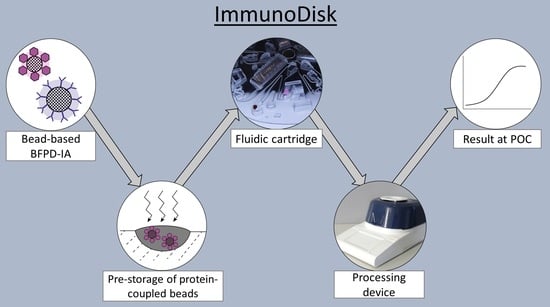
ImmunoDisk—A Fully Automated Bead-Based Immunoassay Cartridge with All Reagents Pre-Stored
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bound-Free Phase Detection Immunoassay
2.2. Preparation of Magnetic Beads for the CRP Assay
2.3. Preparation of Fluorescent Beads for the CRP Assay
2.4. Parametric Investigation of Pre-Storage of Protein-Coupled Beads
2.5. Drying Buffer for the Magnetic Beads
2.6. Drying Buffer for the Fluorescent Beads
2.7. ImmunoDisk Cartridge Fabrication
3. Results and Discussion
3.1. ImmunoDisk—Description of Fluidic Workflow
3.2. Fluidic Characterization
3.3. Multipurpose Chamber
3.4. Storage Chamber on the Disk
3.5. Study on Pre-Storage of Protein-Coupled Beads
3.6. Parametric Study on Drying Buffers
3.7. Investigation of Pre-Storage Temperature and Duration Conditions
3.8. Sample-to-Answer CRP Detection on the ImmunoDisk
3.9. Overall Assessment of the ImmunoDisk
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luong, J.; Vashist, S.K. (Eds.) Handbook of Immunoassay Technologies: Approaches, Performances, and Applications; Academic Press an imprint of Elsevier: London, UK, 2018. [Google Scholar]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary biomarkers detection: Analytical and immunological methods overview. TrAC-Trends Anal. Chem. 2019, 113, 301–316. [Google Scholar] [CrossRef]
- Silbereisen, A.; Alassiri, S.; Bao, K.; Grossmann, J.; Nanni, P.; Fernandez, C.; Tervahartiala, T.; Nascimento, G.G.; Belibasakis, G.N.; Heikkinen, A.-M.; et al. Label-Free Quantitative Proteomics versus Antibody-Based Assays to Measure Neutrophil-Derived Enzymes in Saliva. Proteom. Clin. Appl. 2020, 14, e1900050. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R. ELISA: Methods and Protocols; Humana Press: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- O’Kennedy, R.; Murphy, C. Immunoassays: Development, Applications and Future Trends; Pan Stanford Publishing: Milton, GA, USA, 2017. [Google Scholar]
- Bostanci, N.; Mitsakakis, K.; Afacan, B.; Bao, K.; Johannsen, B.; Baumgartner, D.; Müller, L.; Kotolová, H.; Emingil, G.; Karpíšek, M. Validation and verification of predictive salivary biomarkers for oral health. Sci. Rep. 2021, 11, 6406. [Google Scholar] [CrossRef] [PubMed]
- Lhopitallier, L.; Kronenberg, A.; Meuwly, J.-Y.; Locatelli, I.; Mueller, Y.; Senn, N.; D’Acremont, V.; Boillat-Blanco, N. Procalcitonin and lung ultrasonography point-of-care testing to determine antibiotic prescription in patients with lower respiratory tract infection in primary care: Pragmatic cluster randomised trial. BMJ 2021, 374, n2132. [Google Scholar] [CrossRef]
- Teggert, A.; Datta, H.; Ali, Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines 2020, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Bostanci, N.; Selevsek, N.; Wolski, W.; Grossmann, J.; Bao, K.; Wahlander, A.; Trachsel, C.; Schlapbach, R.; Öztürk, V.Ö.; Afacan, B.; et al. Targeted Proteomics Guided by Label-free Quantitative Proteome Analysis in Saliva Reveal Transition Signatures from Health to Periodontal Disease. Mol. Cell. Proteom. 2018, 17, 1392–1409. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.T.; Zhang, Y. Bead-based microfluidic immunoassays: The next generation. Biosens. Bioelectron. 2007, 22, 1197–1204. [Google Scholar] [CrossRef]
