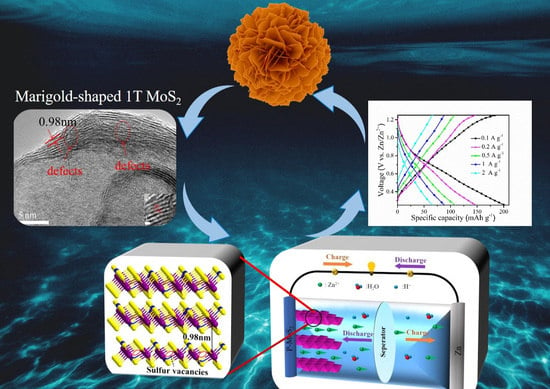
Enlarged Interlayer Spacing of Marigold-Shaped 1T-MoS2 with Sulfur Vacancies via Oxygen-Assisted Phosphorus Embedding for Rechargeable Zinc-Ion Batteries
Abstract
:1. Introduction
2. Methods
2.1. Experimental Section
2.2. Material Characterizations
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Composition and Structure
3.2. Electrochemical Performance of Aqueous Zn-Ion Batteries and Kinetic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Huang, Y.; Chang, Z.; Liu, W.; Huang, W.; Dong, L.; Kang, F.; Xu, C. Layer-by-layer zinc metal anodes to achieve long-life zinc-ion batteries. Chem. Eng. J. 2022, 431, 133902. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous Zinc-Ion Storage in MoS2 by Tuning the Intercalation Energy. Nano Lett. 2019, 19, 3199–3206. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; He, P.; Zhang, Z.; Chen, T. Edge-rich vertical graphene nanosheets templating V2O5 for highly durable zinc ion battery. Carbon 2021, 172, 207–213. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and Transition Metal Dichalcogenides as Functional Zinc-Ion Battery Cathode: A Perspective. Small Methods 2021, 5, 2000815. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, D.; Das, S.; Kumar, A.; Patil, S.; Nanda, K.K.; Sarma, D.D.; Shukla, A. Expanding Interlayer Spacing in MoS2 for Realizing an Advanced Supercapacitor. ACS Energy Lett. 2019, 4, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, G.; Zhang, Y.-W. Thermal conduction across the one-dimensional interface between a MoS2 monolayer and metal electrode. Nano Res. 2016, 9, 2372–2383. [Google Scholar] [CrossRef]
- Jin Jang, Y.; Park, S.-K. Rational design of hierarchical Ni-Mo bimetallic Selenide/N-doped carbon microspheres toward high–performance potassium ion batteries. Appl. Surf. Sci. 2022, 583, 152491. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Zhang, M.; Sun, Q.; Li, S.; Norouzi Banis, M.; Han, X.; Dong, Q.; Yang, J.; Wang, G.; et al. Enhanced sodium storage capability enabled by super wide-interlayer-spacing MoS2 integrated on carbon fibers. Nano Energy 2017, 41, 66–74. [Google Scholar] [CrossRef]
- Liu, W.; Hao, J.; Xu, C.; Mou, J.; Dong, L.; Jiang, F.; Kang, Z.; Wu, J.; Jiang, B.; Kang, F. Investigation of zinc ion storage of transition metal oxides, sulfides, and borides in zinc ion battery systems. Chem. Commun. 2017, 53, 6872–6874. [Google Scholar] [CrossRef]
- Khan, M.F.; Miriyala, N.; Lee, J.; Hassanpourfard, M.; Kumar, A.; Thundat, T. Heat capacity measurements of sub-nanoliter volumes of liquids using bimaterial microchannel cantilevers. Appl. Phys. Lett. 2016, 108, 211906. [Google Scholar] [CrossRef]
- Oh, H.G.; Park, S.-K. Co-MOF derived MoSe2@CoSe2/N-doped carbon nanorods as high-performance anode materials for potassium ion batteries. Int. J. Energy Res. 2022, 46, 10677–10688. [Google Scholar] [CrossRef]
- Shao, F.; Huang, Y.; Wang, X.; Li, Z.; Huang, X.; Huang, W.; Dong, L.; Kang, F.; Liu, W.; Xu, C. MoS2 with high 1T phase content enables fast reversible zinc-ion storage via pseudocapacitance. Chem. Eng. J. 2022, 448, 137688. [Google Scholar] [CrossRef]
- Liu, J.; Gong, N.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Vertically aligned 1 T phase MoS2 nanosheet array for high-performance rechargeable aqueous Zn-ion batteries. Chem. Eng. J. 2022, 428, 130981. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, S.; Guo, J.; Ling, Y.; Zhang, Y.; Gong, W.; Wei, L.; Wang, C.; Zhang, Q.; et al. Cobalt-doped MoS2·nH2O nanosheets induced heterogeneous phases as high-rate capability and long-term cyclability cathodes for wearable zinc-ion batteries. Energy Storage Mater. 2023, 55, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-Like Heterostructures of MoS2/Graphene with Enlarged Interlayer Spacing and Enhanced Hydrophilicity as High-Performance Cathodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Cui, K.; Shen, Q.; Li, P.; Qu, X.; Jiao, L. Molecular Engineering on MoS2 Enables Large Interlayers and Unlocked Basal Planes for High-Performance Aqueous Zn-Ion Storage. Angew. Chem. Int. Ed. 2021, 60, 20286–20293. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Li, B.; Zhang, C.; Du, Z.; Yin, H.; Bi, X.; Yang, S. Ultrastable In-Plane 1T–2H MoS2 Heterostructures for Enhanced Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1801345. [Google Scholar] [CrossRef]
