A Modified Electrochemical Sensor Based on N,S-Doped Carbon Dots/Carbon Nanotube-Poly(Amidoamine) Dendrimer Hybrids for Imatinib Mesylate Determination
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of Modifiers
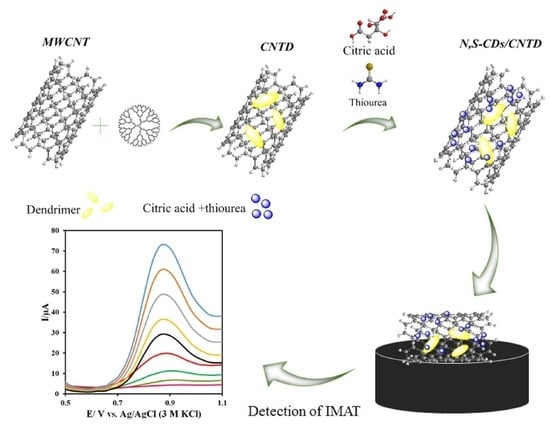
2.2.1. Preparation of CNT-NH2
2.2.2. Preparation of CNTD
2.2.3. Preparation of N,S-CDs/CNTD
2.3. Preparation of the Sensor
2.4. Measurements and Analyses
3. Results
3.1. Characterization
3.2. Electrochemical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghalkhani, M.; Sohouli, E. Synthesis of the decorated carbon nano onions with aminated MCM-41/Fe3O4 NPs: Morphology and electrochemical sensing performance for methotrexate analysis. Microporous Mesoporous Mater. 2021, 331, 111658. [Google Scholar] [CrossRef]
- Cahill, K.N.; Katz, H.R.; Cui, J.; Lai, J.; Kazani, S.; Crosby-Thompson, A.; Garofalo, D.; Castro, M.; Jarjour, N.; DiMango, E. KIT inhibition by imatinib in patients with severe refractory asthma. N. Engl. J. Med. 2017, 376, 1911–1920. [Google Scholar] [CrossRef]
- Bende, G.; Kollipara, S.; Movva, S.; Moorthy, G.; Saha, R. Validation of an HPLC method for determination of imatinib mesylate in rat serum and its application in a pharmacokinetic study. J. Chromatogr. Sci. 2010, 48, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Al-nayili, A.; Rzoqy, M. Local silica sand as a silica source in the synthesis of Y zeolite. Asia-Pac. J. Chem. Eng. 2022, 17, e2824. [Google Scholar] [CrossRef]
- Buclin, T.; Thoma, Y.; Widmer, N.; André, P.; Guidi, M.; Csajka, C.; Decosterd, L.A. The steps to therapeutic drug monitoring: A structured approach illustrated with imatinib. Front. Pharmacol. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; Mariño-Enríquez, A.; Tao, D.L.; Ketzer, J.; Eilers, G.; Zhu, M.; Yu, C.; Mannan, A.M.; Rubin, B.P.; Demetri, G.D. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer 2019, 120, 612–620. [Google Scholar] [CrossRef]
- Ajel, M.K.; Al-Nayili, A. Synthesis, characterization of Ag-WO3/bentonite nanocomposites and their application in photocatalytic degradation of humic acid in water. Environ. Sci. Pollut. Res. 2023, 30, 20775–20789. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Huang, L.; Ye, L.; Zhou, Z.; Xiang, G.; Xu, L. Determination of imatinib mesylate and related compounds by field amplified sample stacking with large volume sample injection capillary electrophoresis. J. Pharm. Biomed. Anal. 2012, 70, 26–31. [Google Scholar] [CrossRef]
- Roth, O.; Spreux-Varoquaux, O.; Bouchet, S.; Rousselot, P.; Castaigne, S.; Rigaudeau, S.; Raggueneau, V.; Therond, P.; Devillier, P.; Molimard, M. Imatinib assay by HPLC with photodiode-array UV detection in plasma from patients with chronic myeloid leukemia: Comparison with LC-MS/MS. Clin. Chim. Acta 2010, 411, 140–146. [Google Scholar] [CrossRef]
- Karim, S.E.A.; El-Nashar, R.M.; Abadi, A.H. Potentiometric determination of imatinib under batch and flow injection analysis conditions. Int. J. Electrochem. Sci. 2012, 7, 9668–9681. [Google Scholar]
- Karim, S.S.E.A.; Farghaly, R.A.; El-Nashar, R.M.; Abadi, A.H. Spectrophotometric Determination of Imatinib Mesylate using Charge Transfer Complexs in Pure Form and Pharmaceutical Formulation. Chem. Rapid Commun. 2014, 2, 55–63. [Google Scholar]
- Rahimi Kakavandi, N.; Asadi, T.; Jannat, B.; Abdi, K.; Ghazi-Khansari, M.; Shahali, H.; Naraki, K. Method development for determination of imatinib and its major metabolite, N-desmethyl imatinib, in biological and environmental samples by SA–SHS–LPME and HPLC. Biomed. Chromatogr. 2021, 35, e5088. [Google Scholar] [CrossRef] [PubMed]
