Metal-Organic Frameworks-Based Optical Nanosensors for Analytical and Bioanalytical Applications
Abstract
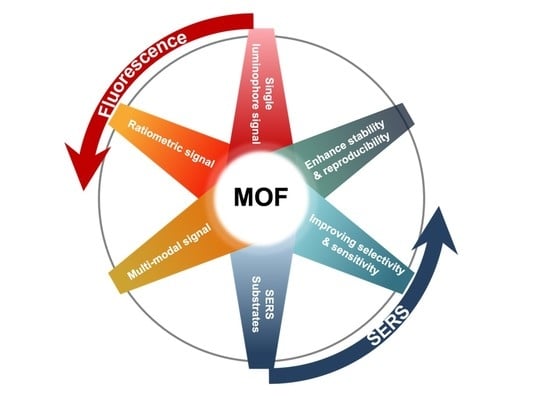
:1. Introduction
2. MOFs-Based Nanosensors for Luminescence Applications
2.1. Single Luminophore Signal
2.2. Ratiometric Signal
2.3. Multi-Modal Signal
| MOF | Synthesis Method | Luminescence Center | λex/em (nm) | Target Molecules | LOD | Ref. |
|---|---|---|---|---|---|---|
| ZnMOF | solvothermal | TCPE | HCl vapor | 2.63 ppm | [6] | |
| CoMOF | histidine | 2 × 10−6 M | ||||
| Eu-ZnMOF | solvothermal | Eu & BPDC | 330/433 & 615 | vanillylmandelic acid | [32] | |
| TbTATAB | solvothermal | Tb & linker | Hg2+ | 4.4 nM | [39] | |
| CDs@ZIF-8 | one-pot room temperature crystal growth | CD | 365/480 | quercetin | 3.5 nM | [40] |
| Ag12bpy-NH2 | bpy-NH2 | 370 | O2 | 11.4 mPa | [42] | |
| Tb(III)@Cd-MOF | solvothermal | Tb3+ | 325/544 | diphenyl phosphate | 0.022 mg/mL | [47] |
| MIL-101(Cr) | hydrothermal | Cy3 | 525/570 | tetrodotoxin | 0.006 ng/mL | [60] |
| ZIF-8⊃4-MU & Flu | solvothermal | 4-MU & Flu | 360/380–450 & 500–570 | temperature | [77] | |
| Zr-MOF | Suzuki coupling | Zr-MOF-ABt Zr-MOF-BDP | 340/403 & 530 | peroxynitrite | [78] | |
| 340/403 & 610 | ||||||
| R-UiO | Suzuki coupling | DBP-Pt/RITC | 514/570 & 630 | O2 | [79] | |
| EuMOF-FITC | hydrothermal | Eu3+ & FITC | 380/525 & 611 | biogenic amine | 1.11 mg/L | [80] |
| Eu3+/Tb3+ MOFs | solvothermal | Eu & Tb | 280/547 & 491, 616 & 592 | Fe3+ | 3.86 μM | [83] |
| Eu0.0069Tb0.9931-DMBDC | solvothermal | Eu & Tb | 355/613 & 545 | temperature | [84] | |
| Ru@MIL-101(Al)-NH2 | one-pot | Ru & linker | 300/465 & 615 | water | 0.02% v/v | [89] |
| UC-PB | ligand-exchange and controllable complexation | UCNP | 980/540 & 654 | H2S | 50 nM | [94] |
| ZnMOF | solvothermal | Linker | 370 | Fe3+ | 28 μM | [101] |
| Pb2+ | 600 μM | |||||
| Cr2O72− | 43 μM | |||||
| CrO42− | 45 μM | |||||
| CdMOF | Fe3+; Pb2+; Cr2O72−; CrO42− | 57 μM; 370 μM; 71 μM; 31 μM | ||||
| ZJU-168(Tb or Eu) | solvothermal | Tb &linker | 340/430 & 544 | Glutamic acid | 3.6 μM | [102] |
| Eu & linker | 340/430 & 614 | 4.3 μM | ||||
| F-UiO | solvothermal | FITC | 488 & 435/520 | pH | [103] | |
| BSA + KFP@ZIF-8/HP +primer + MB | one-pot room temperature crystal growth | Cy5 | survivin mRNA | 2.3 pM | [104] |
3. MOFs-Based Nanosensors in SERS Applications
3.1. SERS Substrates
3.2. Other Effects of MOFs for Improving Selectivity and Sensitivity
3.3. Enhancement of the Stability, Homogeneity, and Reproducibility of SERS Substrates
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stich, M.I.J.; Fischer, L.H.; Wolfbeis, O.S. Multiple fluorescent chemical sensing and imaging. Chem. Soc. Rev. 2010, 39, 3102–3114. [Google Scholar]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.; Lin, J. Yolk-shell structured Au nanostar@metal-organic framework for synergistic chemo-photothermal therapy in the second near-infrared window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef]
- Sun, H.; Yu, B.; Pan, X.; Zhu, X.; Liu, Z. Recent progress in metal–organic frameworks-based materials toward surface-enhanced Raman spectroscopy. Appl. Spectrosc. Rev. 2022, 57, 513–528. [Google Scholar] [CrossRef]
- He, J.; Dong, J.; Hu, Y.; Li, G.; Hu, Y. Design of Raman tag-bridged core-shell Au@Cu3(BTC)2 nanoparticles for Raman imaging and synergistic chemo-photothermal therapy. Nanoscale 2019, 11, 6089–6100. [Google Scholar] [PubMed]
- Zhu, Z.H.; Ni, Z.; Zou, H.H.; Feng, G.; Tang, B.Z. Smart metal–organic frameworks with reversible luminescence/magnetic switch behavior for HCl vapor detection. Adv. Funct. Mater. 2021, 31, 2106925. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous androbust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 341, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, Y.Z.; Zhang, K.; Zhang, L.; Su, C.Y. Engineering porphyrin metal-organic framework composites as multifunctional platforms for CO2 adsorption and activation. J. Am. Chem. Soc. 2020, 142, 14548–14556. [Google Scholar]
