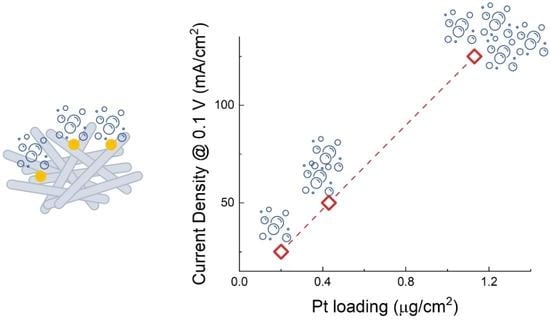
WO3 Nanorods Decorated with Very Small Amount of Pt for Effective Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of WO3 Nanorods and Pt Nanoparticles
2.2. Electrode Preparation
2.3. Characterization of the Pt-Decorated WO3 Nanorods
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Morphological and Structural Analysis
3.2. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 Economy Realizable in the Foreseeable Future? Part III: H2 Usage Technologies, Applications, and Challenges and Opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and Correctness in the Evaluation of Electrocatalytic Water Splitting: Revisiting Activity Parameters with a Critical Assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, X.; Liu, Y.; Wei, H.; Ma, J.; Chen, M.; Liu, X.; Zhang, W.; Wang, G.; Ren, F.; et al. WO3-Based Materials as Electrocatalysts for Hydrogen Evolution Reaction. Front. Mater. 2020, 7, 105. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced Water Splitting Performance of Biomass Activated Carbon-Anchored WO3 Nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the Scaling-up of Water Splitting Catalysts. Nat. Energy 2019, 4, 430–433. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Sang, W.; He, Z.; Wei, Q.; Chen, B.; Li, H.; Cao, C.; Huang, R.; Yan, X.; Pan, B.; et al. Conductive Tungsten Oxide Nanosheets for Highly Efficient Hydrogen Evolution. Nano Lett. 2017, 17, 7968–7973. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Ping, S.; Shao, Z. Materials Reports: Energy Designing Single-Atom Catalysts toward Improved Alkaline Hydrogen Evolution Reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Xie, X.; Jiang, Y.F.; Yuan, C.Z.; Jiang, N.; Zhao, S.J.; Jia, L.; Xu, A.W. Ultralow Pt Loaded Molybdenum Dioxide/Carbon Nanotubes for Highly Efficient and Durable Hydrogen Evolution Reaction. J. Phys. Chem. C 2017, 121, 24979–24986. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Kim, Y.; Kim, H.W.; Choi, M.; Park, N.; Chang, H.; Kwon, Y.; Park, J.H.; Kim, H.J. In Situ Electrochemically Synthesized Pt-MoO3−x Nanostructure Catalysts for Efficient Hydrogen Evolution Reaction. J. Catal. 2020, 381, 1–13. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Salama, T.M.; Hegazy, M.A.; Abou Shahba, R.M.; Mohamed, S.H. Synthesis of Hexagonal WO3 Nanocrystals with Various Morphologies and Their Enhanced Electrocatalytic Activities toward Hydrogen Evolution. Int. J. Hydrogen Energy 2019, 44, 4724–4736. [Google Scholar] [CrossRef]
- Mineo, G.; Scuderi, M.; Bruno, E.; Mirabella, S. Engineering Hexagonal/Monoclinic WO3 Phase Junctions for Improved Electrochemical Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 9702–9710. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, G.; Wang, D.; Chen, Z.; Zhao, M.; Xi, G. Crystallographic Phase and Morphology Dependent Hydrothermal Synthesis of Tungsten Oxide for Robust Hydrogen Evolution Reaction. J. Alloys Compd. 2021, 875, 160054. [Google Scholar] [CrossRef]
- Sharma, L.; Kumar, P.; Halder, A. Phase and Vacancy Modulation in Tungsten Oxide: Electrochemical Hydrogen Evolution. ChemElectroChem 2019, 6, 3420–3428. [Google Scholar] [CrossRef]