- Pecoraro, V.; Banfi, G.; Germagnoli, L.; Trenti, T. A systematic evaluation of immunoassay point-of-care testing to define impact on patients’ outcomes. Ann. Clin. Biochem. 2017, 54, 420–431. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, V.M.; Moraes, R.B.; Stein, A.T.; Wendland, E.M. Accuracy of C-Reactive protein as a bacterial infection marker in critically immunosuppressed patients: A systematic review and meta-analysis. J. Crit. Care 2017, 42, 129–137. [Google Scholar] [CrossRef]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [Green Version]
- Maguire, I.; O’Kennedy, R.; Ducrée, J.; Regan, F. A review of centrifugal microfluidics in environmental monitoring. Anal. Methods 2018, 10, 1497–1515. [Google Scholar] [CrossRef]
- Zehnle, S.; Rombach, M.; Zengerle, R.; von Stetten, F.; Paust, N. Network simulation-based optimization of centrifugo-pneumatic blood plasma separation. Biomicrofluidics 2017, 11, 24114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannsen, B.; Müller, L.; Baumgartner, D.; Karkossa, L.; Früh, S.M.; Bostanci, N.; Karpíšek, M.; Zengerle, R.; Paust, N.; Mitsakakis, K. Automated Pre-Analytic Processing of Whole Saliva Using Magnet-Beating for Point-of-Care Protein Biomarker Analysis. Micromachines 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czilwik, G.; Vashist, S.K.; Klein, V.; Buderer, A.; Roth, G.; von Stetten, F.; Zengerle, R.; Mark, D. Magnetic chemiluminescent immunoassay for human C-reactive protein on the centrifugal microfluidics platform. RSC Adv. 2015, 5, 61906–61912. [Google Scholar] [CrossRef]
- Hemmi, A.; Usui, T.; Moto, A.; Tobita, T.; Soh, N.; Nakano, K.; Zeng, H.; Uchiyama, K.; Imato, T.; Nakajima, H. A surface plasmon resonance sensor on a compact disk-type microfluidic device. J. Sep. Sci. 2011, 34, 2913–2919. [Google Scholar] [CrossRef]
- Honda, N.; Lindberg, U.; Andersson, P.; Hoffmann, S.; Takei, H. Simultaneous multiple immunoassays in a compact disc-shaped microfluidic device based on centrifugal force. Clin. Chem. 2005, 51, 1955–1961. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.; Aeinehvand, M.M.; Uddin, S.M.; Benzina, A.; Rothan, H.A.; Yusof, R.; Koole, L.H.; Madou, M.J.; Djordjevic, I.; Ibrahim, F. Microsphere integrated microfluidic disk: Synergy of two techniques for rapid and ultrasensitive dengue detection. Sci. Rep. 2015, 5, 16485. [Google Scholar] [CrossRef]
- Kim, T.-H.; Abi-Samra, K.; Sunkara, V.; Park, D.-K.; Amasia, M.; Kim, N.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Flow-enhanced electrochemical immunosensors on centrifugal microfluidic platforms. Lab Chip 2013, 13, 3747–3754. [Google Scholar] [CrossRef]
- Lai, S.; Wang, S.; Luo, J.; Lee, L.J.; Yang, S.-T.; Madou, M.J. Design of a compact disk-like microfluidic platform for enzyme-linked immunosorbent assay. Anal. Chem. 2004, 76, 1832–1837. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, J.-N.; Park, J.-M.; Lee, J.-G.; Kim, S.; Cho, Y.-K.; Ko, C. A fully automated immunoassay from whole blood on a disc. Lab Chip 2009, 9, 1548–1555. [Google Scholar] [CrossRef]
- Lin, Q.; Wu, J.; Fang, X.; Kong, J. Washing-free centrifugal microchip fluorescence immunoassay for rapid and point-of-care detection of protein. Anal. Chim. Acta 2020, 1118, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Lopez-Calle, E.; Espindola, P.; Boehm, C.; Brueckner, T.; Spinke, J.; Marcinowski, M.; Keller, T.; Tgetgel, A.; Herbert, N.; et al. A fully integrated microfluidic platform for highly sensitive analysis of immunochemical parameters. Analyst 2017, 142, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Nwankire, C.E.; Donohoe, G.G.; Zhang, X.; Siegrist, J.; Somers, M.; Kurzbuch, D.; Monaghan, R.; Kitsara, M.; Burger, R.; Hearty, S.; et al. At-line bioprocess monitoring by immunoassay with rotationally controlled serial siphoning and integrated supercritical angle fluorescence optics. Anal. Chim. Acta 2013, 781, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Sunkara, V.; Kim, T.-H.; Hwang, H.; Cho, Y.-K. Lab-on-a-disc for fully integrated multiplex immunoassays. Anal. Chem. 2012, 84, 2133–2140. [Google Scholar] [CrossRef]
