Facile Synthesis of Battery-Type CuMn2O4 Nanosheet Arrays on Ni Foam as an Efficient Binder-Free Electrode Material for High-Rate Supercapacitors
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Fabrication of Battery-Type CuMn2O4 NSA Material on Ni Foam Surface
2.3. Material Characterization and Electrochemical Measurements
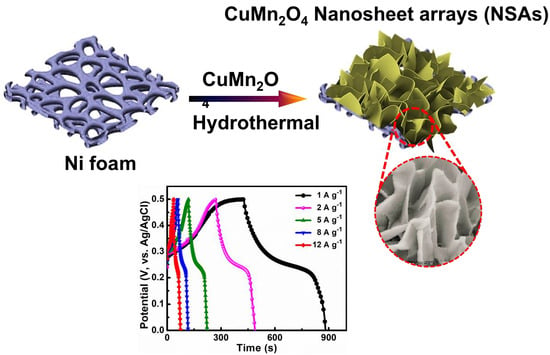
3. Results and Discussion
| Battery-Type Electrode | Preparation Route | Specific Capacity (mA h g−1) | Cycling Stability (Cycles) | Ref. |
|---|---|---|---|---|
| NiCo2O4 flower-like | Hydrothermal | 34.02 at 1 A g−1 | 78.30% (6000) | [42] |
| CuCo2O4 ultrathin nanosheets | Hydrothermal | 84.22 at 1 A g−1 | 71.80% (5000) | [43] |
| NiMn2O4 microspheres | Microwave-assisted | 138.83 at 1 A g−1 | 85.80% (6000) | [44] |
| CuNiO2 dandelion flower-like | Hydrothermal | 111.52 at 2 A g−1 | 89.13% (3000) | [45] |
| CuCo2O4 microspheres | Hydrothermal | 86.37 at 1 A g−1 | 93.00% (6000) | [46] |
| FeCo2O4 chopsticks-like | Hydrothermal | 113.32 at 1 A g−1 | 93.68% (4000) | [47] |
| CuMn2O4 microspheres | Micro/nano MnCO3 precursor | 73 at 1 A g−1 | 96% (3000) | [26] |
| Spinel CuMn2O4-RGO nanosheets | Sol-gel with physical griding | 95 at 1 A g−1 | 75.5% (1000) | [27] |
| CuMn2O4 | Hydrothermal | 125.56 at 1 A g−1 | 92.15% (5000) | Present work |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Liu, P.; Xie, Q.; Zhang, G.; Zheng, H.; Cai, Y.; Li, Z.; Wang, L.; Zhu, Z.Z.; Mai, L.; et al. Double-shell Li-rich layered oxide hollow microspheres with sandwich-like carbon@spinel@layered@spinel@carbon shells as high-rate lithium ion battery cathode. Nano Energy 2019, 59, 184–196. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Dinh, K.N.; Yan, Q.Y.; Ma, J.M. Synthesis, characterizations and utilization of oxygen-deficient metal oxides for lithium/sodium-ion batteries and supercapacitors. Coord. Chem. Rev. 2019, 397, 138–167. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, X.; Wu, N.; Zhao, X.; Gong, J. Dandelion-Like CuCo2O4@ NiMn LDH Core/Shell Nanoflowers for Excellent Battery-Type Supercapacitor. Nanomaterials 2023, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.H.; Zhang, G.X.; Zhou, T.; Sun, S.H. Recent developments of planar microsupercapacitors: Fabrication, properties and applications. Adv. Funct. Mater. 2020, 30, 1910000. [Google Scholar] [CrossRef]
- Gong, Y.N.; Li, D.L.; Luo, C.Z.; Fu, Q.; Pan, C.X. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.i.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Wan, L.; Liang, K.; Tay, B.K.; Kong, L.; Yan, X. An Asymmetric Supercapacitor with Both Ultra-High Gravimetric and Volumetric Energy Density Based on 3D Ni(OH)2/MnO2@Carbon Nanotube and Activated Polyaniline-Derived Carbon. ACS Appl. Mater. Interfaces 2017, 9, 668–676. [Google Scholar] [CrossRef]
- Hsu, C.T.; Hu, C.C.; Wu, T.H.; Chen, J.C.; Rajkumar, M. How the Electrochemical Reversibility of a Battery-type Material Affects the Charge Balance and Performances of Asymmetric Supercapacitors. Electrochim. Acta 2014, 146, 759–768. [Google Scholar] [CrossRef]
