.3390/antibiotics11111623
Chicago/Turabian StyleAbo-zeid, Yasmin, Amr Amer, Marwa Reda Bakkar, Boushra El-Houssieny, and Wedad Sakran. 2022. "Antimicrobial Activity of Azithromycin Encapsulated into PLGA NPs: A Potential Strategy to Overcome Efflux Resistance" Antibiotics 11, no. 11: 1623. https://doi.org/10.3390/antibiotics11111623