Probing the Hepatitis B Virus E-Antigen with a Nanopore Sensor Based on Collisional Events Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Protein Preparation
2.2.1. HBeAg Cloning
2.2.2. HBeAg Expression
2.2.3. HBeAg Purification
2.2.4. HBeAg Quantification
2.3. Single-Molecule Electrophysiology
2.4. Representation of Molecular Structures
3. Results and Discussion
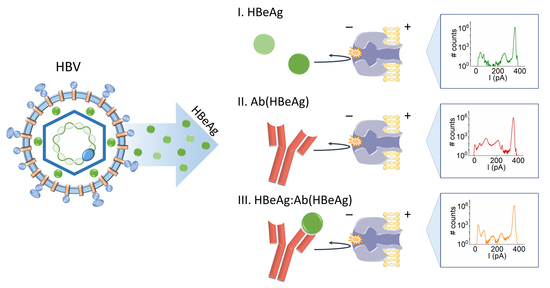
- Experimental Principle
- HBeAg and Ab(HBeAg) proteins interact with the α-HL’s vestibule entrance and generate collisional-based alterations of the ionic current
- Nanopore detection of HBeAg–Ab(HBeAg) complexes
- Noise analysis of ionic current fluctuations generated by HBeAg, Ab(HBeAg), or HBeAg–Ab(HBeAg) complex interactions with the α-HL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasianowicz, J.J.; Brandin, E.; Branton, D.; Deamer, D.W. Characterization of Individual Polynucleotide Molecules Using a Membrane Channel. Proc. Natl. Acad. Sci. USA 1996, 93, 13770–13773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.; et al. The Potential and Challenges of Nanopore Sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Ying, Y.-L.; Hu, Z.-L.; Liao, D.-F.; Tian, H.; Long, Y.-T. Discrimination of Oligonucleotides of Different Lengths with a Wild-Type Aerolysin Nanopore. Nat. Nanotechnol. 2016, 11, 713–718. [Google Scholar] [CrossRef]
- Stoddart, D.; Heron, A.J.; Mikhailova, E.; Maglia, G.; Bayley, H. Single-Nucleotide Discrimination in Immobilized DNA Oligonucleotides with a Biological Nanopore. Proc. Natl. Acad. Sci. USA 2009, 106, 7702–7707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howorka, S.; Cheley, S.; Bayley, H. Sequence-Specific Detection of Individual DNA Strands Using Engineered Nanopores. Nat. Biotechnol. 2001, 19, 636–639. [Google Scholar] [CrossRef]
- Venkatesan, B.M.; Bashir, R. Nanopore Sensors for Nucleic Acid Analysis. Nat. Nanotechnol. 2011, 6, 615–624. [Google Scholar] [CrossRef]
- Manrao, E.A.; Derrington, I.M.; Laszlo, A.H.; Langford, K.W.; Hopper, M.K.; Gillgren, N.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Reading DNA at Single-Nucleotide Resolution with a Mutant MspA Nanopore and Phi29 DNA Polymerase. Nat. Biotechnol. 2012, 30, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, F.; Li, J.; Wu, H.-C.; Liang, X.-J.; Guo, P. Solid-State and Biological Nanopore for Real-Time Sensing of Single Chemical and Sequencing of DNA. Nano Today 2013, 8, 56–74. [Google Scholar] [CrossRef] [Green Version]
- Mereuta, L.; Asandei, A.; Schiopu, I.; Park, Y.; Luchian, T. Nanopore-Assisted, Sequence-Specific Detection, and Single-Molecule Hybridization Analysis of Short, Single-Stranded DNAs. Anal. Chem. 2019, 91, 8630–8637. [Google Scholar] [CrossRef]
- Mereuta, L.; Asandei, A.; Dragomir, I.; Park, J.; Park, Y.; Luchian, T. A Nanopore Sensor for Multiplexed Detection of Short Polynucleotides Based on Length-Variable, Poly-Arginine-Conjugated Peptide Nucleic Acids. Anal. Chem. 2022, 94, 8774–8782. [Google Scholar] [CrossRef]
