Microfluidic-Based Electrochemical Immunosensing of Ferritin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
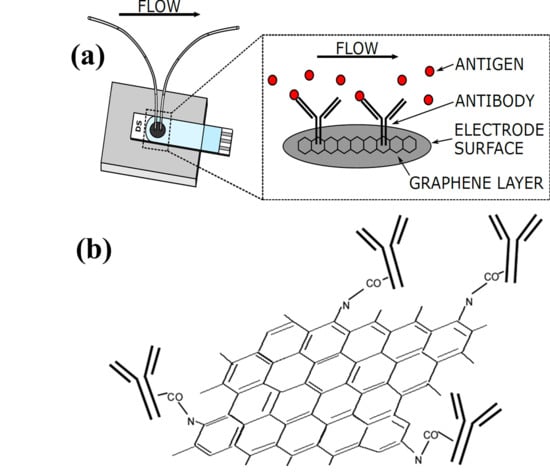
2.2. Fabrication of Microfluidics Flow Cell and Surface Modification of Electrode
2.3. Electrochemical Measurements
2.4. Computer Simulations
3. Results and Discussion
3.1. Characterization of Amine-Functionalized Graphene Oxide (NH2-GO)
3.2. Computer Simulations
3.3. Electrochemical Characterization
Effect of Scan Rate
Effect of Flow Rate
Stability of NH2-GO Coating
Effect of Functionalization and Antibody Immobilization
Electrochemical Detection of Ferritin and Sensor Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics
References
- Kaur, K. Anaemia ‘a silent killer’among women in India: Present scenario. Eur. J. Zool. Res. 2014, 3, 32–36. [Google Scholar]
- Winzerling, J.J.; Pham, D.Q.D. 4.10-Ferritin. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 341–356. [Google Scholar]
- Winzerling, J.J.; Pham, D.Q.D. Ferritin☆. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ho, C.-H.; Yuan, C.-C.; Yeh, S.-H. Serum ferritin levels and their significance in normal full-term pregnant women. Int. J. Gynecol. Obstet. 1987, 25, 291–295. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Biddulph, J.P.; Rafnsson, S.B.; Trivella, M.; Nihoyannopoulos, P.; Demakakos, P. The association of ferritin with cardiovascular and all-cause mortality in community-dwellers: The English longitudinal study of ageing. PLoS ONE 2017, 12, e0178994. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kuragano, T.; Nanami, M.; Otaki, Y.; Nonoguchi, H.; Hasuike, Y. Importance of Ferritin for Optimizing Anemia Therapy in Chronic Kidney Disease. Am. J. Nephrol. 2010, 32, 439–446. [Google Scholar] [CrossRef]
- Evensen, K.J.; Swaak, T.J.G.; Nossent, J.C. Increased ferritin response in adult Still’s disease: Specificity and relationship to outcome. Scand. J. Rheumatol. 2007, 36, 107–110. [Google Scholar] [CrossRef]
- Emmenegger, U.; Frey, U.; Reimers, A.; Fux, C.; Semela, D.; Cottagnoud, P.; Spaeth, P.J.; Neftel, K. Hyperferritinemia as indicator for intravenous immunoglobulin treatment in reactive macrophage activation syndromes. Am. J. Hematol. 2001, 68, 4–10. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta (BBA) Rev. Cancer 2013, 1836, 245–254. [Google Scholar] [CrossRef]
- Barnett, M.D.; Gordon, Y.B.; A Amess, J.; Mollin, D.L. Measurement of ferritin in serum by radioimmunoassay. J. Clin. Pathol. 1978, 31, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. CHAPTER 3—Electrochemical glucose biosensors. In Electrochemical Sensors, Biosensors and their Biomedical Applications; Zhang, X., Ju, H., Wang, J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 57–69. [Google Scholar]
- Song, T.-T.; Wang, W.; Meng, L.-L.; Liu, Y.; Jia, X.-B.; Mao, X. Electrochemical detection of human ferritin based on gold nanorod reporter probe and cotton thread immunoassay device. Chin. Chem. Lett. 2017, 28, 226–230. [Google Scholar] [CrossRef]
- Matysiak-Brynda, E.; Wagner, B.; Bystrzejewski, M.; Grudzinski, I.P.; Nowicka, A.M. The importance of antibody orientation in the electrochemical detection of ferritin. Biosens. Bioelectron. 2018, 109, 83–89. [Google Scholar] [CrossRef]
