High-Performance Nitric Oxide Gas Sensors Based on an Ultrathin Nanoporous Poly(3-hexylthiophene) Film
Abstract
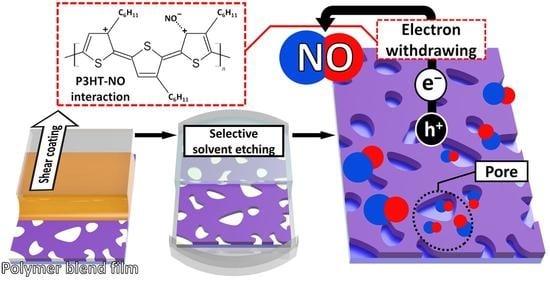
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. CP-Based OFET Gas Sensor Fabrication
2.3. Gas Sensing Evaluation and Characterization of the OFET Devices
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, S.H.; Girma, H.G.; Sim, K.M.; Yoon, S.; Park, J.M.; Kong, H.; Chung, D.S. Polymer-based flexible NO x sensors with ppb-level detection at room temperature using breath-figure molding. Nanoscale 2019, 11, 17709–17717. [Google Scholar] [CrossRef] [PubMed]
- Petruci, J.F.d.S.; Tütüncü, E.; Cardoso, A.A.; Mizaikoff, B. Real-Time and Simultaneous Monitoring of NO, NO2, and N2O Using Substrate–Integrated Hollow Waveguides Coupled to a Compact Fourier Transform Infrared (FT-IR) Spectrometer. Appl. Spectrosc. 2019, 73, 98–103. [Google Scholar] [PubMed]
- Lee, B. Highlights of the clean air act amendments off 1990. J. Air Waste Manag. Assoc. 1991, 41, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.; Wang, J.; Wang, L.; He, J.; Jiang, C. Response enhancement mechanism of NO2 gas sensing in ultrathin pentacene field-effect transistors. Org. Electron. 2015, 24, 96–100. [Google Scholar] [CrossRef]
- Mane, A.; Moholkar, A. Effect of solution concentration on physicochemical and NO2 gas sensing properties of sprayed MoO3 nanobelts. Thin Solid Films 2018, 648, 50–61. [Google Scholar] [CrossRef]
- Suceska, M.; Tumara, B.S.; Skrlec, V.; Stankovic, S. Prediction of concentration of toxic gases produced by detonation of commercial explosives by thermochemical equilibrium calculations. Def. Technol. 2021, 18, 2181–2189. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- Hamed, A.M.; Wahab, B.I. Measuring the Acid Rain in Heet City of Iraq. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Raipur, India, 26–30 June 2022; p. 012022. [Google Scholar]
- Panigrahi, T.H.; Sahoo, S.R.; Murmu, G.; Maity, D.; Saha, S. Current challenges and developments of inorganic/organic materials for the abatement of toxic nitrogen oxides (NOx)–A critical review. Prog. Solid. State Chem. 2022, 68, 100380. [Google Scholar] [CrossRef]
- García-Morin, M.; Manrique-Martin, G.; Ropero, P.; Bardón-Cancho, E.; García-Rovés, R.Á.; Beléndez, C.; Cela, E. Hb M-Saskatoon: An unusual cause of cyanosis in a Spanish child. Pediatr. Hematol. Oncol. J. 2019, 4, 23–26. [Google Scholar] [CrossRef]
- Porrini, C.; Ramarao, N.; Tran, S.-L. Dr. NO and Mr. Toxic–the versatile role of nitric oxide. Biol. Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef]
- Khot, S.; Phalake, S.; Mahadik, S.; Baragale, M.; Jagadale, S.; Burungale, V.; Navale, Y.; Patil, V.; Patil, V.; Patil, P. Synthesis of CuO thin film sensors by spray pyrolysis method for NO2 gas detection. Mater. Today Proc. 2021, 43, 2694–2697. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Shendage, S.; Patil, V.; Vanalakar, S.; Patil, S.; Harale, N.; Bhosale, J.; Kim, J.; Patil, P. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B Chem. 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High surface area MoS2/graphene hybrid aerogel for ultrasensitive NO2 detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Diao, Y.; Yang, Z.; He, J.; Wang, J.; Liu, C.; Liu, F.; Lu, H.; Yan, X.; Sun, P. Enhanced room temperature gas sensor based on Au-loaded mesoporous In2O3 nanospheres@ polyaniline core-shell nanohybrid assembled on flexible PET substrate for NH3 detection. Sens. Actuators B Chem. 2018, 276, 526–533. [Google Scholar] [CrossRef]