- Park, Y.-S.; Sunkara, V.; Kim, Y.; Lee, W.S.; Han, J.-R.; Cho, Y.-K. Fully Automated Centrifugal Microfluidic Device for Ultrasensitive Protein Detection from Whole Blood. J. Vis. Exp. 2016, 110, 54143. [Google Scholar] [CrossRef] [Green Version]
- Riegger, L.; Grumann, M.; Nann, T.; Riegler, J.; Ehlert, O.; Bessler, W.; Mittenbuehler, K.; Urban, G.; Pastewka, L.; Brenner, T.; et al. Read-out concepts for multiplexed bead-based fluorescence immunoassays on centrifugal microfluidic platforms. Sens. Actuators A-Phys. 2006, 126, 455–462. [Google Scholar] [CrossRef]
- Schaff, U.Y.; Sommer, G.J. Whole blood immunoassay based on centrifugal bead sedimentation. Clin. Chem. 2011, 57, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.-H.; Wu, H.-C.; Chang, C.-Y.; Huang, W.-H.; Yang, Y.-F. An enzyme-linked immunosorbent assay on a centrifugal platform using magnetic beads. Biomicrofluidics 2014, 8, 52110. [Google Scholar] [CrossRef] [Green Version]
- Uddin, R.; Donolato, M.; Hwu, E.-T.; Hansen, M.F.; Boisen, A. Combined detection of C-reactive protein and PBMC quantification from whole blood in an integrated lab-on-a-disc microfluidic platform. Sens. Actuators B-Chem. 2018, 272, 634–642. [Google Scholar] [CrossRef]
- Wang, K.; Liang, R.; Chen, H.; Lu, S.; Jia, S.; Wang, W. A microfluidic immunoassay system on a centrifugal platform. Sens. Actuators B-Chem. 2017, 251, 242–249. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, Y.-H.; Shih, C.-H. Disk-based enzyme-linked immunosorbent assays using the liquid-aliquoting and siphoning-evacuation technique. Biomicrofluidics 2018, 12, 54101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Czilwik, G.; Klein, V.; Mitsakakis, K.; Zengerle, R.; Paust, N. C-reactive protein and interleukin 6 microfluidic immunoassays with on-chip pre-stored reagents and centrifugo-pneumatic liquid control. Lab Chip 2017, 17, 1666–1677. [Google Scholar] [CrossRef]
- Abe, T.; Okamoto, S.; Taniguchi, A.; Fukui, M.; Yamaguchi, A.; Utsumi, Y.; Ukita, Y. A lab in a bento box: An autonomous centrifugal microfluidic system for an enzyme-linked immunosorbent assay. Anal. Methods 2020, 12, 4858–4866. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jung, J.; Hahn, Y.K.; Kim, S.K.; Lee, Y.; Lee, J.; Lee, T.-H.; Park, J.-Y.; Seo, H.; Lee, J.N.; et al. A centrifugally actuated point-of-care testing system for the surface acoustic wave immunosensing of cardiac troponin I. Analyst 2013, 138, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Reith, P.; Kijanka, G.; Akujobi, V.; Abgrall, P.; Ducrée, J. Array-based capture, distribution, counting and multiplexed assaying of beads on a centrifugal microfluidic platform. Lab Chip 2012, 12, 1289–1295. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Kinahan, D.J.; Mishra, R.; Mangwanya, F.; Kilcawley, N.; Ferreira, M.; Ducrée, J. Label-free, spatially multiplexed SPR detection of immunoassays on a highly integrated centrifugal Lab-on-a-Disc platform. Biosens. Bioelectron. 2018, 119, 86–93. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Deng, J.; Li, X.; Qu, Y.; Xu, L.; Luo, Y.; Lu, Y.; Liu, T.; Zhao, W.; et al. Measurement of Carcinoembryonic Antigen in Clinical Serum Samples Using a Centrifugal Microfluidic Device. Micromachines 2018, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 2010, 110, 1518–1563. [Google Scholar] [CrossRef]