- Luchian, T.; Mereuta, L.; Park, Y.; Asandei, A.; Schiopu, I. Single-molecule, Hybridization-based Strategies for Short Nucleic Acids Detection and Recognition with Nanopores. Proteomics 2021, 22, e2100046. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Akeson, M.; Branton, D. Three Decades of Nanopore Sequencing. Nat. Biotechnol. 2016, 34, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.-L.; Zhang, J.; Gao, R.; Long, Y.-T. Nanopore-Based Sequencing and Detection of Nucleic Acids. Angew. Chem. Int. Ed. 2013, 52, 13154–13161. [Google Scholar] [CrossRef]
- Wanunu, M. Nanopores: A Journey towards DNA Sequencing. Phys. Life Rev. 2012, 9, 125–158. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, E.; Dong, Z.; Tennant, C.; Timp, G. Reading the Primary Structure of a Protein with 0.07 Nm3 Resolution Using a Subnanometre-Diameter Pore. Nat. Nanotechnol. 2016, 11, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, H.; Sarthak, K.; Ensslen, T.; Piguet, F.; Manivet, P.; Pelta, J.; Behrends, J.C.; Aksimentiev, A.; Oukhaled, A. Electrical Recognition of the Twenty Proteinogenic Amino Acids Using an Aerolysin Nanopore. Nat. Biotechnol. 2020, 38, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Afshar Bakshloo, M.; Kasianowicz, J.J.; Pastoriza-Gallego, M.; Mathé, J.; Daniel, R.; Piguet, F.; Oukhaled, A. Nanopore-Based Protein Identification. J. Am. Chem. Soc. 2022, 144, 2716–2725. [Google Scholar] [CrossRef]
- Asandei, A.; Rossini, A.E.; Chinappi, M.; Park, Y.; Luchian, T. Protein Nanopore-Based Discrimination between Selected Neutral Amino Acids from Polypeptides. Langmuir 2017, 33, 14451–14459. [Google Scholar] [CrossRef] [Green Version]
- Motone, K.; Cardozo, N.; Nivala, J. Herding Cats: Label-Based Approaches in Protein Translocation through Nanopore Sensors for Single-Molecule Protein Sequence Analysis. Iscience 2021, 24, 103032. [Google Scholar] [CrossRef]
- Oukhaled, A.; Bacri, L.; Pastoriza-Gallego, M.; Betton, J.-M.; Pelta, J. Sensing Proteins through Nanopores: Fundamental to Applications. ACS Chem. Biol. 2012, 7, 1935–1949. [Google Scholar] [CrossRef]
- Waduge, P.; Hu, R.; Bandarkar, P.; Yamazaki, H.; Cressiot, B.; Zhao, Q.; Whitford, P.C.; Wanunu, M. Nanopore-Based Measurements of Protein Size, Fluctuations, and Conformational Changes. ACS Nano 2017, 11, 5706–5716. [Google Scholar] [CrossRef]
- Oukhaled, A.; Cressiot, B.; Bacri, L.; Pastoriza-Gallego, M.; Betton, J.-M.; Bourhis, E.; Jede, R.; Gierak, J.; Auvray, L.; Pelta, J. Dynamics of Completely Unfolded and Native Proteins through Solid-State Nanopores as a Function of Electric Driving Force. ACS Nano 2011, 5, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Talaga, D.S.; Li, J. Single-Molecule Protein Unfolding in Solid State Nanopores. J. Am. Chem. Soc. 2009, 131, 9287–9297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zhao, Q.; Kang, X.; Guan, X. Probing Mercury(II)-DNA Interactions by Nanopore Stochastic Sensing. J. Phys. Chem. B 2013, 117, 4763–4769. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Zeng, T.; Liu, L.; Zhao, K.; Zhao, Y.; Liu, X.; Wu, H.-C. Highly Sensitive and Selective DNA-Based Detection of Mercury(II) with α-Hemolysin Nanopore. J. Am. Chem. Soc. 2011, 133, 18312–18317. [Google Scholar] [CrossRef] [PubMed]