- Liu, C.; Xing, J.; Akakuru, O.U.; Luo, L.; Sun, S.; Zou, R.; Yu, Z.; Fang, Q.; Wu, A. Nanozymes-engineered metal-organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett. 2019, 19, 5674–5682. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Yang, J.; Gao, Y.; Huang, T.; Luan, X.; Wang, Y.; Song, Y. Gold nanoparticles doped metal-organic frameworks as near-infrared light-enhanced cascade nanozyme against hypoxic tumors. Nano Res. 2020, 13, 653–660. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Di, Z.; Zhang, G.; Sun, L.D.; Li, L.; Yan, C.H. Engineering of upconverted metal-organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors. J. Am. Chem. Soc. 2020, 142, 3939–3946. [Google Scholar] [PubMed]
- Zhou, H.; Fu, C.; Chen, X.; Tan, L.; Yu, J.; Wu, Q.; Su, L.; Huang, Z.; Cao, F.; Ren, X.; et al. Mitochondria-targeted zirconium metal-organic frameworks for enhancing the efficacy of microwave thermal therapy against tumors. Biomater. Sci. 2018, 6, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, F.; Zhang, J.; Wang, C.; Li, L. A biomimetic coordination nanoplatform for controlled encapsulation and delivery of drug-gene combinations. Angew. Chem. Int. Ed. 2019, 58, 8804–8808. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Patil, S.; Alyami, M.; Alamoudi, K.O.; Aleisa, F.A.; Merzaban, J.S.; Li, M.; Khashab, N.M. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J. Am. Chem. Soc. 2017, 140, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, Y.; Wang, S.; Li, P.; Mirkin, C.A.; Farha, O.K. DNA-functionalized metal-organic framework nanoparticles for intracellular delivery of proteins. J. Am. Chem. Soc. 2019, 141, 2215–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhao, P.; Yang, G.; Chen, X.; Jiang, Y.; Jiang, X.; Wu, Y.; Liu, Y.; Zhang, W.; Bu, W. Reconstructing the intracellular pH microenvironment for enhancing photodynamic therapy. Mater. Horiz. 2020, 7, 1180–1185. [Google Scholar] [CrossRef]
- Chen, Q.W.; Liu, X.H.; Fan, J.X.; Peng, S.Y.; Wang, J.W.; Wang, X.N.; Zhang, C.; Liu, C.J.; Zhang, X.Z. Self-mineralized photothermal bacteria hybridizing with mitochondria-targeted metal–organic frameworks for augmenting photothermal tumor therapy. Adv. Funct. Mater. 2020, 30, 1909806. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, Y.; Wen, C.; Sun, Y.Q.; Yin, X.B. Triple cascade nanocatalyst with laser-activatable O-2 supply and photothermal enhancement for effective catalytic therapy against hypoxic tumor. Biomaterials 2022, 280, 121308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Xu, C.; Lin, C.; Sun, K.; Wang, J.; Chen, X.; Li, L.; Whittaker, A.K.; Xu, H.B. Controlled synthesis of up-conversion luminescent Gd/Tm-MOFs for pH-responsive drug delivery and UCL/MRI dual-modal imaging. Dalton Trans. 2018, 47, 11253–11263. [Google Scholar] [CrossRef]
- Deng, J.; Wang, K.; Wang, M.; Yu, P.; Mao, L. Mitochondria targeted nanoscale zeolitic imidazole framework-90 for ATP imaging in live cells. J. Am. Chem. Soc. 2017, 139, 5877–5882. [Google Scholar]
- Wang, Y.-M.; Liu, W.; Yin, X.-B. Self-limiting growth nanoscale coordination polymers for fluorescence and magnetic resonance dual-modality imaging. Adv. Funct. Mater. 2016, 26, 8463–8470. [Google Scholar] [CrossRef]
- Zhang, G.; Shan, D.; Dong, H.; Cosnier, S.; Al-Ghanim, K.A.; Ahmad, Z.; Mahboob, S.; Zhang, X. DNA-Mediated Nanoscale Metal−Organic frameworks for ultrasensitive photoelectrochemical enzyme-free immunoassay. Anal. Chem. 2018, 90, 12284–12291. [Google Scholar] [CrossRef] [PubMed]
- Sen Bishwas, M.; Malik, M.; Poddar, P. Raman spectroscopy-based sensitive, fast and reversible vapour phase detection of explosives adsorbed on metal–organic frameworks UiO-67. New J. Chem. 2021, 45, 7145–7153. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al(iii)/In(iii)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A new 3D 8-connected Cd(ii) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Micropor. Mesopor. Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal–organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Dong, M.-J.; Zhao, M.; Ou, S.; Zou, C.; Wu, C.-D. A luminescent Dye@MOF platform: Emission fingerprint relationships of volatile organic molecules. Angew. Chem. Int. Ed. 2014, 53, 1575–1579. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.N.; Niu, D.; Gu, J.; Lin, S.; Li, Y.; Shi, J. Structure engineering of a lanthanide-based metal-organic framework for the regulation of dynamic ranges and sensitivities for pheochromocytoma diagnosis. Adv. Mater. 2020, 32, e2000791. [Google Scholar] [CrossRef]