- Natarajan, S.; Ulaganathan, M.; Bajaj, H.C.; Aravindan, V. Transformation of spent Li-ion battery into high energy supercapacitors in asymmetric configuration. Chem. Electron. Chem. 2019, 6, 5283–5292. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.S.; Aravindan, V. Biomass-derived carbon: A value added journey towards constructing high-energy supercapacitors in an asymmetric fashion. ChemSusChem 2019, 12, 4353–4382. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, S.; Mu, S.; Chen, T.; Qiao, Y.; Yu, S.; Gao, F. Synthesis of capsule-like porous hollow nano-nickel cobalt sulfides via cation exchange based on the kirkendall effect for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 9721–9732. [Google Scholar] [CrossRef]
- Nagaraju, G.; Sekhar, S.C.; Bharat, L.K.; Yu, J.S. Wearable Fabrics with Self-Branched Bimetallic Layered Double Hydroxide Coaxial Nanostructures for Hybrid Supercapacitors. ACS Nano 2017, 11, 10860–10874. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. High-performance pouch-type hybrid supercapacitor based on hierarchical NiO-Co3O4-NiO composite nanoarchitectures as an advanced electrode material. Nano Energy 2018, 48, 81–92. [Google Scholar] [CrossRef]
- Sun, J.; Tian, X.; Xu, C.; Chen, H. Porous CuCo2O4 microtubes as a promising battery-type electrode material for high-performance hybrid supercapacitors. J. Materiomics 2021, 7, 1358–1368. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Vinodh, R.; Obaidat, I.M.; Kim, H.J. Facile synthesis of hierarchical flower-like NiMoO4-CoMoO4 nanosheet arrays on nickel foam as an efficient electrode for high rate hybrid supercapacitors. J. Energy Storage 2020, 30, 101550. [Google Scholar] [CrossRef]
- Nam, H.W.; Gopi, C.V.V.M.; Sambasivam, S.; Vinodh, R.; Raghavendra, K.V.G.; Kim, H.J.; Obaidat, I.M.; Kim, S. Binder-free honeycomb-like FeMoO4 nanosheet arrays with dual properties of both battery-type and pseudocapacitive-type performances for supercapacitor applications. J. Energy Storage 2020, 27, 101055. [Google Scholar] [CrossRef]
- Song, C.S.; Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Kalla, R.M.N.; Obaidat, I.M.; Kim, H.J. Morphology-dependent binder-free CuNiO2 electrode material with excellent electrochemical performances for supercapacitors. J. Energy Storage 2019, 26, 101037. [Google Scholar] [CrossRef]
- Zhang, M.M.; Song, Z.X.; Liu, H.; Ma, T.J. Biomass-derived highly porous nitrogen-doped graphene orderly supported NiMn2O4 nanocrystals as efficient electrode materials for asymmetric supercapacitors. Appl. Srf. Sci. 2020, 507, 145065. [Google Scholar] [CrossRef]
- Lia, L.; Jiang, G.X.; Ma, J.M. CuMn2O4/graphene nanosheets as excellent anode for lithium-ion battery. Mater. Res. Bull 2018, 104, 53–59. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Harilal, M.; Arshid, N.; Jagadish, P.; Khalid, M.; Li, L.P. Rapid microwave-assisted synthesis of MnCo2O4 nanoflakes as a cathode for battery-supercapacitor hybrid. J. Energy Storage 2021, 44, 103566. [Google Scholar] [CrossRef]
- Krishna, B.N.V.; Bhagwan, J.; Yu, J.S. Sol-Gel Routed NiMn2O4 Nanofabric Electrode Materials for Supercapacitors. J. Electrochem. Soc. 2019, 166, A1950. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Yu, L.; Zhang, Y.; Hou, D.; Li, T. Facile synthesis of NiMn2O4 nanosheet arrays grown on nickel foam as novel electrode materials for high-performance supercapacitors. Ceram. Int. 2016, 42, 14963–14969. [Google Scholar] [CrossRef]
