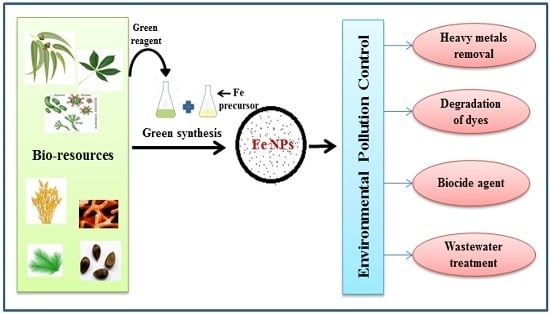
Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications
Abstract
:1. Introduction
2. Green Routes for the Synthesis of Metallic Iron Nanoparticles
2.1. Synthesis by Biocompatible Green Reagents
2.2. Synthesis by Microorganisms
2.3. Synthesis of Iron Nanoparticles from Plant Biomaterials
2.4. Other Plant Materials
2.5. Possible Mechanism of Nanoparticles Synthesis
3. Environmental Applications of Green Iron Nanoparticles
3.1. Degradation of Dyes
3.2. Removal of Heavy Metals
3.3. Wastewater Treatment
3.4. Antibacterial Activity
3.5. Stabilised/Immobilised Plant Mediated FeNPs for Degradation of Pollutants
4. Environmental Implications of Iron Nanoparticles
5. Conclusions and Future Perspective
Acknowledgments
Authors Contribution
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller |
| COD | Chemical oxygen demand |
| EDS | Energy dispersive spectroscopy |
| EDDS | Ethylenediamine disuccinic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| FTIR | Fourier transforminfrared |
| GC-MS | Gas chromatography-mass spectrometry |
| HAADF | Highly advanced techniques like the high angle annular dark field |
| HR-TEM | High resolution transmission electron microscope |
| LDH | Lactate dehydrogenase |
| MTS | Methyl tetrazolium |
| MCB | Monochlorobenzene |
| NPs | Nanoparticles |
| nZVI | Nanoscale zero-valent iron |
| PAA | Polyacrylic acid |
| PVDF | Polyvinylidene fluoride |
| SAED | Selected area electron diffraction |
| SCE | Saturated calomel electrode |
| SEM | Scanning electron microscope |
| TCE | Trichloroethylene |
| TEM | Transmission electron microscope |
| TOC | Total organic carbon |
| XAS | X-ray absorption spectroscopy technique |
| XRD | X-ray diffraction |
| ZVMI | Zero valent metallic iron |
References
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Rotello, V.M. Nanoparticles: Building Blocks for Nanotechnology; Springer Science & Business Media: New York, NY, USA, 2004. [Google Scholar]
- Virkutyte, J.; Varma, R.S. Chapter 2 Environmentally Friendly Preparation of Metal Nanoparticles. In Sustainable Preparation of Metal Nanoparticles: Methods and Applications; The Royal Society of Chemistry: London, UK, 2013; pp. 7–33. [Google Scholar]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Ramezani, F.; Kazemi, B. Biosynthesis of silver nanoparticles by Geotricum sp. J. Clust. Sci. 2011, 22, 225–232. [Google Scholar] [CrossRef]
- Lin, K.-S.; Chang, N.-B.; Chuang, T.-D. Fine structure characterization of zero-valent iron nanoparticles for decontamination of nitrites and nitrates in wastewater and groundwater. Sci. Technol. Adv. Mater. 2008, 9, 025015. [Google Scholar] [CrossRef]
- Gui, M.; Smuleac, V.; Ormsbee, L.E.; Sedlak, D.L.; Bhattacharyya, D. Iron oxide nanoparticle synthesis in aqueous and membrane systems for oxidative degradation of trichloroethylene from water. J. Nanopart. Res. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- He, F.; Zhao, D. Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environ. Sci. Technol. 2005, 39, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Shi, Y.; Zhang, S.; Jiang, K.; Yang, S.; Li, Z.; Takayama-Muromachi, E. Biopolymer-assisted green synthesis of iron oxide nanoparticles and their magnetic properties. J. Phys. Chem. C 2008, 112, 10398–10401. [Google Scholar] [CrossRef]
- Jegan, A.; Ramasubbu, A.; Saravanan, S.; Vasanthkumar, S. One-pot synthesis and characterization of biopolymer—Iron oxide nanocomposite. Int. J. Nano Dimens. 2011, 2, 105–110. [Google Scholar]