- Asandei, A.; Iftemi, S.; Mereuta, L.; Schiopu, I.; Luchian, T. Probing of Various Physiologically Relevant Metals: Amyloid-β Peptide Interactions with a Lipid Membrane-Immobilized Protein Nanopore. J. Membr. Biol. 2014, 247, 523–530. [Google Scholar] [CrossRef]
- Braha, O.; Gu, L.-Q.; Zhou, L.; Lu, X.; Cheley, S.; Bayley, H. Simultaneous Stochastic Sensing of Divalent Metal Ions. Nat. Biotechnol. 2000, 18, 1005–1007. [Google Scholar] [CrossRef]
- Asandei, A.; Mereuta, L.; Luchian, T. The Kinetics of Ampicillin Complexation by γ-Cyclodextrins. A Single Molecule Approach. J. Phys. Chem. B 2011, 115, 10173–10181. [Google Scholar] [CrossRef]
- Reiner, J.E.; Kasianowicz, J.J.; Nablo, B.J.; Robertson, J.W.F. Theory for Polymer Analysis Using Nanopore-Based Single-Molecule Mass Spectrometry. Proc. Natl. Acad. Sci. USA 2010, 107, 12080–12085. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.W.F.; Rodrigues, C.G.; Stanford, V.M.; Rubinson, K.A.; Krasilnikov, O.V.; Kasianowicz, J.J. Single-Molecule Mass Spectrometry in Solution Using a Solitary Nanopore. Proc. Natl. Acad. Sci. USA 2007, 104, 8207–8211. [Google Scholar] [CrossRef] [Green Version]
- Darvish, A.; Lee, J.S.; Peng, B.; Saharia, J.; VenkatKalyana Sundaram, R.; Goyal, G.; Bandara, N.; Ahn, C.W.; Kim, J.; Dutta, P.; et al. Mechanical Characterization of HIV-1 with a Solid-state Nanopore Sensor. Electrophoresis 2019, 40, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yamamoto, T. Quantification of Virus Particles Using Nanopore-Based Resistive-Pulse Sensing Techniques. Front. Microbiol. 2016, 7, 1500. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Li, L.; Tan, Z.; Zlotnick, A.; Jacobson, S.C. Characterization of Hepatitis B Virus Capsids by Resistive-Pulse Sensing. J. Am. Chem. Soc. 2011, 133, 1618–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjmandi, N.; Van Roy, W.; Lagae, L. Measuring Mass of Nanoparticles and Viruses in Liquids with Nanometer-Scale Pores. Anal. Chem. 2014, 86, 4637–4641. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Y.; Zhou, Q.; Wang, R.; Xia, B.; Ma, D.; Luo, K.; Liu, Q. Translocation of Rigid Rod-Shaped Virus through Various Solid-State Nanopores. Anal. Chem. 2016, 88, 2502–2510. [Google Scholar] [CrossRef]
- McMullen, A.; de Haan, H.W.; Tang, J.X.; Stein, D. Stiff Filamentous Virus Translocations through Solid-State Nanopores. Nat. Commun. 2014, 5, 4171. [Google Scholar] [CrossRef] [Green Version]
- Arima, A.; Tsutsui, M.; Harlisa, I.H.; Yoshida, T.; Tanaka, M.; Yokota, K.; Tonomura, W.; Taniguchi, M.; Okochi, M.; Washio, T.; et al. Selective Detections of Single-Viruses Using Solid-State Nanopores. Sci. Rep. 2018, 8, 16305. [Google Scholar] [CrossRef]
- Coulter, W.H. Means for Counting Particles Suspended in a Fluid. Available online: https://patents.google.com/patent/US2656508A/en (accessed on 13 July 2022).