- Hu, M.-L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal–organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598–1632. [Google Scholar] [CrossRef]
- Guo, B.B.; Yin, J.C.; Li, N.; Fu, Z.X.; Han, X.; Xu, J.; Bu, X.H. Recent progress in luminous particle-encapsulated host–guest metal-organic frameworks for optical applications. Adv. Opt. Mater. 2021, 9, 2100283. [Google Scholar] [CrossRef]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter metal–organic framework-based ratiometric fluorescent sensors. Adv. Mater. 2019, 32, 1805871. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, J.; Chen, Y.; Yang, P.; Lin, J. Lanthanide-activated nanoconstructs for optical multiplexing. Coordin. Chem. Rev. 2020, 415, 213328. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yin, X.M.; Yang, S.L.; Zhang, L.; Bu, R.; Gao, E.Q. Chromic and fluorescence-responsive metal-organic frameworks afforded by N-amination modification. Acs Appl. Mater. Inter. 2021, 13, 20380–20387. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Lv, X.; Liu, F.; Feng, L.; Liu, M.; Cai, Y.; Liu, H.; Wang, J.; Yang, Y.; Wang, H. Silver nanoclusters encapsulated into metal–organic frameworks with enhanced fluorescence and specific ion accumulation toward the microdot array-based fluorimetric analysis of copper in blood. ACS Sensors 2018, 3, 441–450. [Google Scholar] [CrossRef]
- Xia, T.; Song, T.; Zhang, G.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. A terbium metal-organic framework for highly selective and sensitive luminescence sensing of Hg2+ ions in aqueous solution. Chem. Eur. J. 2016, 22, 18429–18434. [Google Scholar] [CrossRef]
- Xu, L.; Fang, G.; Liu, J.; Pan, M.; Wang, R.; Wang, S. One-pot synthesis of nanoscale carbon dots-embedded metal–organic frameworks at room temperature for enhanced chemical sensing. J. Mater. Chem. A 2016, 4, 15880–15887. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, N.; Wang, Y.; Bai, F.Y.; Xing, Y.H.; Sun, L.X. Ln-MOFs with window-shaped channels based on triazine tricarboxylic acid as a linker for the highly efficient capture of cationic dyes and iodine. Inorg. Chem. Front. 2021, 8, 1736–1746. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Si, Y.; Yang, J.-S.; Zhang, C.; Han, Z.; Luo, P.; Wang, Z.-Y.; Zang, S.-Q.; Mak, T.C.W. Ligand engineering to achieve enhanced ratiometric oxygen sensing in a silver cluster-based metal-organic framework. Nat. Commun. 2020, 11, 3678. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, C.; Wu, Y.; Yin, Y.; Wu, H.; Li, H.; Fan, Y.; Wu, J.; Li, S.; Huang, X.; et al. Fabrication of two-dimensional metal-organic framework nanosheets through crystal dissolution-growth kinetics. Acs Appl. Mater. Inter. 2022, 14, 7192–7199. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2011, 112, 1126–1162. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid detection of the biomarkers for carcinoid tumors by a water stable luminescent lanthanide metal-organic framework sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Yan, B. Luminescence response mode and chemical sensing mechanism for lanthanide-functionalized metal–organic framework hybrids. Inorg. Chem. Front. 2021, 8, 201–233. [Google Scholar] [CrossRef]