- Nadagouda, M.N.; Varma, R.S. A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous Vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Savasari, M.; Emadi, M.; Bahmanyar, M.A.; Biparva, P. Optimization of Cd(II) removal from aqueous solution by ascorbic acid-stabilized zero valent iron nanoparticles using response surface methodology. J. Ind. Eng. Chem. 2015, 21, 1403–1409. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in mri. Appl. Nanosci. 2014, 5, 435–441. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Krishna, R.; Bardhan, N.; Bahadur, D.; Gracio, J. Wet-chemical green synthesis of l-lysine amino acid stabilized biocompatible iron-oxide magnetic nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 6645–6651. [Google Scholar] [CrossRef] [PubMed]
- Siskova, K.M.; Straska, J.; Krizek, M.; Tucek, J.; Machala, L.; Zboril, R. Formation of zero-valent iron nanoparticles mediated by amino acids. Procedia Environ. Sci. 2013, 18, 809–817. [Google Scholar] [CrossRef]
- Sayyad, A.S.; Balakrishnan, K.; Ci, L.; Kabbani, A.T.; Vajtai, R.; Ajayan, P.M. Synthesis of iron nanoparticles from hemoglobin and myoglobin. Nanotechnology 2012, 23, 055602. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shen, Y.; Xie, A.; Zhang, W. Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2010, 322, 1828–1833. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe(III) precursor by a sucrose bifunctional hydrothermal method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Yan, Q.; Street, J.; Yu, F. Synthesis of carbon-encapsulated iron nanoparticles from wood derived sugars by hydrothermal carbonization (HTC) and their application to convert bio-syngas into liquid hydrocarbons. Biomass Bioenergy 2015, 83, 85–95. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Rius, J.L.; Zorrilla, C. Tannin biosynthesis of iron oxide nanoparticles. Appl. Phys. A 2010, 100, 453–459. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.; Wani, A.; Shouche, Y.; Joy, P.A.; Prasad, B.L.V.; Sastry, M. Bacterial aerobic synthesis of nanocrystalline magnetite. J. Am. Chem. Soc. 2005, 127, 9326–9327. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.A.; Parikh, R.Y.; Baidakova, M.; Jouen, S.; Hannoyer, B.; Enoki, T.; Prasad, B.; Shouche, Y.S.; Ogale, S.; Sastry, M. Bacteria-mediated precursor-dependent biosynthesis of superparamagnetic iron oxide and iron sulfide nanoparticles. Langmuir 2008, 24, 5787–5794. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.W.; Rawn, C.J.; Rondinone, A.J.; Love, L.J.; Roh, Y.; Everett, S.M.; Lauf, R.J.; Phelps, T.J. Large-scale production of magnetic nanoparticles using bacterial fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P.A.; Augustine, R.; Kannan, M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioprocess Eng. 2012, 17, 835–840. [Google Scholar] [CrossRef]
- Elcey, C.; Kuruvilla, A.T.; Thomas, D. Synthesis of magnetite nanoparticles from optimized iron reducing bacteria isolated from iron ore mining sites. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 408–417. [Google Scholar]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular biosynthesis of magnetite using fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.K.; Kumar, P.; Burman, U.; Joshi, P.; Agrawal, A.; Raliya, R.; Tarafdar, J.C. Magnesium and iron nanoparticles production using microorganisms and various salts. Mater. Sci. Poland 2012, 30, 254–258. [Google Scholar] [CrossRef]
- Pavani, K.V.; Kumar, N.S. Adsorption of iron and synthesis of iron nanoparticles by Aspergillus species kvp 12. Am. J. Nanomater. 2013, 1, 24–26. [Google Scholar]