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: Potentials and pitfalls. Proteom. Clin. Appl. 2015, 9, 406–422. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Christopoulos, T.K. Immunoassay; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Slagle, K.M.; Ghosn, S.J. Immunoassays: Tools for Sensitive, Specific, and Accurate Test Results. Lab. Med. 1996, 27, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, B.; Karpíšek, M.; Baumgartner, D.; Klein, V.; Bostanci, N.; Paust, N.; Früh, S.M.; Zengerle, R.; Mitsakakis, K. One-step, wash-free, bead-based immunoassay employing bound-free phase detection. Anal. Chim. Acta 2021, 1153, 338280. [Google Scholar] [CrossRef] [PubMed]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Boss, D.S.; Musson, R.E.A.; Demir, A.Y. Influence of point-of-care C-reactive protein testing on antibiotic prescription habits in primary care in the Netherlands. Fam. Pract. 2018, 35, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Eccles, S.; Pincus, C.; Higgins, B.; Woodhead, M. Diagnosis and management of community and hospital acquired pneumonia in adults: Summary of NICE guidance. BMJ 2014, 349, g6722. [Google Scholar] [CrossRef]
- Johannsen, B.; Mark, D.; Boillat-Blanco, N.; Fresco, A.; Baumgartner, D.; Zengerle, R.; Mitsakakis, K. Rapid Diagnosis of Respiratory Tract Infections Using a Point-of-Care Platform Incorporating a Clinical Decision Support Algorithm. Stud. Health Technol. Inform. 2020, 273, 234–239. [Google Scholar]
- Prins, H.J.; Duijkers, R.; van der Valk, P.; Schoorl, M.; Daniels, J.M.A.; van der Werf, T.S.; Boersma, W.G. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur. Respir. J. 2019, 53, 1802014. [Google Scholar] [CrossRef]
- Schwarz, I.; Zehnle, S.; Hutzenlaub, T.; Zengerle, R.; Paust, N. System-level network simulation for robust centrifugal-microfluidic lab-on-a-chip systems. Lab Chip 2016, 16, 1873–1885. [Google Scholar] [CrossRef]
- Focke, M.; Stumpf, F.; Faltin, B.; Reith, P.; Bamarni, D.; Wadle, S.; Müller, C.; Reinecke, H.; Schrenzel, J.; Francois, P.; et al. Microstructuring of polymer films for sensitive genotyping by real-time PCR on a centrifugal microfluidic platform. Lab Chip 2010, 10, 2519–2526. [Google Scholar] [CrossRef]
- van Oordt, T.; Barb, Y.; Smetana, J.; Zengerle, R.; von Stetten, F. Miniature stick-packaging—an industrial technology for pre-storage and release of reagents in lab-on-a-chip systems. Lab Chip 2013, 13, 2888–2892. [Google Scholar] [CrossRef]
- Zehnle, S.; Schwemmer, F.; Roth, G.; von Stetten, F.; Zengerle, R.; Paust, P. Centrifugo-dynamic inward pumping of liquids on a centrifugal microfluidic platform. Lab Chip 2012, 12, 5142–5145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.F.; Zehnle, S.; Juelg, P.; Hutzenlaub, T.; Zengerle, R.; Paust, N. Review on pneumatic operations in centrifugal microfluidics. Lab Chip 2019, 19, 3745–3770. [Google Scholar] [CrossRef] [PubMed]
- Grumann, M.; Geipel, A.; Riegger, L.; Zengerle, R.; Ducrée, J. Batch-mode mixing on centrifugal microfluidic platforms. Lab Chip 2005, 5, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, K.H.; Kang, Q.J.; Chen, Q. Contact angles in the pseudopotential lattice Boltzmann modeling of wetting. Phys. Rev. E 2014, 90, 53301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, A.E.; Sullivan, W.F. Centrifugal Sedimentation Method for Particle Size Distribution. Ind. Eng. Chem. Anal. Ed. 1946, 18, 360–364. [Google Scholar] [CrossRef]