- Wang, X.; Tao, G.; Meng, Y. Nanogold hollow microsphere-based electrochemical immunosensor for the detection of ferritin in human serum. Microchim. Acta 2009, 167, 147. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Hu, M.; Xiao, Y. An immunosensor for ferritin based on agarose hydrogel. Biosens. Bioelectron. 2006, 21, 2180–2183. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, R.; Chai, Y.; Zhuo, Y.; Hong, C.; Liu, Z.; Su, H. Porous redox-active Cu2O–SiO2 nanostructured film: Preparation, characterization and application for a label-free amperometric ferritin immunosensor. Talanta 2009, 78, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tang, D.; Su, B.; Tang, J.; Chen, G. Glucose oxidase-doped magnetic silica nanostrutures as labels for localized signal amplification of electrochemical immunosensors. Nanoscale 2010, 2, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.-C.; Pan, T.-M.; Lee, C.-H.; Chao, T.-S. Label-free and real-time detection of ferritin using a horn-like polycrystalline-silicon nanowire field-effect transistor biosensor. Sens. Actuators B Chem. 2016, 230, 398–404. [Google Scholar] [CrossRef]
- Garg, M.; Chatterjee, M.; Sharma, A.L.; Singh, S. Label-free approach for electrochemical ferritin sensing using biosurfactant stabilized tungsten disulfide quantum dots. Biosens. Bioelectron. 2020, 151, 111979. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Zitka, O.; Klimanek, M.; Vrba, R.; Adam, V. Microfluidic electrochemical devices for pollution analysis—A review. Sens. Actuators B Chem. 2017, 246, 578–590. [Google Scholar] [CrossRef]
- Fragoso, A.; Latta, D.; Laboria, N.; Von Germar, F.; Hansen-Hagge, T.E.; Kemmner, W.; Gärtner, C.; Klemm, R.; Drese, K.S.; Sullivan, C.O. Integrated microfluidic platform for the electrochemical detection of breast cancer markers in patient serum samples. Lab Chip 2011, 11, 625–631. [Google Scholar] [CrossRef]
- Soo Ko, J.; Yoon, H.C.; Yang, H.; Pyo, H.-B.; Chung, K.H.; Kim, S.J.; Kim, Y.T. A polymer-based microfluidic device for immunosensing biochips. Lab Chip 2003, 3, 106–113. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.; Vestergaard, M.d.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.V.; Bertolino, F.A.; Fernández-Baldo, M.A.; Messina, G.A.; Salinas, E.; Sanz, M.I.; Raba, J. A microfluidic device based on a screen-printed carbon electrode with electrodeposited gold nanoparticles for the detection of IgG anti-Trypanosoma cruziantibodies. Analyst 2011, 136, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S. Disposable microfluidic immuno-biochip for rapid electrochemical detection of tumor necrosis factor alpha biomarker. Sens. Actuators B Chem. 2015, 221, 1406–1411. [Google Scholar] [CrossRef]
- Vasiliadou, R.; Esfahani, M.M.N.; Brown, N.J.; Welham, K.J. A Disposable Microfluidic Device with a Screen Printed Electrode for Mimicking Phase II Metabolism. Sensors 2016, 16, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wang, Z.; Cui, X.; Jiang, L.; Zhi, Y.; Ding, X.; Nie, Z.; Zhou, P.; Cui, D. Microfluidic Device Directly Fabricated on Screen-Printed Electrodes for Ultrasensitive Electrochemical Sensing of PSA. Nanoscale Res. Lett. 2019, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Peacock, M.; Leonhardt, S.; Damiati, L.; Baghdadi, M.A.; Becker, H.; Kodzius, R.; Schuster, B. Embedded Disposable Functionalized Electrochemical Biosensor with a 3D-Printed Flow Cell for Detection of Hepatic Oval Cells (HOCs). Genes 2018, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Patinglag, L.; Esfahani, M.M.N.; Ragunathan, K.; He, P.; Brown, N.J.; Archibald, S.J.; Pamme, N.; Tarn, M.D. On-chip electrochemical detection of glucose towards the miniaturised quality control of carbohydrate-based radiotracers. Analyst 2020. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.; Kumar, M.; Jyoti; Agarwal, A.; Mizaikoff, B. Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem. Eng. J. 2017, 313, 283–292. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Deep, A. A graphene electrode functionalized with aminoterephthalic acid for impedimetric immunosensing of Escherichia coli. Mikrochim. Acta 2019, 186, 800. [Google Scholar] [CrossRef]