- Al-nayili, A.; Muhammad, N.J. Perovskite’s LaNiMnO6/montmorillonite K10 nanocomposites: Synthesis and enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2023, 155, 107254. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Gharagozlou, M.; Sohouli, E.; Khosrowshahi, E.M. Preparation of an electrochemical sensor based on a HKUST-1/CoFe2O4/SiO2-modified carbon paste electrode for determination of Azaperone. Microchem. J. 2022, 175, 107199. [Google Scholar] [CrossRef]
- Sohouli, E.; Keihan, A.H.; Shahdost-fard, F.; Naghian, E.; Plonska-Brzezinska, M.E.; Rahimi-Nasrabadi, M.; Ahmadi, F. A glassy carbon electrode modified with carbon nanoonions for electrochemical determination of fentanyl. Mater. Sci. Eng. C 2020, 110, 110684. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B. Stimuli-chromism of photoswitches in smart polymers: Recent advances and applications as chemosensors. Prog. Polym. Sci. 2019, 98, 101149. [Google Scholar] [CrossRef]
- Dezhakam, E.; Khalilzadeh, B.; Mahdipour, M.; Isildak, I.; Yousefi, H.; Ahmadi, M.; Naseri, A.; Rahbarghazi, R. Electrochemical biosensors in exosome analysis; a short journey to the present and future trends in early-stage evaluation of cancers. Biosens. Bioelectron. 2022, 222, 114980. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Liang, M.; Zheng, H.; Zhu, C.; Wang, Y. Detection of imatinib based on electrochemical sensor constructed using biosynthesized graphene-silver nanocomposite. Front. Chem. 2021, 9, 670074. [Google Scholar] [CrossRef]
- Zaidan, B.A.H.; Sohouli, E.; Mazaheri, S. A Novel Capping Agent in Preparation and Characterization of CuAl2O4/CuO Nanocomposite and its Application for Electrochemical Detection of Dopamine. Anal. Bioanal. Electrochem. 2019, 11, 108–122. [Google Scholar]
- Gbair, G.A.; Alshamsi, H.A. Facile green synthesis of CuO-ZnO nanocomposites from Argyreia nervosa leaves extract for photocatalytic degradation of Rhodamine B dye. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H. Highly sensitive electrochemical sensor based on La3+-doped Co3O4 nanocubes for determination of sudan I content in food samples. Food Chem. 2019, 286, 191–196. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K. Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: A docking/experimental investigation. Ind. Eng. Chem. Res. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Albdiry, M.; Al-Nayili, A. Ternary sulfonated graphene/polyaniline/carbon nanotubes nanocomposites for high performance of supercapacitor electrodes. Polym. Bull. 2022, 1–14. [Google Scholar] [CrossRef]
- Al-Abidy, M.; Al-Nayili, A. Enhancement of photocatalytic activities of ZnFe2O4 composite by incorporating halloysite nanotubes for effective elimination of aqueous organic pollutants. Environ. Monit. Assess. 2023, 195, 190. [Google Scholar] [CrossRef]
- Alam, A.U.; Clyne, D.; Jin, H.; Hu, N.-X.; Deen, M.J. Fully integrated, simple, and low-cost electrochemical sensor array for in situ water quality monitoring. ACS Sens. 2020, 5, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S. Development of an electrochemical biosensor for phylogenetic analysis of Amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Albdiry, M. Identification of active structure and catalytic efficiency of MCM-22 zeolite detemplated by two different processes. J. Porous Mater. 2021, 28, 1439–1448. [Google Scholar] [CrossRef]
- Alwan, S.H.; Salem, K.H.; Alshamsi, H.A. Visible light-driven photocatalytic degradation of Rhodamine B dye onto TiO2/rGO nanocomposites. Mater. Today Commun. 2022, 33, 104558. [Google Scholar] [CrossRef]
- Dayekh, N.S.; Al-Nayili, A. Heterogeneous photocatalytic degradation of phenol over Pd/rGO sheets. AIP Conf. Proc. 2022, 2398, 030010. [Google Scholar]