- Qu, X.-L.; Yan, B. Cd-based metal–organic framework containing uncoordinated carbonyl groups as lanthanide postsynthetic modification sites and chemical sensing of diphenyl phosphate as a flame-retardant biomarker. Inorg. Chem. 2020, 59, 15088–15100. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, B.; Luo, J.; Liu, B. Design and property modulation of metal–organic frameworks with aggregation-induced emission. ACS Mater. Lett. 2020, 3, 77–89. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, F.; Wang, W.; Nie, W.; You, W.; Ye, X. Simple synthesis of dual-emission CsPbBr3@EuBTC composite for latent fingerprints and optical anti-counterfeiting applications. Mater. Today Commun. 2022, 33, 104493. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, H.; Wu, J.; Zhai, G.; Li, Z.; Luan, Y.; Garg, S. CuS@MOF-based well-designed quercetin delivery system for chemo-photothermal therapy. Acs Appl. Mater. Inter. 2018, 10, 34513–34523. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Shang, Y.; Li, Y.H.; Cui, Y.; Yin, X.B. An “all-in-one” antitumor and anti-recurrence/metastasis nanomedicine with multi-drug co-loading and burst drug release for multi-modality therapy. Chem. Sci. 2018, 9, 7210–7217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, C.; Wu, X.; Sun, M.; Zhang, H.; Yuan, A.; Xu, L.; Xu, C.; Kuang, H. Chiral core-shell upconversion nanoparticle@MOF nanoassemblies for quantification and bioimaging of reactive oxygen species in vivo. J. Am. Chem. Soc. 2019, 141, 19373–19378. [Google Scholar] [CrossRef]
- He, L.; Brasino, M.; Mao, C.; Cho, S.; Park, W.; Goodwin, A.P.; Cha, J.N. DNA-assembled core-satellite upconverting-metal-organic framework nanoparticle superstructures for efficient photodynamic therapy. Small 2017, 13, 1700504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, J.; He, L.; Liu, Y.; Liu, Y.; Chen, C.; Tang, Z. Core-shell upconversion nanoparticle@metal-organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging. Adv. Mater. 2015, 27, 4075–4080. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, P.; Wang, C.; Goh, Y.T.; Fang, Z.; Messersmith, P.B.; Duan, H. Versatile core-shell nanoparticle@metal-organic framework nanohybrids: Exploiting mussel-inspired polydopamine for tailored structural integration. ACS Nano 2015, 9, 6951–6960. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Cai, X.; Sun, C.; Liang, S.; Shao, S.; Huang, S.; Cheng, Z.; Pang, M.; Xing, B.; Kheraif, A.A.A.; et al. O2-loaded pH-responsive multifunctional nanodrug carrier for overcoming hypoxia and highly efficient chemo-photodynamic cancer therapy. Chem. Mater. 2018, 31, 483–490. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef]
- Liang, S.; Sun, C.; Yang, P.; Ma, P.; Huang, S.; Cheng, Z.; Yu, X.; Lin, J. Core-shell structured upconversion nanocrystal-dendrimer composite as a carrier for mitochondria targeting and catalase enhanced anti-cancer photodynamic therapy. Biomaterials 2020, 240, 119850. [Google Scholar] [CrossRef]
- Liu, S.; Huo, Y.; Deng, S.; Li, G.; Li, S.; Huang, L.; Ren, S.; Gao, Z. A facile dual-mode aptasensor based on AuNPs@MIL-101 nanohybrids for ultrasensitive fluorescence and surface-enhanced Raman spectroscopy detection of tetrodotoxin. Biosens. Bioelectron. 2022, 201, 113891. [Google Scholar] [CrossRef]
- Shang, W.; Zeng, C.; Du, Y.; Hui, H.; Liang, X.; Chi, C.; Wang, K.; Wang, Z.; Tian, J. Core-shell gold nanorod@metal-organic framework nanoprobes for multimodality diagnosis of glioma. Adv. Mater. 2017, 29, 1604381. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Cao, M.; Zhang, X.; Lin, J.; Tian, Q.; Yang, S. pH and glutathione synergistically triggered release and self-assembly of Au nanospheres for tumor theranostics. Acs Appl. Mater. Inter. 2020, 12, 8050–8061. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, A.; Chen, X.; Wang, T. Understanding the role of metal-organic frameworks in surface-enhanced raman scattering application. Small 2020, 16, e2004802. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Su, B.; Liu, C.; Song, Q.; Luo, D.; Mo, G.; Wang, T. Selective surface enhanced Raman scattering for quantitative detection of lung cancer biomarkers in superparticle@MOF structure. Adv. Mater. 2018, 30, 1702275. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Zhang, D.; Wang, C.-e.; Tang, Z.; Zou, M.; Yang, X.; Gong, H.; Yu, Z.; Jin, S.; Liang, P. Ag@MIL-101(Cr) film substrate with high SERS enhancement effect and uniformity. J. Phys. Chem. C 2021, 125, 7297–7304. [Google Scholar] [CrossRef]