- Cheng, C.; Cheng, Y.; Lai, G. CuMn2O4 hierarchical microspheres as remarkable electrode of supercapacitors. Mater. Lett. 2022, 317, 132102. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, A.; Zhang, W.; Chang, J.; Liu, C.; Gu, L.; Duo, X.; Pan, F.; Luo, S. CuMn2O4 spinel anchored on graphene nanosheets as a novel electrode material for supercapacitor. J. Energy Storage 2021, 34, 102181. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, B.; Hu, L.; Xu, Z.; Li, Q.; Wang, T. High-performance supercapacitor and lithium-ion battery based on 3D hierarchical NH4F-induced nickel cobaltate nanosheet–nanowire cluster arrays as self-supported electrodes. Nanoscale 2013, 5, 9812–9820. [Google Scholar] [CrossRef]
- Gopi, C.V.M.; Ramesh, R.; Kim, H.J. Designing nanosheet manganese cobaltate@manganese cobaltate nanosheet arrays as a battery-type electrode material towards high-performance supercapacitors. J. Energy Storage 2022, 47, 103603. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, C.W.; Kim, S.Y.; Reddy, A.E.; Gopi, C.V.V.M. Facile synthesis of unique diamond-like structured CdMn2O4@CdMn2O4 composite material for high performance supercapacitors. Materials Lett. 2018, 210, 143–147. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kalla, R.M.N.; Kim, H.J. One-pot synthesis of copper oxide-cobalt oxide core-shell nanocactus-like heterostructures as binder-free electrode materials for high-rate hybrid supercapacitors. Mater. Today Energy 2019, 14, 100358. [Google Scholar] [CrossRef]
- Sambasivam, S.; Gopi, C.V.M.; Arbi, H.M.; Kumar, Y.A.; Kim, H.J.; Zahmi, S.A.; Obaidat, I.M. Binder-free hierarchical core-shell-like CoMn2O4@MnS nanowire arrays on nickel foam as a battery-type electrode material for high-performance supercapacitors. J. Energy Storage 2021, 36, 102377. [Google Scholar] [CrossRef]
- Yousef, R.; Al-Zoubi, A.; Sad-Din, N. A Study of Structural Properties of CuMn2O4 Synthesized by Solid State Method. Adv. Phys. Theor. Appl. 2018, 71, 24–30. [Google Scholar]
- Zhu, B.; Qin, Y.; Du, J.; Zhang, F.; Lei, X. Ammonia Etching to Generate Oxygen Vacancies on CuMn2O4 for Highly Efficient Electrocatalytic Oxidation of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2021, 9, 11790–11797. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, L.; Wang, C.Y.; Lin, Z.; Lou, X.W.D. Hierarchical Tubular Structures Composed of Mn-Based Mixed Metal Oxide Nanoflakes with Enhanced Electrochemical Properties. Adv. Funct. Mater. 2015, 25, 5184–5189. [Google Scholar] [CrossRef]
- Guan, B.Y.; Kushima, A.; Yu, L.; Li, S.; Li, J.; Lou, X.W.D. Coordination Polymers Derived General Synthesis of Multishelled Mixed Metal-Oxide Particles for Hybrid Supercapacitors. Adv. Mater. 2017, 29, 1605902. [Google Scholar] [CrossRef]
- Fu, W.B.; Zhao, Y.Y.; Mei, J.F.; Wang, F.J.; Han, W.H.; Wang, F.C.; Xie, E.Q. Honeycomb-like Ni3S2 nanosheet arrays for high-performance hybrid supercapacitors. Electrochim. Acta 2018, 283, 737–743. [Google Scholar] [CrossRef]
- Gao, M.J.; Le, K.; Xu, D.M.; Wang, Z.; Wang, F.L.; Liu, W.; Yu, H.J.; Liu, J.R.; Chen, C.Z. Controlled sulfidation towards achieving core-shell 1D-NiMoO4 @ 2D-NiMoS4 architecture for high-performance asymmetric supercapacitor. J. Alloys Compd. 2019, 804, 27–34. [Google Scholar] [CrossRef]