- Mohamed, Y.M.; Azzam, A.M.; Amin, B.H.; Safwat, N.A. Mycosynthesis of iron nanoparticles by Alternaria alternata and its antibacterial activity. Afr. J. Biotechnol. 2015, 14, 1234–1241. [Google Scholar] [CrossRef]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyam, V.; Subashchandrabose, S.R.; Thavamani, P.; Megharaj, M.; Chen, Z.; Naidu, R. Chlorococcum sp. MM11—A novel phyco-nanofactory for the synthesis of iron nanoparticles. J. Appl. Phycol. 2015, 27, 1861–1869. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Kalaiarasi, R.; Jayallakshmi, N.; Venkatachalam, P. Phytosynthesis of nanoparticles and its applications. Plant Cell Biotechnol. Mol. Biol. 2010, 11, 1–16. [Google Scholar]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Mukunthan, K.S.; Balaji, S. Silver nanoparticles shoot up from the root of Daucus carrota (L.). Int. J. Green Nanotechnol. 2012, 4, 54–61. [Google Scholar] [CrossRef]
- Zambre, A.; Upendran, A.; Shukla, R.; Chanda, N.; Katti, K.K.; Cutler, C.; Kannan, R.; Katti, K.V. Chapter 6 Green Nanotechnology—A Sustainable Approach in the Nanorevolution. In Sustainable Preparation of Metal Nanoparticles: Methods and Applications; The Royal Society of Chemistry: London, UK, 2013; pp. 144–156. [Google Scholar]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Markova, Z.; Novak, P.; Kaslik, J.; Plachtova, P.; Brazdova, M.; Jancula, D.; Siskova, K.M.; Machala, L.; Marsalek, B.; Zboril, R.; et al. Iron(II,III)—Polyphenol complex nanoparticles derived from green tea with remarkable ecotoxicological impact. ACS Sustain. Chem. Eng. 2014, 2, 1674–1680. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In vitro biocompatibility of nanoscale zerovalent iron particles (nZVI) synthesized using tea polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445–446, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, M.; Nayak, P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachta indica (neem). World J. Nano Sci. Technol. 2013, 2, 6–9. [Google Scholar]
- Wang, Z. Iron complex nanoparticles synthesized by eucalyptus leaves. ACS Sustain. Chem. Eng. 2013, 1, 1551–1554. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Megharaj, M. Characterization of iron-polyphenol nanoparticles synthesized by three plant extracts and their fenton oxidation of azo dye. ACS Sustain. Chem. Eng. 2014, 2, 1022–1025. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Biomolecules in grape leaf extract involved in one-step synthesis of iron-based nanoparticles. RSC Adv. 2014, 4, 53467–53474. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. A green and facile approach for synthesis of magnetite nanoparticles. Nanosci. Nanotechnol. 2012, 2, 208–213. [Google Scholar] [CrossRef]
- Pattanayak, M.; Nayak, P.L. Ecofriendly green synthesis of iron nanoparticles from various plants and spices extract. J. Plant Anim. Environ. Sci. 2013, 3, 68–76. [Google Scholar]
- Senthil, M.; Ramesh, C. Biogenic synthesis of Fe3O4 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Dig. J. Nanomater. Biostruct. 2012, 7, 1655–1660. [Google Scholar]
- Rao, A.; Bankar, A.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Removal of hexavalent chromium ions by Yarrowia lipolytica cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles. J. Contam. Hydrol. 2013, 146, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Makarova, S.S.; Love, A.J.; Sinitsyna, O.V.; Dudnik, A.O.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants. Langmuir 2014, 30, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Iron oxide nanoparticles synthesized by controlled bio-precipitation using leaf extract of garlic vine (Mansoa alliacea). Mater. Sci. Semicond. Process. 2016, 53, 79–83. [Google Scholar] [CrossRef]
- Mohan Kumar, K.; Mandal, B.K.; Siva Kumar, K.; Sreedhara Reddy, P.; Sreedhar, B. Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta A 2013, 102, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication. J. Saudi Chem. Soc. 2014, 18, 364–369. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Natesh Kumar, B.; Prasad, C.H.; Venkateswarlu, P.; Jyothi, N.V.V. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Physica. B 2014, 449, 67–71. [Google Scholar] [CrossRef]