- Biswas, S.; Chen, Y.; Xie, Y.; Sun, X.; Wang, Y. Ultrasmall Au(0) inserted hollow PCN-222 MOF for the high-sensitive detection of estradiol. Anal. Chem. 2020, 92, 4566–4572. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Wu, Y.; Jia, M.; Zhou, C.; Xu, Z. Ultrafine metal species confined in metal–organic frameworks: Fabrication, characterization and photocatalytic applications. Coordin. Chem. Rev. 2021, 439, 213924. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, H.; Wang, F.; Zhu, M.; Meng, H.; Wan, P.; Feng, L. Target-responsive smart nanomaterials via a Au–S binding encapsulation strategy for electrochemical/colorimetric dual-mode paper-based analytical devices. Anal. Chem. 2022, 94, 2569–2577. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-enhanced raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef]
- De Marchi, S.; Vázquez-Iglesias, L.; Bodelón, G.; Pérez-Juste, I.; Fernández, L.Á.; Pérez-Juste, J.; Pastoriza-Santos, I. Programmable modular assembly of functional proteins on Raman-encoded zeolitic imidazolate framework-8 (ZIF-8) nanoparticles as SERS tags. Chem. Mater. 2020, 32, 5739–5749. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Lam, S.H.; Wang, J.; Wen, S.; Yam, C.; Shao, L.; Wang, J. Site-selective deposition of metal–organic frameworks on gold nanobipyramids for surface-enhanced Raman scattering. Nano Lett. 2021, 21, 8205–8212. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Lim, H.; Na, J.; Eguchi, M.; Kim, H.-J.; Elashnikov, R.; Postnikov, P.; Svorcik, V.; Semyonov, O.; Miliutina, E.; et al. Enantioselective SERS sensing of pseudoephedrine in blood plasma biomatrix by hierarchical mesoporous Au films coated with a homochiral MOF. Biosens. Bioelectron. 2021, 180, 113109. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, L.; Kang, S.-Z.; Li, X. A special zinc metal-organic frameworks-controlled composite nanosensor for highly sensitive and stable SERS detection. Appl. Surf. Sci. 2021, 550, 149302. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Li, G.; Zhang, R. A composite prepared from gold nanoparticles and a metal organic framework (type MOF-74) for determination of 4-nitrothiophenol by surface-enhanced Raman spectroscopy. Microchim. Acta 2019, 186, 477. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and sensitive SERS platform with analyte enrichment and filtration function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, P.; Yang, L.; Huang, C.; Li, Y. Facile in situ synthesis of silver nanoparticles on the surface of metal–organic framework for ultrasensitive surface-Enhanced Raman scattering detection of dopamine. Anal. Chem. 2015, 87, 12177–12182. [Google Scholar] [CrossRef]
- Cao, W.; Cui, Y.; Yang, Y.; Qian, G. Dyes encapsulated nanoscale metal–organic frameworks for multimode temperature sensing with high spatial resolution. ACS Mater. Lett. 2021, 3, 1426–1432. [Google Scholar] [CrossRef]
- Ding, Z.; Tan, J.; Feng, G.; Yuan, Z.; Wu, C.; Zhang, X. Nanoscale metal–organic frameworks coated with poly(vinyl alcohol) for ratiometric peroxynitrite sensing through FRET. Chem. Sci. 2017, 8, 5101–5106. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Wang, Y.; Duan, X.; Lu, K.; Micheroni, D.; Hu, A.; Lin, W. Nanoscale metal–organic frameworks for ratiometric oxygen sensing in live cells. J. Am. Chem. Soc. 2016, 138, 2158–2161. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, D.; Ye, Y.; Qiu, Y.; Liu, J.; Huang, L.; Liang, B.; Chen, B. A fluorescent metal–organic framework for food real-time visual monitoring. Adv. Mater. 2021, 33, 2008020. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.L.; Li, Y.A.; Dong, Y.B. Diiodo-bodipy-encapsulated nanoscale metal-organic framework for pH-driven selective and mitochondria targeted photodynamic therapy. Inorg. Chem. 2018, 57, 10137–10145. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Lim, W.Q.; Phua, S.Z.F.; Xu, P.; Chen, Q.; Guo, Z.; Zhao, Y. A mesoporous nanoenzyme derived from metal-organic frameworks with endogenous oxygen generation to alleviate tumor hhypoxia for significantly enhanced photodynamic therapy. Adv. Mater. 2019, 31, e1901893. [Google Scholar]
- Su, Y.; Yu, J.; Li, Y.; Phua, S.F.Z.; Liu, G.; Lim, W.Q.; Yang, X.; Ganguly, R.; Dang, C.; Yang, C.; et al. Versatile bimetallic lanthanide metal-organic frameworks for tunable emission and efficient fluorescence sensing. Commun. Chem. 2018, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef]