- Karami, M.; Ghanbari, M.; Alshamsi, H.A.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. The effect of CuI–PbI2 nanocomposite fabricated by the sonochemical route on electrochemical hydrogen storage characteristics. Int. J. Hydrogen Energy 2021, 46, 19074–19084. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Saleh-Mohammadnia, M.; Roghani-Mamaqani, H.; Mardani, H.; Rezvani-Moghaddam, A.; Hemmati, S.; Salami-Kalajahi, M. Fluorescent cellulosic composites based on carbon dots: Recent advances, developments, and applications. Carbohydr. Polym. 2022, 294, 119768. [Google Scholar] [CrossRef] [PubMed]
- Abdulhusain, Z.H.; Alshamsi, H.A.; Salavati-Niasari, M. Silver and zinc oxide decorated on reduced graphene oxide: Simple synthesis of a ternary heterojunction nanocomposite as an effective visible-active photocatalyst. Int. J. Hydrogen Energy 2022, 47, 34036–34047. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, X.; Ma, J.; Gu, Y.; Qian, Z.; Chen, J.; Feng, H. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Adv. 2014, 4, 5465–5468. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Albo Hay Allah, M.A.; Alshamsi, H.A. Green synthesis of ZnO NPs using Pontederia crassipes leaf extract: Characterization, their adsorption behavior and anti-cancer property. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, J.; Dong, Y.; Xie, P.; Zhang, Y.; Cai, Z. Nitrogen and sulfur co-doped carbon-dot-assisted laser desorption/ionization time-of-flight mass spectrometry imaging for profiling bisphenol S distribution in mouse tissues. Anal. Chem. 2018, 90, 10872–10880. [Google Scholar] [CrossRef]
- Dara, M.; Hassanpour, M.; Alshamsi, H.A.; Baladi, M.; Salavati-Niasari, M. Green sol–gel auto combustion synthesis and characterization of double perovskite Tb2ZnMnO6 nanoparticles and a brief study of photocatalytic activity. RSC Adv. 2021, 11, 8228–8238. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Nguyen, A.T.N.; Shim, J.H. All carbon hybrid N-doped carbon dots/carbon nanotube structures as an efficient catalyst for the oxygen reduction reaction. RSC Adv. 2021, 11, 12520–12530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, J.; Xiao, H.; Hu, T.; Jia, J.; Wu, H. Facile in situ synthesis of a carbon quantum dot/graphene heterostructure as an efficient metal-free electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tian, Z.; Li, J.; Ruan, H.; Ma, Y.; Yang, Z.; Qu, Y. Synergistically enhanced activity of graphene quantum dot/multi-walled carbon nanotube composites as metal-free catalysts for oxygen reduction reaction. Nanoscale 2014, 6, 2603–2607. [Google Scholar] [CrossRef]
- Alwan, S.H.; Alshamsi, H.A. In situ synthesis NiO/F-MWCNTs nanocomposite for adsorption of malachite green dye from polluted water. Carbon Lett. 2022, 32, 1073–1084. [Google Scholar] [CrossRef]
- Senokos, E.; Reguero, V.; Palma, J.; Vilatela, J.; Marcilla, R. Macroscopic fibres of CNTs as electrodes for multifunctional electric double layer capacitors: From quantum capacitance to device performance. Nanoscale 2016, 8, 3620–3628. [Google Scholar] [CrossRef]
- Pour, B.H.; Haghnazari, N.; Keshavarzi, F.; Ahmadi, E.; Zarif, B.R. High sensitive electrochemical sensor for imatinib based on metal-organic frameworks and multiwall carbon nanotubes nanocomposite. Microchem. J. 2021, 165, 106147. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Ghaedi, M.; Kokhdan, S.N.; Vashaee, D. Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic Fe–Ag/TiO2 nanoparticles. Dalton Trans. 2019, 48, 898–907. [Google Scholar] [CrossRef]
- Li, F.; Peng, J.; Zheng, Q.; Guo, X.; Tang, H.; Yao, S. Carbon nanotube-polyamidoamine dendrimer hybrid-modified electrodes for highly sensitive electrochemical detection of microRNA24. Anal. Chem. 2015, 87, 4806–4813. [Google Scholar] [CrossRef]