- Becerra, R.H.; Zorrilla, C.; Ascencio, J.A. Production of iron oxide nanoparticles by a biosynthesis method: An environmentally friendly route. J. Phys. Chem. 2007, 111, 16147–16153. [Google Scholar]
- Herrera-Becerra, R.; Zorrilla, C.; Rius, J.L.; Ascencio, J.A. Electron microscopy characterization of biosynthesized iron oxide nanoparticles. Appl. Phys. A 2008, 91, 241–246. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous Sorghum bran extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Rao, Y.S.; Balaji, T.; Prathima, B.; Jyothi, N.V.V. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Ahmmad, B.; Leonard, K.; Shariful Islam, M.; Kurawaki, J.; Muruganandham, M.; Ohkubo, T.; Kuroda, Y. Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv. Powder Technol. 2013, 24, 160–167. [Google Scholar] [CrossRef]
- Phumying, S.; Labuayai, S.; Thomas, C.; Amornkitbamrung, V.; Swatsitang, E.; Maensiri, S. Aloe vera plant-extracted solution hydrothermal synthesis and magnetic properties of magnetite (Fe3O4) nanoparticles. Appl. Phys. A 2012, 111, 1187–1193. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using Oolong tea extract for the degradation of malachite green. Spectrochim. Acta A 2013, 117, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, C.; Mallavarapu, M. Characterization of iron–polyphenol complex nanoparticles synthesized by sage (Salvia officinalis) leaves. Environ. Technol. Innov. 2015, 4, 92–97. [Google Scholar] [CrossRef]
- Kuang, Y.; Wang, Q.; Chen, Z.; Megharaj, M.; Naidu, R. Heterogeneous fenton-like oxidation of monochlorobenzene using green synthesis of iron nanoparticles. J. Colloid Interface Sci. 2013, 410, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized conditions impacting on the reactivity of Fe NPs for the degradation of malachite green. Spectrochim. Acta A 2015, 137, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Prasad, T.N.; Reddy, A.V.; Ravindra Reddy, B.; Madhavi, G. Application of phytogenic zerovalent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim. Acta A 2013, 116, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mystrioti, C.; Xenidis, A.; Papassiopi, N. Reduction of hexavalent chromium with polyphenol-coated nano zero-valent iron: Column studies. Desalination Water Treat. 2014, 56, 1162–1170. [Google Scholar] [CrossRef]
- Mystrioti, C.; Papassiopi, N.; Xenidis, A.; Dermatas, D.; Chrysochoou, M. Column study for the evaluation of the transport properties of polyphenol-coated nanoiron. J. Hazard. Mater. 2015, 281, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yuan, M.; Yang, B.; Liu, Z.; Huang, J.; Sun, D. Plant-mediated synthesis of highly active iron nanoparticles for Cr(VI) removal: Investigation of the leading biomolecules. Chemosphere 2016, 150, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Chrysochoou, M.; McGuirea, M.; Dahalb, G. Transport characteristics of green-tea nano-scale zero valent iron as a function of soil mineralogy. Chem. Eng. Trans. 2012, 28, 122–126. [Google Scholar]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of Fe nanoparticles using Eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea andeucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Kiruba Daniel, S.C.G.; Vinothini, G.; Subramanian, N.; Nehru, K.; Sivakumar, M. Biosynthesis of Cu, ZVI, and Ag nanoparticles using Dodonaea viscosa extract for antibacterial activity against human pathogens. J. Nanopart. Res. 2012, 15, 1319. [Google Scholar] [CrossRef]
- Ponder, S.M.; Darab, J.G.; Mallouk, T.E. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ. Sci. Technol. 2000, 34, 2564–2569. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.; Chen, G. Removal of Cr(VI) by magnetite. Water Sci. Technol. 2004, 50, 139–146. [Google Scholar] [PubMed]