- Ooka, H.; Huang, J.; Exner, K.S. The Sabatier Principle in Electrocatalysis: Basics, Limitations, and Extensions. Front. Energy Res. 2021, 9, 654460. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Y.; Liu, Y.; Chen, Z.; Zhang, M. CoSe2/Wse2/WO3 Hybrid Nanowires on Carbon Cloth for Efficient Hydrogen Evolution Reaction. J. Alloys Compd. 2018, 768, 889–895. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Do, H.H.; Tekalgne, M.; Van Le, Q.; Nguyen, T.P.; Hong, S.H.; Cho, J.H.; Van Dao, D.; Ahn, S.H.; Kim, S.Y. WS2–WC–WO3 Nano-Hollow Spheres as an Efficient and Durable Catalyst for Hydrogen Evolution Reaction. Nano Converg. 2021, 8, 28. [Google Scholar] [CrossRef]
- Shang, X.; Rao, Y.; Lu, S.S.; Dong, B.; Zhang, L.M.; Liu, X.H.; Li, X.; Liu, Y.R.; Chai, Y.M.; Liu, C.G. Novel WS2/WO3 Heterostructured Nanosheets as Efficient Electrocatalyst for Hydrogen Evolution Reaction. Mater. Chem. Phys. 2017, 197, 123–128. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Liu, P.; Zhu, X.; Li, X.; Ali, R.N.; Xiang, B. Enhanced Electrocatalytic Activity of WO3@NPRGO Composite in a Hydrogen Evolution Reaction. Appl. Surf. Sci. 2019, 463, 275–282. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, C.S.; Jung, H.; Joo, O.S.; Min, B.K.; Kim, J.H.; Hwang, Y.J. Insight into Charge Separation in WO3/BiVO4 Heterojunction for Solar Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 19780–19790. [Google Scholar] [CrossRef]
- Mineo, G.; Moulaee, K.; Neri, G.; Mirabella, S.; Bruno, E. H2 Detection Mechanism in Chemoresistive Sensor Based on Low-Cost Synthesized WO3 Nanorods. Sens. Actuators B 2021, 348, 130704. [Google Scholar] [CrossRef]
- Bruno, L.; Battiato, S.; Scuderi, M.; Priolo, F.; Terrasi, A.; Mirabella, S. Physical Insights into Alkaline Overall Water Splitting with NiO Microflowers Electrodes with Ultra-Low Amount of Pt Catalyst. Int. J. Hydrogen Energy 2022, 47, 33988–33998. [Google Scholar] [CrossRef]
- Available online: http://www.genplot.com/download.htm (accessed on 1 February 2023).
- Yang, M.; Li, J.; Ke, G.; Liu, B.; Dong, F.; Yang, L.; He, H.; Zhou, Y. WO3 Homojunction Photoanode: Integrating the Advantages of WO3 Different Facets for Efficient Water Oxidation. J. Energy Chem. 2021, 56, 37–45. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Noda, S. The Significance of Properly Reporting Turnover Frequency in Electrocatalysis Research. Angew. Chem. Int. Ed. 2021, 60, 23051–23067. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Anderson, L.; Chen, Y.; Pan, M.; Abel Chuang, P.Y. New Insights into Evaluating Catalyst Activity and Stability for Oxygen Evolution Reactions in Alkaline Media. Sustain. Energy Fuels 2018, 2, 237–251. [Google Scholar] [CrossRef]
- Battiato, S.; Bruno, L.; Terrasi, A.; Mirabella, S. Superior Performances of Electroless-Deposited Ni-P Films Decorated with an Ultralow Content of Pt for Water-Splitting Reactions. ACS Appl. Energy Mater. 2022, 5, 2391–2399. [Google Scholar] [CrossRef]