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas sensors based on chemi-resistive hybrid functional nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Qu, G.; Mohammadi, E.; Mei, J.; Diao, Y. Solution-processed nanoporous organic semiconductor thin films: Toward health and environmental monitoring of volatile markers. Adv. Funct. Mater. 2017, 27, 1701117. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Zhu, X.; Zhou, X.; Chi, L. An ultrasensitive organic semiconductor NO2 sensor based on crystalline TIPS-Pentacene films. Adv. Mater. 2017, 29, 1703192. [Google Scholar] [CrossRef]
- Tran, V.V.; Jeong, G.; Kim, K.S.; Kim, J.; Jung, H.-R.; Park, B.; Park, J.-J.; Chang, M. Facile Strategy for Modulating the Nanoporous Structure of Ultrathin π-Conjugated Polymer Films for High-Performance Gas Sensors. ACS Sens. 2021, 7, 175–185. [Google Scholar] [CrossRef]
- Darshan, V.; Rajeev, V.; Unni, K.N. Enhanced performance of room temperature ammonia sensors using morphology-controlled organic field-effect transistors. Org. Electron. 2021, 98, 106280. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, S.; Huang, J. OFET chemical sensors: Chemical sensors based on ultrathin organic field-effect transistors. Polym. Int. 2021, 70, 414–425. [Google Scholar] [CrossRef]
- Pernites, R.B.; Foster, E.L.; Felipe, M.J.L.; Robinson, M.; Advincula, R.C. Patterned surfaces combining polymer brushes and conducting polymer via colloidal template electropolymerization. Adv. Mater. 2011, 23, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Bai, H.; Li, L. Breath figure: A nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro-and nanoscale patterning. Nat. Protoc. 2010, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, L.; Zhuang, Z.; Chen, X.; Ni, M.; Li, Y.; Cui, Y.; Zhan, P.; Yuan, C.; Ge, H. A new strategy of lithography based on phase separation of polymer blends. Sci. Rep. 2015, 5, 15947. [Google Scholar] [CrossRef] [Green Version]
- Hulkkonen, H.H.; Salminen, T.; Niemi, T. Block copolymer patterning for creating porous silicon thin films with tunable refractive indices. ACS Appl. Mater. Interfaces 2017, 9, 31260–31265. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Song, J.; Jang, H.-J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-effect transistors. Chem. Rev. 2018, 119, 3–35. [Google Scholar] [CrossRef]
- Chang, M.; Lee, J.; Kleinhenz, N.; Fu, B.; Reichmanis, E. Photoinduced anisotropic supramolecular assembly and enhanced charge transport of poly (3-hexylthiophene) thin films. Adv. Funct. Mater. 2014, 24, 4457–4465. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, H.; Liu, A.; Zhu, H.; Li, B.; Minari, T.; Balestra, F.; Ghibaudo, G.; Noh, Y.Y. Essential effects on the mobility extraction reliability for organic transistors. Adv. Funct. Mater. 2018, 28, 1803907. [Google Scholar] [CrossRef]