- Scott, D.J.; Harding, S.E.; Rowe, A.J.; Scott, D.; Rowe, A. (Eds.) Introduction to Differential Sedimentation. In Analytical Ultracentrifugation; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 270–290. [Google Scholar]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of protein pharmaceuticals: An update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef]
- Baumgartner, D.; Johannsen, B.; Specht, M.; Lüddecke, J.; Rombach, M.; Hin, S.; Paust, N.; von Stetten, F.; Zengerle, R.; Herz, C.; et al. OralDisk: A Chair-Side Compatible Molecular Platform Using Whole Saliva for Monitoring Oral Health at the Dental Practice. Biosensors 2021, 11, 423. [Google Scholar] [CrossRef]
- Hin, S.; Lopez-Jimena, B.; Bakheit, M.; Klein, V.; Stack, S.; Fall, C.; Sall, A.; Enan, K.; Mustafa, M.; Gillies, L.; et al. Fully automated point-of-care differential diagnosis of acute febrile illness. PLoS Negl. Trop. Dis. 2021, 15, e0009177. [Google Scholar] [CrossRef]
- Rombach, M.; Hin, S.; Specht, M.; Johannsen, B.; Lüddecke, J.; Paust, N.; Zengerle, R.; Roux, L.; Sutcliffe, T.; Peham, J.R.; et al. RespiDisk: A point-of-care platform for fully automated detection of respiratory tract infection pathogens in clinical samples. Analyst 2020, 145, 7040–7047. [Google Scholar] [CrossRef]
- Molecular Probes, FluoSpheres® Fluorescent Microspheres: Product Information. 2005. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp05000.pdf (accessed on 11 May 2022).
- Jeyachandran, Y.L.; Mielczarski, J.A.; Mielczarski, E.; Rai, B. Efficiency of blocking of non-specific interaction of different proteins by BSA adsorbed on hydrophobic and hydrophilic surfaces. J. Colloid Interface Sci. 2010, 341, 136–142. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, L.L. Preferential solvent interactions between proteins and polyethylene glycols. J. Biol. Chem. 1981, 256, 625–631. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Mechanism of poly(ethylene glycol) interaction with proteins. Biochemistry 1985, 24, 6756–6762. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Timasheff, S.N. Steric exclusion is the principal source of the preferential hydration of proteins in the presence of polyethylene glycols. Protein Sci. 1992, 1, 1133–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, S.; Raman Suri, C.; Sahoo, D.K. Molecular mechanism of polyethylene glycol mediated stabilization of protein. Biochem. Biophys. Res. Commun. 2010, 392, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Agrawal, A. Computational analysis of C-reactive protein for assessment of molecular dynamics and interaction properties. Cell Biochem. Biophys. 2013, 67, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, K.A.; Melbye, H.; Kelly, M.J.; Ceynowa, C.; Mölstad, S.; Hood, K.; Butler, C.C. Influence of CRP testing and clinical findings on antibiotic prescribing in adults presenting with acute cough in primary care. Scand. J. Prim. Health Care 2010, 28, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Alcoba, G.; Keitel, K.; Maspoli, V.; Lacroix, L.; Manzano, S.; Gehri, M.; Tabin, R.; Gervaix, A.; Galetto-Lacour, A. A three-step diagnosis of pediatric pneumonia at the emergency department using clinical predictors, C-reactive protein, and pneumococcal PCR. Eur. J. Pediatr. 2017, 176, 815–824. [Google Scholar] [CrossRef]
- Hahn-Schickard-Gesellschaft für Angewandte Forschung e.V., Lab-on-a-Chip Foundry. Available online: https://www.hahn-schickard.de/en/service-portfolio/production/lab-on-a-chip-foundry (accessed on 11 May 2022).