- Tang, S.C.; Lo, I.M. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Suthersan, S.S.; Payne, F.C. In Situ Remediation Engineering; Taylor & Francis: Oxfordshire, UK, 2004; p. 304. [Google Scholar]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.K.; Shukla, R.C.; Singh, S.B. Removal of arsenic(III) from water with clay-supported zerovalent iron nanoparticles synthesized with the help of tea liquor. Ind. Eng. Chem. Res. 2013, 52, 10052–10058. [Google Scholar] [CrossRef]
- Prasad, K.S.; Gandhi, P.; Selvaraj, K. Synthesis of green nano iron particles (GnIP) and their application in adsorptive removal of As(III) and As(V) from aqueous solution. Appl. Surf. Sci. 2014, 317, 1052–1059. [Google Scholar] [CrossRef]
- Martínez-Cabanas, M.; López-García, M.; Barriada, J.L.; Herrero, R.; Sastre de Vicente, M.E. Green synthesis of iron oxide nanoparticles. Development of magnetic hybrid materials for efficient As(V) removal. Chem. Eng. J. 2016, 301, 83–91. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Carbone, S.; Gatti, A.; Vianello, G.; Nannipieri, P. Toxicity of metal oxide (CeO2, Fe3O4, SnO2) engineered nanoparticles on soil microbial biomass and their distribution in soil. Soil Biol. Biochem. 2013, 60, 87–94. [Google Scholar] [CrossRef]
- Sacca, M.L.; Fajardo, C.; Costa, G.; Lobo, C.; Nande, M.; Martin, M. Integrating classical and molecular approaches to evaluate the impact of nanosized zero-valent iron (nZVI) on soil organisms. Chemosphere 2014, 104, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Sacca, M.L.; Martinez-Gomariz, M.; Costa, G.; Nande, M.; Martin, M. Transcriptional and proteomic stress responses of a soil bacterium Bacillus cereus to nanosized zero-valent iron (nZVI) particles. Chemosphere 2013, 93, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.-Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Greden, K.; Alvarez, P.J.J.; Gregory, K.B.; Lowry, G.V. Adsorbed polymer and NOM limits adhesion and toxicity of nano scale zerovalent iron to E. coli. Environ. Sci. Technol. 2010, 44, 3462–3467. [Google Scholar] [CrossRef] [PubMed]
- Phenrat, T.; Long, T.C.; Lowry, G.V.; Veronesi, B. Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ. Sci. Technol. 2009, 43, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Wu, W.L.; Wu, K.C. The zerovalent iron nanoparticle causes higher developmental toxicity than its oxidation products in early life stages of Medaka fish. Water Res. 2013, 47, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J.; Koser, J.; Arndt, D.; Filser, J. The coating makes the difference: Acute effects of iron oxide nanoparticles on Daphnia magna. Sci. Total Environ. 2014, 484, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, L.; Dewez, D. Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. BioMed Res. Int. 2013, 2013, 647974. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Q.; Wu, Y.; Fu, J.; Wang, T.; Jiang, G. Effects of waterborne nano-iron on medaka (Oryzias latipes): Antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol. Environ. Saf. 2009, 72, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Remya, A.S.; Ramesh, M.; Saravanan, M.; Poopal, R.K.; Bharathi, S.; Nataraj, D. Iron oxide nanoparticles to an indian major carp, Labeo rohita: Impacts on hematology, iono regulation and gill Na+/K+ atpase activity. J. King Saud Univ. Sci. 2015, 27, 151–160. [Google Scholar] [CrossRef]