- Emerson, J.A.; Toolan, D.T.; Howse, J.R.; Furst, E.M.; Epps, T.H., III. Determination of solvent–polymer and polymer–polymer Flory–Huggins interaction parameters for poly (3-hexylthiophene) via solvent vapor swelling. Macromolecules 2013, 46, 6533–6540. [Google Scholar] [CrossRef]
- Niu, X.; Li, N.; Chen, Q.; Zhou, H. Insights into large-scale fabrication methods in perovskite photovoltaics. Adv. Energy Sustain. Res. 2021, 2, 2000046. [Google Scholar] [CrossRef]
- Heo, K.; Miesch, C.; Na, J.-H.; Emrick, T.; Hayward, R.C. Assembly of P3HT/CdSe nanowire networks in an insulating polymer host. Soft Matter 2018, 14, 5327–5332. [Google Scholar] [CrossRef]
- Otulakowski, L.; Dworak, A.; Forys, A.; Gadzinowski, M.; Slomkowski, S.; Basinska, T.; Trzebicka, B. Micellization of polystyrene-b-polyglycidol in dioxane and water/dioxane solutions. Polymers 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. 2001, 59, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Yoo, R.; Lee, H.-S.; Kim, W.; Park, Y.; Koo, A.; Jin, S.-H.; Pham, T.V.; Kim, M.J.; Maeng, S.; Lee, W. Selective detection of nitrogen-containing compound gases. Sensors 2019, 19, 3565. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Jenjeti, R.N.; Choutipalli, V.S.K.; Subramanian, V.; Sampath, S. Conductometric NOx sensor based on exfoliated two-dimensional layered MnPSe3. Sens. Actuators B Chem. 2021, 347, 130633. [Google Scholar] [CrossRef]
- Kwon, E.H.; An, H.; Park, M.B.; Kim, M.; Park, Y.D. Conjugated polymer–zeolite hybrids for robust gas sensors: Effect of zeolite surface area on NO2 sensing ability. Chem. Eng. J. 2021, 420, 129588. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Zhang, L.; Zeng, H. Ammonia gas sensor based on pentacene organic field-effect transistor. Sens. Actuators B Chem. 2012, 173, 133–138. [Google Scholar] [CrossRef]
- Verma, A.; Sahu, P.K.; Chaudhary, V.; Singh, A.K.; Mishra, V.; Prakash, R. Fabrication and Characterization of P3HT/MoS₂ Thin-Film Based Ammonia Sensor Operated at Room Temperature. IEEE Sens. J. 2022, 22, 10361–10369. [Google Scholar] [CrossRef]
- Jeong, G.; Choi, S.; Jang, M.; Chang, M. Thermal annealing effects on the morphology and charge transport of polymer semiconductor nanowires aligned in an insulating polymer matrix. Dye. Pigment. 2021, 185, 108962. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, J.W.; Jo, G.; Ma, B.C.; Chang, M. Conjugated polymer/paraffin blends for organic field-effect transistors with high environmental stability. Nanoscale 2019, 11, 10004–10016. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Choi, D.; Wang, G.; Kleinhenz, N.; Persson, N.; Park, B.; Reichmanis, E. Photoinduced anisotropic assembly of conjugated polymers in insulating polymer blends. ACS Appl. Mater. Interfaces 2015, 7, 14095–14103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, B.; Huang, L.; Huang, W.; Wang, Z.; Zhu, W.; Chen, Y.; Mao, Y.; Facchetti, A.; Marks, T.J. Breath figure–derived porous semiconducting films for organic electronics. Sci. Adv. 2020, 6, eaaz1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Yang, Z.; Li, Z.; Zhuang, X.; Akinwande, D.; Yu, J. Improved room temperature NO2 sensing performance of organic field-effect transistor by directly blending a hole-transporting/electron-blocking polymer into the active layer. ACS Appl. Mater. Interfaces 2018, 10, 38280–38286. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.J.; Shin, S.Y.; Van Tran, V.; Park, B.; Yoon, H.; Chang, M. Preparation of conjugated polymer/reduced graphene oxide nanocomposites for high-performance volatile organic compound sensors. Chem. Eng. J. 2021, 425, 131424. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, X.; Shi, W.; Yang, X.; Li, L.; Yu, J. Poly (3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B Chem. 2016, 225, 10–15. [Google Scholar] [CrossRef]