- Huang, X.; Leng, M.; Xiao, W.; Li, M.; Ding, J.; Tan, T.L.; Lee, W.S.V.; Xue, J. Activating Basal Planes and S-Terminated Edges of MoS2 toward More Efficient Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1604943. [Google Scholar] [CrossRef]
- Nipane, A.; Karmakar, D.; Kaushik, N.; Karande, S.; Lodha, S. Few-Layer MoS2 p-Type Devices Enabled by Selective Doping Using Low Energy Phosphorus Implantation. ACS Nano 2016, 10, 2128–2137. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, L.; Han, B.; Zhang, H.; Chen, J.; Xie, F.; Wang, N.; Jin, Y.; Meng, H. Cobalt-doped molybdenum disulfide with rich defects and extended layered structure for rechargeable zinc-ion batteries. J. Alloy Compd. 2022, 916, 165487. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, J.; Zhao, L.; Li, G.; Chen, D.; Han, W.; Chu, Y.; Cui, X.; Li, C.; Zhang, Y. Few-layered MoS2 with S-vacancies anchored on N-doped carbon flower for high performance sodium storage. J. Alloy Compd. 2022, 895, 162514. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Si, H.; Zhang, Q.; Wu, J.; Gao, L.; Wei, X.; Sun, Y.; Liao, Q.; Zhang, Z.; et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. J. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef]
- Xu, W.; Sun, C.; Zhao, K.; Cheng, X.; Rawal, S.; Xu, Y.; Wang, Y. Defect engineering activating (Boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019, 16, 527–534. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Z.; Wu, Z.; Wang, Z.; Liu, Z. Hydrothermal synthesis of MoS2 nanoflowers as highly efficient hydrogen evolution reaction catalysts. J. Power Sources 2014, 264, 229–234. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef]
- Song, H.; Li, T.; He, T.; Wang, Z.; Fang, D.; Wang, Y.; Li, X.L.; Zhang, D.; Hu, J.; Huang, S. Cooperative catalytic Mo-S-Co heterojunctions with sulfur vacancies for kinetically boosted lithium-sulfur battery. Chem. Eng. J. 2022, 450, 138115. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Mi, H.; Luo, S.; Zhang, P.; Lin, Z.; Li, J.; Zhang, H. Robust SnO2−x Nanoparticle-Impregnated Carbon Nanofibers with Outstanding Electrochemical Performance for Advanced Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 8901–8905. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.T.; Zhou, W.; Loh, K.P. Phase Restructuring in Transition Metal Dichalcogenides for Highly Stable Energy Storage. ACS Nano 2016, 10, 9208–9215. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Su, D.; Zhou, R.; Sun, B.; Wang, G.; Qiao, S.Z. Highly Ordered Mesoporous MoS2 with Expanded Spacing of the (002) Crystal Plane for Ultrafast Lithium Ion Storage. Adv. Energy Mater. 2012, 2, 970–975. [Google Scholar] [CrossRef]
- Shi, S.; Sun, Z.; Hu, Y.H. Synthesis, stabilization and applications of 2-dimensional 1T metallic MoS2. J. Mater. Chem. A 2018, 6, 23932–23977. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, D.; Ouyang, B.; Raja, A.; Song, J.; Heinz, T.F.; Brus, L.E. Probing the Dynamics of the Metallic-to-Semiconducting Structural Phase Transformation in MoS2 Crystals. Nano Lett. 2015, 15, 5081–5088. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, J.; He, J.; Luo, R.; Wang, D.; Che, X.; Bu, K.; Zhao, W.; Liu, P.; Mu, G.; et al. Structure Re-determination and Superconductivity Observation of Bulk 1T MoS2. Angew. Chem. Int. Ed. 2018, 57, 1232–1235. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef]
- Xue, H.; Meng, A.; Chen, C.; Xue, H.; Li, Z.; Wang, C. Phosphorus-doped MoS2 with sulfur vacancy defects for enhanced electrochemical water splitting. Sci. China Mater. 2022, 65, 712–720. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; You, Z.; Hou, Z.; Yang, J.; Wei, C.; Kang, F. Boosting zinc-ion intercalation in hydrated MoS2 nanosheets toward substantially improved performance. Energy Storage Mater. 2021, 35, 731–738. [Google Scholar] [CrossRef]
- Angamuthu, G.; Rengarajan, V. MoS2 mediated nitrogen enriched composite material for high and fast Li-ion storage. Appl. Surf. Sci. 2020, 525, 146437. [Google Scholar] [CrossRef]