- Feng, T.; Ye, Y.; Liu, X.; Cui, H.; Li, Z.; Zhang, Y.; Liang, B.; Li, H.; Chen, B. A robust mixed-lanthanide polyMOF membrane for ratiometric temperature sensing. Angew. Chem. Int. Ed. 2020, 59, 21752–21757. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-B.; Sun, Y.-Q.; Yu, H.; Cheng, Y.; Wen, C. Design and multiple applications of mixed-ligand metal–organic frameworks with dual emission. Anal. Chem. 2022, 94, 4938–4947. [Google Scholar] [CrossRef]
- Yin, H.Q.; Yin, X.B. Metal-organic frameworks with multiple luminescence emissions: Designs and applications. Acc. Chem. Res. 2020, 53, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, F.; Yin, X.-B. A ratiometric fluorescence platform based on boric-acid-functional Eu-MOF for sensitive detection of H2O2 and glucose. Biosens. Bioelectron. 2019, 135, 208–215. [Google Scholar] [CrossRef]
- Yin, H.-Q.; Yang, J.-C.; Yin, X.-B. Ratiometric fluorescence sensing and real-time detection of water in organic solvents with one-pot synthesis of Ru@MIL-101(Al)–NH2. Anal. Chem. 2017, 89, 13434–13440. [Google Scholar] [CrossRef]
- Wen, C.; Yin, F.; Cheng, Y.; Yu, H.; Sun, Y.Q.; Yin, X.B. Construction of NaYF4 Library for Morphology-Controlled Multimodality Applications. Small 2021, 17, e2103206. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, K.; Han, X.; He, Y.; Chen, B. Recent progress on porous MOFs for process-efficient hydrocarbon separation, luminescent sensing, and information encryption. Chem. Commun. 2022, 58, 747–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, C.Y.; Mo, J.T.; Fu, P.Y.; Zhao, Y.W.; Yin, S.Y.; Jiang, J.J.; Pan, M.; Su, C.Y. White-light emission from dual-way photon energy conversion in a dye-encapsulated metal–organic framework. Angew. Chem. Int. Ed. 2019, 58, 9752–9757. [Google Scholar] [CrossRef]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Q.; Zhai, X.; Mao, F.; Jiang, A.; Zhou, J. Rationally designed pure-inorganic upconversion nanoprobes for ultra-highly selective hydrogen sulfide imaging and elimination in vivo. Chem. Sci. 2019, 10, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yin, X.-B. Mixed-ligand metal–organic frameworks for all-in-one theranostics with controlled drug delivery and enhanced photodynamic therapy. ACS Appl. Mater. Interfaces 2022, 14, 26528–26535. [Google Scholar] [CrossRef]
- Wang, Y.M.; Liu, W.; Yin, X.B. Multifunctional mixed-metal nanoscale coordination polymers for triple-modality imaging-guided photodynamic therapy. Chem. Sci. 2017, 8, 3891–3897. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Li, N.; Wang, Y.; Xie, Z.; Huang, H.; Yang, G.; Li, T.; Qin, X.; Li, S.; Yang, H.; et al. Zeolitic imidazolate frameworks-based nanomaterials for biosensing, cancer imaging and phototheranostics. Appl. Mater. Today 2021, 23, 100995. [Google Scholar] [CrossRef]
- Cheng, Y.; Wen, C.; Sun, Y.Q.; Yu, H.; Yin, X.B. Mixed-metal MOF-derived hollow porous nanocomposite for trimodality imaging guided reactive oxygen species-augmented synergistic therapy. Adv. Funct. Mater. 2021, 31, 2104378. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Shi, Y.; Cui, Y.; Chen, B.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhong, C.; et al. Novel biological functions of ZIF-NP as a delivery vehicle: High pulmonary accumulation, favorable biocompatibility, and improved therapeutic outcome. Adv. Funct. Mater. 2016, 26, 2715–2727. [Google Scholar] [CrossRef]
- Yang, P.; Men, Y.; Tian, Y.; Cao, Y.; Zhang, L.; Yao, X.; Yang, W. Metal–organic framework nanoparticles with near-infrared dye for multimodal imaging and guided phototherapy. Acs Appl. Mater. Inter. 2019, 11, 11209–11219. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Zhao, Z.; Sun, Z.; Pu, Y.; Wang, Y.; Wang, X. Multifunctional MOF-based probes for efficient detection and discrimination of Pb2+, Fe3+ and Cr2O72−/CrO42−. Dalton Trans. 2021, 50, 12197–12207. [Google Scholar] [CrossRef]
- Xia, T.; Wan, Y.; Li, Y.; Zhang, J. Highly stable lanthanide metal–organic framework as an internal calibrated luminescent sensor for glutamic acid, a neuropathy biomarker. Inorg. Chem. 2020, 59, 8809–8817. [Google Scholar] [CrossRef]