- Gu, L.-Q.; Shim, J.W. Single Molecule Sensing by Nanopores and Nanopore Devices. Analyst 2010, 135, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Siwy, Z.S.; Howorka, S. Engineered Voltage-Responsive Nanopores. Chem. Soc. Rev. 2010, 39, 1115–1132. [Google Scholar] [CrossRef]
- Majd, S.; Yusko, E.C.; Billeh, Y.N.; Macrae, M.X.; Yang, J.; Mayer, M. Applications of Biological Pores in Nanomedicine, Sensing, and Nanoelectronics. Curr. Opin. Biotechnol. 2010, 21, 439–476. [Google Scholar] [CrossRef] [Green Version]
- Luchian, T.; Park, Y.; Asandei, A.; Schiopu, I.; Mereuta, L.; Apetrei, A. Nanoscale Probing of Informational Polymers with Nanopores. Applications to Amyloidogenic Fragments, Peptides, and DNA-PNA Hybrids. Acc. Chem. Res. 2019, 52, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Chen, X.; Guan, X.; Wang, L. Analysis with Biological Nanopore: On-Pore, off-Pore Strategies and Application in Biological Fluids. Talanta 2021, 223, 121684. [Google Scholar] [CrossRef]
- Mayer, S.F.; Cao, C.; Dal Peraro, M. Biological Nanopores for Single-Molecule Sensing. Iscience 2022, 25, 104145. [Google Scholar] [CrossRef] [PubMed]
- Cairns-Gibson, D.F.; Cockroft, S.L. Functionalised Nanopores: Chemical and Biological Modifications. Chem. Sci. 2022, 13, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ying, Y.-L.; Long, Y.-T. Nanopore Confinement for Electrochemical Sensing at the Single-Molecule Level. Curr. Opin. Electrochem. 2018, 7, 172–178. [Google Scholar] [CrossRef]
- Ying, Y.-L.; Long, Y.-T. Nanopore-Based Single-Biomolecule Interfaces: From Information to Knowledge. J. Am. Chem. Soc. 2019, 141, 15720–15729. [Google Scholar] [CrossRef]
- Lee, K.; Park, K.-B.; Kim, H.-J.; Yu, J.-S.; Chae, H.; Kim, H.-M.; Kim, K.-B. Recent Progress in Solid-State Nanopores. Adv. Mater. 2018, 30, 1704680. [Google Scholar] [CrossRef]
- Ohshiro, T.; Taniguchi, M. Review of the Use of Nanodevices to Detect Single Molecules. Anal. Biochem. 2022, 114645. [Google Scholar] [CrossRef]
- Zuo, J.; Song, N.-N.; Wang, J.; Zhao, X.; Cheng, M.-Y.; Wang, Q.; Tang, W.; Yang, Z.; Qiu, K. Review-Single-Molecule Sensors Based on Protein Nanopores. J. Electrochem. Soc. 2021, 168, 126502. [Google Scholar] [CrossRef]
- Saleh, O.A.; Sohn, L.L. Direct Detection of Antibody–Antigen Binding Using an on-Chip Artificial Pore. Proc. Natl. Acad. Sci. USA 2003, 100, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Carbonaro, A.; Sohn, L.L. A Resistive-Pulse Sensor Chip for Multianalyte Immunoassays. Lab Chip 2005, 5, 1155. [Google Scholar] [CrossRef]
- Uram, J.D.; Ke, K.; Hunt, A.J.; Mayer, M. Submicrometer Pore-Based Characterization and Quantification of Antibody-Virus Interactions. Small 2006, 2, 967–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takakura, T.; Yanagi, I.; Goto, Y.; Ishige, Y.; Kohara, Y. Single-Molecule Detection of Proteins with Antigen-Antibody Interaction Using Resistive-Pulse Sensing of Submicron Latex Particles. Appl. Phys. Lett. 2016, 108, 123701. [Google Scholar] [CrossRef]
- Han, A.; Creus, M.; Schürmann, G.; Linder, V.; Ward, T.R.; de Rooij, N.F.; Staufer, U. Label-Free Detection of Single Protein Molecules and Protein−Protein Interactions Using Synthetic Nanopores. Anal. Chem. 2008, 80, 4651–4658. [Google Scholar] [CrossRef] [Green Version]
- Freedman, K.J.; Bastian, A.R.; Chaiken, I.; Kim, M.J. Solid-State Nanopore Detection of Protein Complexes: Applications in Healthcare and Protein Kinetics. Small 2013, 9, 750–759. [Google Scholar] [CrossRef]