- Kukkar, M.; Singh, S.; Kumar, N.; Tuteja, S.K.; Kim, K.-H.; Deep, A. Molybdenum disulfide quantum dot based highly sensitive impedimetric immunoassay for prostate specific antigen. Microchim. Acta 2017, 184, 4647–4654. [Google Scholar] [CrossRef]
- Chrouda, A.; Braiek, M.; Rokbani, K.B.; Bakhrouf, A.; Maaref, A.; Jaffrezic-Renault, N. An Immunosensor for Pathogenic Staphylococcus aureus Based on Antibody Modified Aminophenyl-Au Electrode. ISRN Electrochem. 2013, 2013, 367872. [Google Scholar] [CrossRef]
- Kukkar, M.; Tuteja, S.K.; Kumar, P.; Kim, K.-H.; Bhadwal, A.S.; Deep, A. A novel approach for amine derivatization of MoS2 nanosheets and their application toward label-free immunosensor. Anal. Biochem. 2018, 555, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G. Analytical evaluation of sensor measurements. Anal. Bioanal. Chem. 2018, 410, 5–13. [Google Scholar] [CrossRef] [Green Version]
- IUPAC. Compendium of Analytical Nomenclature (Orange Book). 2002. Available online: http://media.iupac.org/publications/analytical_compendium/ (accessed on 27 July 2020).
- Oleneva, E.; Khaydukova, M.; Ashina, J.; Yaroshenko, I.; Jahatspanian, I.; Legin, A.; Kirsanov, D. A Simple Procedure to Assess Limit of Detection for Multisensor Systems. Sensors 2019, 19, 1359. [Google Scholar] [CrossRef] [Green Version]
- de Vicente, J.; Lavín, Á.; Holgado, M.; Laguna, M.F.; Casquel, R.; Santamaría, B.; Quintero, S.; Hernandez, A.L.; Ramírez, Y.; Bolanos, M.H. The uncertainty and limit of detection in biosensors from immunoassays. Meas. Sci. Technol. 2020, 31, 044004. [Google Scholar] [CrossRef]
- He, D.; Marles-Wright, J. Ferritin family proteins and their use in bionanotechnology. New Biotechnol. 2015, 32, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Reymond, F.; Vollet, C.; Jendelova, P.; Horák, D. Fabrication and characterization of tosyl-activated magnetic and nonmagnetic monodisperse microspheres for use in microfluic-based ferritin immunoassay. Biotechnol. Prog. 2013, 29, 532–542. [Google Scholar] [CrossRef]
- Boonkaew, S.; Teengam, P.; Jampasa, S.; Rengpipat, S.; Siangproh, W.; Chailapakul, O. Cost-effective paper-based electrochemical immunosensor using a label-free assay for sensitive detection of ferritin. Analyst 2020, 145, 5019–5026. [Google Scholar] [CrossRef]





| Concentration Added (ng·mL−1) | Concentration Found (ng·mL−1) | % Found |
|---|---|---|
| 31.25 | 34.47 | 110.30 |
| 62.50 | 70.91 | 113.45 |
| 125 | 120.27 | 96.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, M.; Christensen, M.G.; Iles, A.; Sharma, A.L.; Singh, S.; Pamme, N. Microfluidic-Based Electrochemical Immunosensing of Ferritin. Biosensors 2020, 10, 91. https://doi.org/10.3390/bios10080091
Garg M, Christensen MG, Iles A, Sharma AL, Singh S, Pamme N. Microfluidic-Based Electrochemical Immunosensing of Ferritin. Biosensors. 2020; 10(8):91. https://doi.org/10.3390/bios10080091
Chicago/Turabian StyleGarg, Mayank, Martin Gedsted Christensen, Alexander Iles, Amit L. Sharma, Suman Singh, and Nicole Pamme. 2020. "Microfluidic-Based Electrochemical Immunosensing of Ferritin" Biosensors 10, no. 8: 91. https://doi.org/10.3390/bios10080091