- He, C.; Lu, K.; Lin, W. Nanoscale metal–organic frameworks for real-time intracellular pH sensing in live cells. J. Am. Chem. Soc. 2014, 136, 12253–12256. [Google Scholar] [CrossRef]
- Zhang, J.; He, M.; Nie, C.; He, M.; Pan, Q.; Liu, C.; Hu, Y.; Chen, T.; Chu, X. Biomineralized metal–organic framework nanoparticles enable a primer exchange reaction-based DNA machine to work in living cells for imaging and gene therapy. Chem. Sci. 2020, 11, 7092–7101. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Su, H.-S.; Chang, X.; Xu, B. Surface-enhanced vibrational spectroscopies in electrocatalysis: Fundamentals, challenges, and perspectives. Chin. J. Catal. 2022, 43, 2757–2771. [Google Scholar] [CrossRef]
- Chang, X.; Vijay, S.; Zhao, Y.; Oliveira, N.J.; Chan, K.; Xu, B. Understanding the complementarities of surface-enhanced infrared and Raman spectroscopies in CO adsorption and electrochemical reduction. Nat. Commun. 2022, 13, 2656. [Google Scholar] [CrossRef]
- Chen, S.-H.; He, W.-J.; Zhu, Y.-J.; Song, H.-T. A luminescent turn-off sensor for Cr(VI) anions recognition derived from a Zn(II)-based metal–organic framework. Inorg. Chim. Acta 2021, 525, 120498. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Hassan, M.M.; Ali, S.; Chen, Q. SERS based artificial peroxidase enzyme regulated multiple signal amplified system for quantitative detection of foodborne pathogens. Food Control 2021, 123, 107733. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, J.; Wang, D.; Li, G. Fabrication of gold nanoparticle-embedded metal-organic framework for highly sensitive surface-enhanced Raman scattering detection. Anal. Chem. 2014, 86, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Su, L.; Ma, X.; Duan, Z.; Xiong, Y. A mixed valence state Mo-based metal–organic framework from photoactivation as a surface-enhanced Raman scattering substrate. New J. Chem. 2021, 45, 5121–5126. [Google Scholar] [CrossRef]
- Fu, J.H.; Zhong, Z.; Xie, D.; Guo, Y.J.; Kong, D.X.; Zhao, Z.X.; Zhao, Z.X.; Li, M. SERS-active MIL-100(Fe) sensory array for ultrasensitive and multiplex detection of VOCs. Angew. Chem. Int. Ed. 2020, 59, 20489–20498. [Google Scholar] [CrossRef]
- Osterrieth, J.W.M.; Wright, D.; Noh, H.; Kung, C.W.; Vulpe, D.; Li, A.; Park, J.E.; Van Duyne, R.P.; Moghadam, P.Z.; Baumberg, J.J.; et al. Core-shell gold nanorod@zirconium-based metal-organic framework composites as in situ size-selective Raman probes. J. Am. Chem. Soc. 2019, 141, 3893–3900. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cong, S.; Zheng, Z.; Wang, Z.; Chen, Z.; Zhao, Z. Metal–organic frameworks as surface enhanced Raman scattering substrates with high tailorability. J. Am. Chem. Soc. 2018, 141, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, Y.; Zhang, H.; Luo, X.; Yuan, R.; Yang, X. Charge-transfer resonance and surface defect-dominated WO3 hollow microspheres as SERS substrates for the miRNA 155 assay. Anal. Chem. 2022, 94, 6967–6975. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gong, M.; Shah, F.U.; Laaksonen, A.; An, R.; Ji, X. Phosphonium-based ionic liquid significantly enhances SERS of cytochrome c on TiO2 nanotube arrays. Acs Appl. Mater. Inter. 2022, 14, 27456–27465. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zamani, R.; Rivera Gil, P.; Pelaz, B.; Ibanez, M.; Cadavid, D.; Shavel, A.; Alvarez-Puebla, R.A.; Parak, W.J.; Arbiol, J.; et al. CuTe nanocrystals: Shape and size control, plasmonic properties, and use as SERS probes and photothermal agents. J. Am. Chem. Soc. 2013, 135, 7098–7101. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Yi, Q.; Zhang, J. Chiral carbonaceous nanotubes modified with titania nanocrystals: Plasmon-free and recyclable SERS sensitivity. Angew. Chem. Int. Ed. 2015, 54, 10643–10647. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.J.; Kim, H.; Yi, G.C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of chemical enhancement mechanism in non-plasmonic surface enhanced Raman spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.-H.; Ho, C.