- Ying, Y.-L.; Yu, R.-J.; Hu, Y.-X.; Gao, R.; Long, Y.-T. Single Antibody-Antigen Interactions Monitored via Transient Ionic Current Recording Using Nanopore Sensors. Chem. Commun. 2017, 53, 8620–8623. [Google Scholar] [CrossRef]
- Chuah, K.; Wu, Y.; Vivekchand, S.R.C.; Gaus, K.; Reece, P.J.; Micolich, A.P.; Gooding, J.J. Nanopore Blockade Sensors for Ultrasensitive Detection of Proteins in Complex Biological Samples. Nat. Commun. 2019, 10, 2109. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Haque, F.; Rychahou, P.G.; Evers, B.M.; Guo, P. Engineered Nanopore of Phi29 DNA-Packaging Motor for Real-Time Detection of Single Colon Cancer Specific Antibody in Serum. ACS Nano 2013, 7, 9814–9822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, R.; Gatterdam, V.; Wieneke, R.; Tampé, R.; Rant, U. Stochastic Sensing of Proteins with Receptor-Modified Solid-State Nanopores. Nat. Nanotechnol. 2012, 7, 257–263. [Google Scholar] [CrossRef]
- Yusko, E.C.; Johnson, J.M.; Majd, S.; Prangkio, P.; Rollings, R.C.; Li, J.; Yang, J.; Mayer, M. Controlling Protein Translocation through Nanopores with Bio-Inspired Fluid Walls. Nat. Nanotechnol. 2011, 6, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Apetrei, A.; Ciuca, A.; Lee, J.; Seo, C.H.; Park, Y.; Luchian, T. A Protein Nanopore-Based Approach for Bacteria Sensing. Nanoscale Res. Lett. 2016, 11, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Watts, N.R.; Palmer, I.W.; Kaufman, J.D.; Dearborn, A.D.; Trenbeath, J.L.; Eren, E.; Steven, A.C.; Rader, C.; Wingfield, P.T. Chimeric Rabbit/Human Fab Antibodies against the Hepatitis Be-Antigen and Their Potential Applications in Assays, Characterization, and Therapy. J. Biol. Chem. 2017, 292, 16760–16772. [Google Scholar] [CrossRef] [Green Version]
- Elgouhari, H.M.; Abu-Rajab Tamimi, T.I.; Carey, W.D. Hepatitis B Virus Infection: Understanding Its Epidemiology, Course, and Diagnosis. CCJM 2008, 75, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-J.; Chen, Z.-M.; Wei, M.; Liu, J.-Q.; Li, Z.-L.; Shi, T.-S.; Nian, S.; Fu, R.; Wu, Y.-T.; Zhang, Y.-L.; et al. Specific Determination of Hepatitis B e Antigen by Antibodies Targeting Precore Unique Epitope Facilitates Clinical Diagnosis and Drug Evaluation against Hepatitis B Virus Infection. Emerg. Microbes Infect. 2021, 10, 37–50. [Google Scholar] [CrossRef]
- Lazar, C.; Macovei, A.; Petrescu, S.; Branza-Nichita, N. Activation of ERAD Pathway by Human Hepatitis B Virus Modulates Viral and Subviral Particle Production. PLoS ONE 2012, 7, e34169. [Google Scholar] [CrossRef]
- Dragomir, I.S.; Asandei, A.; Schiopu, I.; Bucataru, I.C.; Mereuta, L.; Luchian, T. The Nanopore-Tweezing-Based, Targeted Detection of Nucleobases on Short Functionalized Peptide Nucleic Acid Sequences. Polymers 2021, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [Green Version]
- DiMattia, M.A.; Watts, N.R.; Stahl, S.J.; Grimes, J.M.; Steven, A.C.; Stuart, D.I.; Wingfield, P.T. Antigenic Switching of Hepatitis B Virus by Alternative Dimerization of the Capsid Protein. Structure 2013, 21, 133–142. [Google Scholar] [CrossRef] [Green Version]
- DiMattia, M.A.; Watts, N.R.; Stahl, S.J.; Grimes, J.M.; Steven, A.C.; Stuart, D.I.; Wingfield, P.T. Crystal Structure of Hepatitis B Virus E-Antigen. Available online: https://www.rcsb.org/structure/3v6z (accessed on 13 July 2022).