- Hou, S.; Yu, J.; Zhuang, X.; Li, D.; Liu, Y.; Gao, Z.; Sun, T.; Wang, F.; Yu, X. Phase separation of P3HT/PMMA blend film for forming semiconducting and dielectric layers in organic thin-film transistors for high-sensitivity NO2 detection. ACS Appl. Mater. Interfaces 2019, 11, 44521–44527. [Google Scholar] [CrossRef]
- Yeh, Y.-M.; Chang, S.-J.; Wang, P.-H.; Hsueh, T.-J. A TSV-Structured Room Temperature p-Type TiO2 Nitric Oxide Gas Sensor. Appl. Sci. 2022, 12, 9946. [Google Scholar] [CrossRef]
- Gupta Chatterjee, S.; Dey, S.; Samanta, D.; Santra, S.; Chatterjee, S.; Guha, P.; Chakraborty, A.K. Near room temperature sensing of nitric oxide using SnO2/Ni-decorated natural cellulosic graphene nanohybrid film. J. Mater. Sci. Mater. Electron. 2018, 29, 20162–20171. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Badi, N.; Imran, M.; Al-Ghamdi, S. Development of high-performance flexible and stretchable sensor based on secondary doped PEDOT–PSS: TiO2 nanocomposite for room-temperature detection of nitric oxide. J. Mater. Sci. Mater. Electron. 2021, 32, 7491–7508. [Google Scholar] [CrossRef]
- Gusain, A.; Joshi, N.J.; Varde, P.; Aswal, D. Flexible NO gas sensor based on conducting polymer poly [N-9′-heptadecanyl-2, 7-carbazole-alt-5, 5-(4′, 7′-di-2-thienyl-2′, 1′, 3′-benzothiadiazole)](PCDTBT). Sens. Actuators B Chem. 2017, 239, 734–745. [Google Scholar] [CrossRef]






| P-P3HT | N-P3HT | |
|---|---|---|
| Film thickness (nm) | 7.8 ± 0.5 | 8.1 ± 0.3 |
| Pore size (µm2) | - | (1.7 ± 0.07) × 10−2 |
| Surface area ratio (%) | 0.061 ± 0.001 | 1.150 ± 0.026 |
| NO Concentration (ppm) | Responsivity (%) | |
|---|---|---|
| P-P3HT | N-P3HT | |
| 0.5 | 5.10 ± 0.01 | 10.1 ± 0.01 |
| 1 | 9.44 ± 0.01 | 17.4 ± 0.01 |
| 2 | 13.1 ± 0.01 | 22.0 ± 0.02 |
| 3 | 15.9 ± 0.01 | 28.2 ± 0.02 |
| 4 | 19.5 ± 0.01 | 31.4 ± 0.03 |
| 5 | 19.7 ± 0.03 | 32.4 ± 0.03 |
| 10 | 28.1 ± 0.06 | 42.5 ± 0.06 |
| 15 | 31.8 ± 0.07 | 41.4 ± 0.07 |
| 20 | 33.6 ± 0.08 | 39.5 ± 0.06 |
| 30 | 36.7 ± 0.09 | 46.6 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.; Shin, S.Y.; Kyokunzire, P.; Cheon, H.J.; Wi, E.; Woo, M.; Chang, M. High-Performance Nitric Oxide Gas Sensors Based on an Ultrathin Nanoporous Poly(3-hexylthiophene) Film. Biosensors 2023, 13, 132. https://doi.org/10.3390/bios13010132
Jeong G, Shin SY, Kyokunzire P, Cheon HJ, Wi E, Woo M, Chang M. High-Performance Nitric Oxide Gas Sensors Based on an Ultrathin Nanoporous Poly(3-hexylthiophene) Film. Biosensors. 2023; 13(1):132. https://doi.org/10.3390/bios13010132
Chicago/Turabian StyleJeong, Ganghoon, Seo Young Shin, Proscovia Kyokunzire, Hyeong Jun Cheon, Eunsol Wi, Minhong Woo, and Mincheol Chang. 2023. "High-Performance Nitric Oxide Gas Sensors Based on an Ultrathin Nanoporous Poly(3-hexylthiophene) Film" Biosensors 13, no. 1: 132. https://doi.org/10.3390/bios13010132