- Trigueiro, J.P.C.; Figueiredo, R.C.; Rojo, J.; Viana, R.M.; Schnitzler, M.C.; Silva, G.G. Carbon nanotube/dendrimer hybrids as electrodes for supercapacitors. J. Solid State Electrochem. 2016, 20, 1991–2000. [Google Scholar] [CrossRef]
- Sun, J.-T.; Hong, C.-Y.; Pan, C.-Y. Surface modification of carbon nanotubes with dendrimers or hyperbranched polymers. Polym. Chem. 2011, 2, 998–1007. [Google Scholar] [CrossRef]
- Bonet-San-Emeterio, M.; Algarra, M.; Petković, M.; Del Valle, M. Modification of electrodes with N-and S-doped carbon dots. Evaluation of the electrochemical response. Talanta 2020, 212, 120806. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, E.; Ghalkhani, M.; Zargar, T.; Joseph, Y.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Plonska-Brzezinska, M.E.; Ehrlich, H. A new electrochemical aptasensor based on gold/nitrogen-doped carbon nano-onions for the detection of Staphylococcus aureus. Electrochim. Acta 2022, 403, 139633. [Google Scholar] [CrossRef]
- Vivekanand, V.; Rao, D.S.; Vaidyanathan, G.; Sekhar, N.; Kelkar, S.A.; Puranik, P.R. A validated LC method for imatinib mesylate. J. Pharm. Biomed. Anal. 2003, 33, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmad, S.; Kohli, K.; Ali, J.; Khar, R. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2005, 39, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, L.; Wu, Z.; Chang, C.; Yang, Z. Determination of sulfonate ester genotoxic impurities in imatinib mesylate by gas chromatography with mass spectrometry. J. Sep. Sci. 2016, 39, 3558–3563. [Google Scholar] [CrossRef]
- Tan, K.L.; Ankathil, R.; Gan, S.H. Method development and validation for the simultaneous determination of imatinib mesylate and N-desmethyl imatinib using rapid resolution high performance liquid chromatography coupled with UV-detection. J. Chromatogr. B 2011, 879, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Castañeda, G.; Lizcano, I. Electrochemical sensor for leukemia drug imatinib determination in urine by adsorptive striping square wave voltammetry using modified screen-printed electrodes. Electrochim. Acta 2018, 269, 668–675. [Google Scholar] [CrossRef]











| Modifier | Method | Liner Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| C18 | LC-UV | 1–40 | 0.11 | [53] |
| - | HPTLC | 0.2–20 | 0.02 | [54] |
| - | GC-MS | 0.02–0.2 | 0.002 | [55] |
| C18 | UHPLC | 0.1–72 | 0.02 | [56] |
| MWCNT/SPCE | SWV | 50–912 | 16 | [57] |
| N,S-CDs/CNTD/GCE | DPV | 0.03–75 | 0.01 | Our work |
| Add (μM) | Obtained (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| 1 | 0.92 | 92% | 4.2% |
| 5 | 4.85 | 97% | 4.1% |
| 10 | 9.78 | 97% | 4.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh Mohammadnia, M.; Roghani-Mamaqani, H.; Ghalkhani, M.; Hemmati, S. A Modified Electrochemical Sensor Based on N,S-Doped Carbon Dots/Carbon Nanotube-Poly(Amidoamine) Dendrimer Hybrids for Imatinib Mesylate Determination. Biosensors 2023, 13, 547. https://doi.org/10.3390/bios13050547
Saleh Mohammadnia M, Roghani-Mamaqani H, Ghalkhani M, Hemmati S. A Modified Electrochemical Sensor Based on N,S-Doped Carbon Dots/Carbon Nanotube-Poly(Amidoamine) Dendrimer Hybrids for Imatinib Mesylate Determination. Biosensors. 2023; 13(5):547. https://doi.org/10.3390/bios13050547
Chicago/Turabian StyleSaleh Mohammadnia, Maryam, Hossein Roghani-Mamaqani, Masoumeh Ghalkhani, and Salar Hemmati. 2023. "A Modified Electrochemical Sensor Based on N,S-Doped Carbon Dots/Carbon Nanotube-Poly(Amidoamine) Dendrimer Hybrids for Imatinib Mesylate Determination" Biosensors 13, no. 5: 547. https://doi.org/10.3390/bios13050547