-H.; Wu, C.-Y.; Chien, C.-H.; Lin, C.-H.; Lee, S. Metal-organic frameworks: A novel SERS substrate. J. Raman Spectrosc. 2013, 44, 1506–1511. [Google Scholar] [CrossRef]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Hou, J.G. Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Doering, W.E.; Nie, S. Single-molecule and single-nanoparticle SERS: Examining the roles of surface active sites and chemical enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- ÇElİK, Y.; Kurt, A. Three dimensional porous expanded graphite/silver nanoparticles nanocomposite platform as a SERS substrate. Appl. Surf. Sci. 2021, 568, 150946. [Google Scholar] [CrossRef]
- Xu, F.; Shang, W.; Xuan, M.; Ma, G.; Ben, Z. Layered filter paper-silver nanoparticle-ZIF-8 composite for efficient multi-mode enrichment and sensitive SERS detection of thiram. Chemosphere 2022, 288, 132635. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Kong, X.; Yao, K.; Liu, L.; Zhang, J.; Guo, Z.; Xu, W.; Fang, Z.; Liu, Y. Photocatalytic performance of the MOF-coating layer on SPR-excited Ag nanowires. ACS Omega 2021, 6, 2882–2889. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Qi, H.; Chen, Y.; Guo, W.; Yu, H.; Chen, H.; Ying, Y. Humidity-independent artificial olfactory array enabled by hydrophobic core-shell dye/MOFs@COFs composites for plant disease diagnosis. ACS Nano 2022, 16, 14297–14307. [Google Scholar] [CrossRef]
- Yang, F.P.; He, Q.T.; Jiang, H.; Li, Z.; Chen, W.; Chen, R.L.; Tang, X.Y.; Cai, Y.P.; Hong, X.J. Rapid and specific enhanced luminescent switch of aniline gas by MOFs assembled from a planar binuclear cadmium(II) metalloligand. Inorg. Chem. 2022, 61, 10844–10851. [Google Scholar] [CrossRef]
- Cheung, R.C.; Wong, J.H.; Ng, T.B. Immobilized metal ion affinity chromatography: A review on its applications. Appl. Microbiol. Biotechnol. 2012, 96, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng.J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Du, L.; Zhang, B.; Deng, W.; Cheng, Y.; Xu, L.; Mai, L. Hierarchically self-assembled MOF network enables continuous ion transport and high mechanical strength. Adv. Energy Mater. 2022, 12, 2200501. [Google Scholar] [CrossRef]










| SERS Substrate | Target Molecules | Enhancement Mechanism | LOD | Ref |
|---|---|---|---|---|
| UiO-67 | 2,4,6-Trinitrophenol (TNP) | CM a | [23] | |
| Au/MOF-199 | acetamiprid | EM b & CM | 10−8 M | [42] |
| Au@MIL-101 (Cr) | tetrodotoxin | EM & CM | 0.008 ng/mL | [60] |
| GSPs@ZIF-8 | aldehyde VOCs | EM & CM | 10−9 M | [64] |
| Ag@MIL-101 (Cr) | nitrofurantion | EM & CM | 10−7 M | [65] |
| APTES@ZIF-67 | benzaldehyde | 10−2 M | [65] | |
| ZIF-8 | methyl orange | 10−4 M | [65] | |
| Au-L/D-AlaZnCl | pseudoephedrine | EM & CM | 10−12 M | [72] |
| Cu2O@SiO2@ZIF-8@Ag | phenol red | EM & CM | 10−12 M | [73] |
| Au/MOF-74 | 4-nitrothiophenol | EM & CM | 69 nmol·L−1 | [74] |
| Au@ZIF-8 | 4-nitrobenzenethiol | EM & CM | 0.1 nM | [75] |
| Ag@MIL-101 (Fe) | dopamine | EM & CM | 0.32 p M | [76] |
| Au/MIL-101 | rhodamine 6G; benzadine | EM & CM | 41.75 fmol; 0.54 fmol | [110] |
| Mo-MOF | crystal violet | CM | 10−6 M | [111] |
| MIL-100 (Fe) | toluene | CM | 2.5 ppm | [112] |
| acetone | 20 ppm | |||
| chloroform | 20 ppm | |||
| isopropanol | 100 ppm | |||
| 4-ethylbenzaldehyde | 26 ppm | |||
| MIL-100 (Fe-Zr) | isopropanol | 50 ppm | [112] | |
| Au@MIL-101 (Fe) | toluene | EM & CM | 0.48 ppb | [112] |
| Au@NU-901 | 4′-mercaptobiphenylcarbonitrile | EM & CM | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Li, R.; Chang, X.; Li, N. Metal-Organic Frameworks-Based Optical Nanosensors for Analytical and Bioanalytical Applications. Biosensors 2023, 13, 128. https://doi.org/10.3390/bios13010128
Wen C, Li R, Chang X, Li N. Metal-Organic Frameworks-Based Optical Nanosensors for Analytical and Bioanalytical Applications. Biosensors. 2023; 13(1):128. https://doi.org/10.3390/bios13010128
Chicago/Turabian StyleWen, Cong, Rongsheng Li, Xiaoxia Chang, and Na Li. 2023. "Metal-Organic Frameworks-Based Optical Nanosensors for Analytical and Bioanalytical Applications" Biosensors 13, no. 1: 128. https://doi.org/10.3390/bios13010128