- Taze, C.; Panetas, I.; Kalogiannis, S.; Feidantsis, K.; Gallios, G.P.; Kastrinaki, G.; Konstandopoulos, A.G.; Vaclavikova, M.; Ivanicova, L.; Kaloyianni, M. Toxicity assessment and comparison between two types of iron oxide nanoparticles in Mytilus galloprovincialis. Aquat. Toxicol. 2016, 172, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Kanarbik, L.; Irha, N.; Kahru, A. Ecotoxicity of nanosized magnetite to crustacean Daphnia magna and duckweed Lemna minor. Hydrobiologia 2015. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ren, H.; Zhang, Y.; Gu, N.; Lin, X. The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J. Soils Sediment. 2011, 11, 1408–1417. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Ecotoxicological effects on earthworms of fresh and aged nano-sized zero-valent iron (nZVI) in soil. Chemosphere 2012, 89, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Gil-Diaz, M.; Costa, G.; Alonso, J.; Guerrero, A.M.; Nande, M.; Lobo, M.C.; Martin, M. Residual impact of aged nzvi on heavy metal-polluted soils. Sci. Total Environ. 2015, 535, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Canivet, L.; Dubot, P.; Garcon, G.; Denayer, F.O. Effects of engineered iron nanoparticles on the bryophyte, Physcomitrella patens (hedw.) bruch & schimp, after foliar exposure. Ecotoxicol. Environ. Saf. 2015, 113, 499–505. [Google Scholar] [PubMed]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Usha Rani, P.; Rajasekharreddy, P. Green synthesis of silver-protein (core-shell) nanoparticles using Piper betle L. Leaf extract and its ecotoxicological studies on daphnia magna. Colloids Surf. A 2011, 389, 188–194. [Google Scholar] [CrossRef]
- Filser, J.; Arndt, D.; Baumann, J.; Geppert, M.; Hackmann, S.; Luther, E.M.; Pade, C.; Prenzel, K.; Wigger, H.; Arning, J.; et al. Intrinsically green iron oxide nanoparticles? From synthesis via (eco-)toxicology to scenario modelling. Nanoscale 2013, 5, 1034–1046. [Google Scholar] [CrossRef] [PubMed]


| Type of Nanoparticles | Biochemical Agents | Size and Morphology | Environmental Application | Reference |
|---|---|---|---|---|
| Stabilised bimetallic Fe/Pd nanoparticles | Starch | 14.1 nm Discrete well dispersed | Degradation of chlorinated hydrocarbons in water | [8] |
| Fe3O4 | Sodium alginate | 27.2 nm Spherical | - | [9] |
| Fe3O4-polymer composite | Agar | 50–200 nm Spherical and hexagonal | - | [10] |
| Fe noble metal nano-shell | Ascorbic acid (Vitamin C) | <100 nm Cubic | - | [11] |
| nZVI | Ascorbic acid | 20 to 75 nm Spherical in chain | Cadmium (Cd) removal | [12] |
| Superparamagnetic Iron oxide(coating and functionalisation) | Ascorbic acid | 5 nm (TEM) 30 nm (Hydrodynamic size) | - | [13] |
| Fe3O4 | l-lysine amino acid | 17.5 nm and spherical Crystalline | [14] | |
| nZVI | l-glutamic acid, l-glutamine, l-arginine and l-cysteine | - | - | [15] |
| Fe NPs | Haemoglobin and myoglobin | 2–5 nm Aggregates | - | [16] |
| Fe3O4 | d-glucose gluconic acid | 12.5 nm Roughly spherical Crystalline | - | [17] |
| Fe3O4 | Glucose and gluconic acid | 4–16 nmCrystalline | - | [18] |
| Carbon encapsulated iron NPs | Wood derived sugar | Nano-sphere 100–150 nm iron-core 10–25 nm | - | [19] |
| Iron oxide | Tannic acid | <10 nm | - | [20] |
| Fe-core shell structure | Chitosan-Gallic acid | ~11 nm Cubic | - | [21] |
| Micro-Organisms | Species Name | Size | Env. Aps | References |
|---|---|---|---|---|
| Bacteria | Actinobacter sp. | 10–40 nm cubic | - | [22] |
| Actinobacter sp. | <50 nm | - | [23] | |
| Thermoanaerobacter sp. | ~13 nm | - | [24] | |
| Bacillus subtilis | 60–80 nm Spherical | - | [25] | |
| Thiobacillus thioparus | - | - | [26] | |
| Fungi | Fusarium oxysporum and Verticillium sp. | 20–50 nm Spherical | - | [27] |
| P. chlamydosporium, A. fumigates, A. wentii, C. lunata and C. globosum | 5–200 nm | - | [28] | |
| Aspergillus | 50–200 nm | - | [29] | |
| Alternaria alternate | ~9 nm | Antibacterial activity | [30] | |
| Algae | Sargassum muticum | 18 ± 4 nm cubic | - | [31] |
| Chlorococcum sp. | 20–50 nm Spherical | Reduction of chromium | [32] |
| Plants | Part Used | Size and Morphology | Environmental Application | Reference |
|---|---|---|---|---|