- Sheng, Z.; Qi, P.; Lu, Y.; Liu, G.; Chen, M.; Gan, X.; Qin, Y.; Hao, K.; Tang, Y. Nitrogen-Doped Metallic MoS2 Derived from a Metal–Organic Framework for Aqueous Rechargeable Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 34495–34506. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Cho, J.; Ryu, S.; Gong, Y.J.; Pyo, S.; Yun, H.; Kim, H.; Lee, J.; Yoo, J.; Kim, Y.S. Nitrogen-doped MoS2 as a catalytic sulfur host for lithium-sulfur batteries. Chem. Eng. J. 2022, 439, 135568. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Li, J.; Cao, L.; Lu, Z.; Zhu, D. Structure Engineering of MoS2 via Simultaneous Oxygen and Phosphorus Incorporation for Improved Hydrogen Evolution. Small 2020, 16, 1905738. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Y.; Li, S.; He, B.; Liu, H.; Zeng, X.; Zhang, J.; Wang, G. P doped MoS2 nanoplates embedded in nitrogen doped carbon nanofibers as an efficient catalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2019, 547, 291–298. [Google Scholar] [CrossRef]
- Chae, M.S.; Heo, J.W.; Lim, S.-C.; Hong, S.-T. Electrochemical Zinc-Ion Intercalation Properties and Crystal Structures of ZnMo6S8 and Zn2Mo6S8 Chevrel Phases in Aqueous Electrolytes. Inorg. Chem. 2016, 55, 3294–3301. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, L.; Zhong, L.; Chen, J.; Li, B.; Wang, W.; Mao, S.X.; Wang, C.; Sprenkle, V.L.; Li, G.; et al. Highly Reversible Zinc-Ion Intercalation into Chevrel Phase Mo6S8 Nanocubes and Applications for Advanced Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 13673–13677. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, B.; Gao, Y.; Zhang, S.; Shin, H.; Zeng, H.; Park, J.H. Aligned Heterointerface-Induced 1T-MoS2 Monolayer with Near-Ideal Gibbs Free for Stable Hydrogen Evolution Reaction. Small 2019, 15, 1804903. [Google Scholar] [CrossRef]
- Xu, J.; Dong, Z.; Huang, K.; Wang, L.; Wei, Z.; Yu, L.; Wu, X. Flexible design of large layer spacing V-MoS2@C cathode for high-energy zinc-ion battery storage. Scr. Mater. 2022, 209, 114368. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Tao, Z.; Zhu, Y.; Tan, Y.; Wang, A.; Zhou, H.; Yang, Y. A nano interlayer spacing and rich defect 1T-MoS2 as cathode for superior performance aqueous zinc-ion batteries. Nanoscale Adv. 2021, 3, 3780–3787. [Google Scholar] [CrossRef]
- Yao, J.; Jin, T.; Li, Y.; Xiao, S.; Huang, B.; Jiang, J. Electrochemical performance of Fe2(SO4)3 as a novel anode material for lithium-ion batteries. J. Alloy Compd. 2021, 886, 161238. [Google Scholar] [CrossRef]
- Yao, J.; Yang, Y.; Li, Y.; Jiang, J.; Xiao, S.; Yang, J. Interconnected α-Fe2O3 nanoparticles prepared from leaching liquor of tin ore tailings as anode materials for lithium-ion batteries. J. Alloy Compd. 2021, 855, 157288. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, K.; Qin, C.; Huang, K. Unraveling the role of structural water in bilayer V2O5 during Zn2+-intercalation: Insights from DFT calculations. J. Mater. Chem. A 2019, 7, 5612–5620. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef]
- Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Li ion kinetic studies on spinel cathodes, Li(M1/6Mn11/6)O4 (M = Mn, Co, CoAl) by GITT and EIS. J. Mater. Chem. 2003, 13, 106–113. [Google Scholar] [CrossRef] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Li, X.; Wu, L.; Zhang, Z.; Chen, Y.; Liu, L.; Cheng, Y. Enlarged Interlayer Spacing of Marigold-Shaped 1T-MoS2 with Sulfur Vacancies via Oxygen-Assisted Phosphorus Embedding for Rechargeable Zinc-Ion Batteries. Nanomaterials 2023, 13, 1185. https://doi.org/10.3390/nano13071185
Xu Q, Li X, Wu L, Zhang Z, Chen Y, Liu L, Cheng Y. Enlarged Interlayer Spacing of Marigold-Shaped 1T-MoS2 with Sulfur Vacancies via Oxygen-Assisted Phosphorus Embedding for Rechargeable Zinc-Ion Batteries. Nanomaterials. 2023; 13(7):1185. https://doi.org/10.3390/nano13071185
Chicago/Turabian StyleXu, Qinhu, Xinyu Li, Luchen Wu, Zhen Zhang, Yong Chen, Ling Liu, and Yong Cheng. 2023. "Enlarged Interlayer Spacing of Marigold-Shaped 1T-MoS2 with Sulfur Vacancies via Oxygen-Assisted Phosphorus Embedding for Rechargeable Zinc-Ion Batteries" Nanomaterials 13, no. 7: 1185. https://doi.org/10.3390/nano13071185