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.J.; McPherson, A. Structure of Immunoglobulin. Available online: https://www.rcsb.org/structure/1IGY (accessed on 13 July 2022).
- Harris, L.J.; Skaletsky, E.; McPherson, A. Crystallographic Structure of an Intact IgG1 Monoclonal Antibody. J. Mol. Biol. 1998, 275, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Alpha-Hemolysin from Staphylococcus Aureus 1996. Available online: https://www.rcsb.org/structure/7ahl (accessed on 13 July 2022).
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of Staphylococcal Alpha-Hemolysin, a Heptameric Transmembrane Pore. Science 1996, 274, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.R.; Conway, J.F.; Cheng, N.; Stahl, S.J.; Steven, A.C.; Wingfield, P.T. Role of the Propeptide in Controlling Conformation and Assembly State of Hepatitis B Virus E-Antigen. J. Mol. Biol. 2011, 409, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Pedone, D.; Firnkes, M.; Rant, U. Data Analysis of Translocation Events in Nanopore Experiments. Anal. Chem. 2009, 81, 9689–9694. [Google Scholar] [CrossRef]
- Hartel, A.J.W.; Shekar, S.; Ong, P.; Schroeder, I.; Thiel, G.; Shepard, K.L. High Bandwidth Approaches in Nanopore and Ion Channel Recordings-A Tutorial Review. Anal. Chim. Acta 2019, 1061, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Uram, J.D.; Ke, K.; Mayer, M. Noise and Bandwidth of Current Recordings from Submicrometer Pores and Nanopores. ACS Nano 2008, 2, 857–872. [Google Scholar] [CrossRef]
- Tsutsui, M.; Yoshida, T.; Yokota, K.; Yasaki, H.; Yasui, T.; Arima, A.; Tonomura, W.; Nagashima, K.; Yanagida, T.; Kaji, N.; et al. Discriminating Single-Bacterial Shape Using Low-Aspect-Ratio Pores. Sci. Rep. 2017, 7, 17371. [Google Scholar] [CrossRef] [Green Version]
- Lastra, L.S.; Bandara, Y.M.N.D.Y.; Sharma, V.; Freedman, K.J. Protein and DNA Yield Current Enhancements, Slow Translocations, and an Enhanced Signal-to-Noise Ratio under a Salt Imbalance. ACS Sens. 2022, 7, 2c00479. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucataru, I.C.; Dragomir, I.; Asandei, A.; Pantazica, A.-M.; Ghionescu, A.; Branza-Nichita, N.; Park, Y.; Luchian, T. Probing the Hepatitis B Virus E-Antigen with a Nanopore Sensor Based on Collisional Events Analysis. Biosensors 2022, 12, 596. https://doi.org/10.3390/bios12080596
Bucataru IC, Dragomir I, Asandei A, Pantazica A-M, Ghionescu A, Branza-Nichita N, Park Y, Luchian T. Probing the Hepatitis B Virus E-Antigen with a Nanopore Sensor Based on Collisional Events Analysis. Biosensors. 2022; 12(8):596. https://doi.org/10.3390/bios12080596
Chicago/Turabian StyleBucataru, Ioana C., Isabela Dragomir, Alina Asandei, Ana-Maria Pantazica, Alina Ghionescu, Norica Branza-Nichita, Yoonkyung Park, and Tudor Luchian. 2022. "Probing the Hepatitis B Virus E-Antigen with a Nanopore Sensor Based on Collisional Events Analysis" Biosensors 12, no. 8: 596. https://doi.org/10.3390/bios12080596