- Auclair, G.; Zegers, I.; Charoud-Got, J.; Munoz-Pineiro, M.; Hanisch, K.; Boulo, S.; Trapmann, S.; Schimmel, H.; Emons, H.; Schreiber, W. The Certification of the Mass Concentration of C-Reactive Protein in Human Serum—Certified Reference Material ERM®-DA474/IFCC; EUR 24922 EN; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Hin, S.; Baumgartner, D.; Specht, M.; Lüddecke, J.; Mahmodi Arjmand, E.; Johannsen, B.; Schiedel, L.; Rombach, M.; Paust, N.; von Stetten, F.; et al. VectorDisk: A Microfluidic Platform Integrating Diagnostic Markers for Evidence-Based Mosquito Control. Processes 2020, 8, 1677. [Google Scholar] [CrossRef]
- Mitsakakis, K. Novel lab-on-a-disk platforms: A powerful tool for molecular fingerprinting of oral and respiratory tract infections. Expert Rev. Mol. Diagn. 2021, 21, 523–526. [Google Scholar] [CrossRef]
- Teh, R.; Tee, W.D.; Tan, E.; Fan, K.; Koh, C.J.; Tambyah, P.A.; Oon, J.; Tee, N.; Soh, A.Y.S.; Siah, K.T.H. Review of the role of gastrointestinal multiplex polymerase chain reaction in the management of diarrheal illness. J. Gastroenterol. Hepatol. 2021, 36, 3286–3297. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, K.I.; Liu, S.; Yan, Z.; Xu, C.; Qiao, Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lee, I.-K.; Liu, J.-W.; Huang, S.-Y.; Wang, L. Utility of C-Reactive Protein Levels for Early Prediction of Dengue Severity in Adults. Biomed. Res. Int. 2015, 2015, 936062. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johannsen, B.; Baumgartner, D.; Karkossa, L.; Paust, N.; Karpíšek, M.; Bostanci, N.; Zengerle, R.; Mitsakakis, K. ImmunoDisk—A Fully Automated Bead-Based Immunoassay Cartridge with All Reagents Pre-Stored. Biosensors 2022, 12, 413. https://doi.org/10.3390/bios12060413
Johannsen B, Baumgartner D, Karkossa L, Paust N, Karpíšek M, Bostanci N, Zengerle R, Mitsakakis K. ImmunoDisk—A Fully Automated Bead-Based Immunoassay Cartridge with All Reagents Pre-Stored. Biosensors. 2022; 12(6):413. https://doi.org/10.3390/bios12060413
Chicago/Turabian StyleJohannsen, Benita, Desirée Baumgartner, Lena Karkossa, Nils Paust, Michal Karpíšek, Nagihan Bostanci, Roland Zengerle, and Konstantinos Mitsakakis. 2022. "ImmunoDisk—A Fully Automated Bead-Based Immunoassay Cartridge with All Reagents Pre-Stored" Biosensors 12, no. 6: 413. https://doi.org/10.3390/bios12060413