- Wang, G.R.; Li, Y.B.; Xu, L.; Jin, Z.L.; Wang, Y.B. Facile synthesis of difunctional NiV LDH@ZIF-67 p-n junction: Serve as prominent photocatalyst for hydrogen evolution and supercapacitor electrode as well. Renew. Energ. 2020, 162, 535–549. [Google Scholar] [CrossRef]
- Sambasivam, S.; Raghavendra, K.V.G.; Yedluri, A.K.; Arbi, H.M.; Narayanaswamy, V.; Gopi, C.V.V.M.; Choi, B.-C.; Kim, H.-J.; Alzahmi, S.; Obaidat, I.M. Facile Fabrication of MnCo2O4/NiO Flower-Like Nanostructure Composites with Improved Energy Storage Capacity for High-Performance Supercapacitors. Nanomaterials 2021, 11, 1424. [Google Scholar] [CrossRef]
- Bhagwan, J.; Nagaraju, G.; Ramulu, B.; Sekhar, S.C.; Yu, J.S. Rapid synthesis of hexagonal NiCo2O4 nanostructures for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 299, 509–517. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, F.; Yan, Q.; Wu, X. Investigation on electrochemical behaviors of NiCo2O4 battery-type supercapacitor electrodes: The role of an aqueous electrolyte. Inorg. Chem. Front. 2017, 4, 1642–1648. [Google Scholar] [CrossRef]
- Sun, J.; Du, X.; Wu, R.; Zhang, Y.; Xu, C.; Chen, H. Bundle-like CuCo2O4 microstructures assembled with ultrathin nanosheets as battery-type electrode materials for high-performance hybrid supercapacitors. ACS Appl. Energy Mater. 2020, 3, 8026–8037. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.J.; Sun, X.N.; Huang, N.B. High-performance spinel NiMn2O4 microshphres self-assembled with nanosheets by microwave-assisted synthesis for supercapacitors. Crystengcomm 2020, 22, 1645–1652. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Joo, H.H.; Vinodha, R.; Kim, H.J.; Sambasivam, S.; Obaidat, I.M. Facile synthesis of flexible and binder-free dandelion flower-like CuNiO2 nanostructures as advanced electrode material for high-performance supercapacitors. J. Energy Storage 2019, 26, 100914. [Google Scholar]
- Li, G.F.; Liu, S.Q.; Pan, Y.; Zhou, T.Y.; Ding, J.D.; Sun, Y.M.; Wang, Y.Q. Self-templated formation of CuCo2O4 triple-shelled hollow microspheres for all-solid-state asymmetric supercapacitor. J. Alloys Compd. 2019, 787, 694–699. [Google Scholar] [CrossRef]
- Huang, T.; Qiu, Z.; Hu, Z.; Zhang, Z. Porous chopsticks-like FeCo2O4 by the hydrothermal method for high-performance asymmetric supercapacitors. J. Energy Storage 2022, 46, 103898. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopi, C.V.V.M.; Ramesh, R.; Vinodh, R.; Alzahmi, S.; Obaidat, I.M. Facile Synthesis of Battery-Type CuMn2O4 Nanosheet Arrays on Ni Foam as an Efficient Binder-Free Electrode Material for High-Rate Supercapacitors. Nanomaterials 2023, 13, 1125. https://doi.org/10.3390/nano13061125
Gopi CVVM, Ramesh R, Vinodh R, Alzahmi S, Obaidat IM. Facile Synthesis of Battery-Type CuMn2O4 Nanosheet Arrays on Ni Foam as an Efficient Binder-Free Electrode Material for High-Rate Supercapacitors. Nanomaterials. 2023; 13(6):1125. https://doi.org/10.3390/nano13061125
Chicago/Turabian StyleGopi, Chandu V. V. Muralee, R. Ramesh, Rajangam Vinodh, Salem Alzahmi, and Ihab M. Obaidat. 2023. "Facile Synthesis of Battery-Type CuMn2O4 Nanosheet Arrays on Ni Foam as an Efficient Binder-Free Electrode Material for High-Rate Supercapacitors" Nanomaterials 13, no. 6: 1125. https://doi.org/10.3390/nano13061125