| Camellia sinensis | Leaf | 5–15 nm Spherical crystalline | Bromothymol blue degradation (organic contamination) | [38] |
| Green tea | Leaf | 40–60 nm amorphous | Degradation of aqueous cationic and anionic dyes | [39] |
| Green tea | Leaf | 70 nm–spherical crystalline | - | [40] |
| Tea | Tea powder | 40–50 nm spherical | - | [41] |
| Azadirachta indica | Leaf | ~100 nm | - | [43] |
| Eucalyptus Tereticornis | Leaf | 40–60 nm Cubic | Adsorption of azo dyes | [44] |
| Eucalyptus tereticornis, Melaleuca nesophila, and Rosemarinus officinalis | Leaf | 50–80 nm spherical | Catalyst for decolourisation of azo dyes | [45] |
| Grape | Leaf | 15–100 nm quasi-spherical shape amorphous | Azo dyes such as acid Orange | [46] |
| Carob | Leaf | 5–8 nm crystalline mono dispersed | - | [47] |
| Azadirachta Indica | Leaf | 50–100 nm Spherical | - | [48] |
| Tridax procumbens | Leaf | 80–100 nm crystalline irregular sphere shapes | Antibacterial | [49] |
| Punica granatum | Leaf | 100–200 nm | Hexavalent chromium removal | [50] |
| Hordeum vulgare and Rumex acetosa | Leaf | 10–40 nm amorphous | - | [51] |
| GarlicVine (Mansoa alliacea) | Leaf | 13.82 nm–15.45 nm crystalline | - | [52] |
| Terminalia chebula | Fruit | <80 nm amorphous chain-like morphology | - | [53] |
| Passiflora tripartitavar. | Fruit | 18.23–24.65 nm spherical crystalline | - | [54] |
| Syzygium cumini | Seed | 9–20 nm spherical crystalline | - | [55] |
| Alfalfa | - | <5 nm | [56] | |
| Alfalfa | - | 1–10 nm | [57] | |
| Sorghum | Bran | 40–50 nm spherical amorphous | Degradation of bromothymol blue | [58] |
| Orange extract | Peel | 30–50 nm crystalline cubic | [59] | |
| Green tea | Leaf | 40–80 nm crystalline | Photo catalytic activity | [60] |
| Aloe vera | - | 6–30 nm cubic spinel structure crystalline | - | [61] |
| Oolong tea | Leaf | 40–50 nm spherical | Degradation of malachite green | [62] |
| Salvia officinalis | Leaf | 5–25 nm spherical | - | [63] |
| Green tea | Leaf | 20–120 nm | Degradation of monochlorobenzene | [64] |
| Green tea | Leaf | 70–80 nm spherical amorphous | Degradation of dye (malachite green) | [65] |
| Eucalyptus globules | Leaf | 50 to 80 nm spherical | Adsorption of hexavalent chromium | [66] |
| Green tea | Leaf | 5–10 nm Spherical | Removal of hexavalent chromium | [67] |
| Green tea | Leaf | - | Transport properties of nano zero-valent iron (nZVI) through soil | [68] |
| S. jambos (L.) Oolong tea, A. moluccana (L.), etc. | Leaf | - | Removal of chromium | [69] |
| Green-Tea | Leaf | - | Soil mineralogy | [70] |
| Eucalyptus | Leaf | 20–80 nm amorphous | Treatment of eutrophic wastewater | [71] |
| Green tea and eucalyptus | Leaf | 20–80 nm quasi-spherical | Nitrates removal | [72] |
| Dodonaea viscose | Leaf | 50–60 nm Spherical | Antibacterial | [73] |
| Plants | Part Used | Size and Morphology | Polymeric Support | Environmental Application | Reference |
|---|---|---|---|---|---|
| Green tea | Leaf | 20–30 nm aggregates | Polyvinylidene fluoride (PVDF) membranes | Degradation of organic trichloroethylene (TCE) pollutant | [78] |
| Commercially available tea | - | 48–70 nm Crystalline | Clay (montmorillonite) | Removal of arsenic | [79] |
| Mentha spicata L. | Leaf | 20-45 nm poly dispersed cubic crystalline | Chitosan | Removal of arsenic | [80] |
| Eucalyptus globulus | Leaf | - | Chitosan | Removal of arsenic | [81] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. https://doi.org/10.3390/nano6110209
Saif S, Tahir A, Chen Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials. 2016; 6(11):209. https://doi.org/10.3390/nano6110209
Chicago/Turabian StyleSaif, Sadia, Arifa Tahir, and Yongsheng Chen. 2016. "Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications" Nanomaterials 6, no. 11: 209. https://doi.org/10.3390/nano6110209