- Costentin, C.; Passard, G.; Savéant, J.M. Benchmarking of Homogeneous Electrocatalysts: Overpotential, Turnover Frequency, Limiting Turnover Number. J. Am. Chem. Soc. 2015, 137, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.T.; Chen, L.X.; Wang, A.J.; Fang, K.M.; Feng, J.J. Ternary PtCoNi Flower-like Networks: One-Step Additive-Free Synthesis and Highly Boosted Electrocatalytic Performance for Hydrogen Evolution and Oxygen Reduction. Int. J. Hydrogen Energy 2017, 42, 25277–25284. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Su, J.; Jiang, P.; Xia, G.; Chen, Q. Enhanced Activity for Hydrogen Evolution Reaction over CoFe Catalysts by Alloying with Small Amount of Pt. ACS Appl. Mater. Interfaces 2017, 9, 3596–3601. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the Electrocatalytic Hydrogen Evolution Activity of the Inert Two-Dimensional MoS2 Surface via Single-Atom Metal Doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H.; et al. Ultrahigh Pt-Mass-Activity Hydrogen Evolution Catalyst Electrodeposited from Bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, H.E.; Cho, A.; Kim, S.; Ye, Y.; Han, J.W.; Lee, H.; Jang, J.H.; Lee, J. Investigation of the Support Effect in Atomically Dispersed Pt on WO3 − x for Utilization of Pt in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 16038–16042. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Hao, Q.; Chen, D.; Chen, T.; Hao, S.; Yang, J.; Ding, H.; Yao, W.; Song, J. Facile Fabrication of Heterostructured Bismuth Titanate Nanocomposites: The Effects of Composition and Band Gap Structure on the Photocatalytic Activity Performance. Catal. Today 2017, 297, 255–263. [Google Scholar] [CrossRef]
- Kang, M.; Liang, J.; Wang, F.; Chen, X.; Lu, Y.; Zhang, J. Structural Design of Hexagonal/Monoclinic WO3 Phase Junction for Photocatalytic Degradation. Mater. Res. Bull. 2020, 121, 110614. [Google Scholar] [CrossRef]



| Pt Dose (×1015 at/cm2) | Pt Loading (μg/cm2) | (mV) | Tafel Slope (mV/dec) | |
|---|---|---|---|---|
| WO3 | - | - | 173 | 104 |
| 5Pt_WO3 | 0.61 | 0.2 | 63 | 75 |
| 10Pt_WO3 | 1.31 | 0.43 | 44 | 37 |
| 20Pt_WO3 | 3.51 | 1.13 | 32 | 31 |
| Pt Content | Electrolyte | (mV) | Tafel Slope (mV/dec) | TOF @ − 15 mV (Hz) | Mass Activity @10 mA/cm2 (A/mg) | Ref. | |
|---|---|---|---|---|---|---|---|
| Pt/MoO2/MWCNTs | 0.47 mg/cm2 | 0.5 M H2SO4 | 60 | 43 | 2.8 @ − 50 mV | 0.2 | [10] |
| PtCoNi FNs | 0.85 mg/cm2 | 0.5 M H2SO4 | 41 | 37 | - | 0.01 | [31] |
| PtCoFe@CN | 0.013 mg/cm2 | 0.5 M H2SO4 | 45 | 32 | - | 0.8 | [32] |
| PtMoS2 | 0.036 mg/cm2 | 0.5 M H2SO4 | 60 | 96 | - | 0.3 | [33] |
| PtCu nanospheres on WO3 nano-array | 0.25 mg/cm2 | 0.5 M H2SO4 | 40 | 46 | 11 @ − 100 mV | 2 | [34] |
| Pt SA/m-WO3 − x | 0.86 g/cm2 | 0.5 M H2SO4 | 47 | 45 | - | 12.8 (@ 0.05 V) | [35] |
| 5Pt_WO3 | 0.2 g/cm2 | 1 M H2SO4 | 63 | 75 | 3 @ − 15 mV | 23 | Our work |
| 10Pt_WO3 | 0.43 g/cm2 | 1 M H2SO4 | 44 | 37 | 4 @ − 15 mV | 17 | Our work |
| 20Pt_WO3 | 1.13 g/cm2 | 1 M H2SO4 | 32 | 31 | 5 @ − 15 mV | 9 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineo, G.; Bruno, L.; Bruno, E.; Mirabella, S. WO3 Nanorods Decorated with Very Small Amount of Pt for Effective Hydrogen Evolution Reaction. Nanomaterials 2023, 13, 1071. https://doi.org/10.3390/nano13061071
Mineo G, Bruno L, Bruno E, Mirabella S. WO3 Nanorods Decorated with Very Small Amount of Pt for Effective Hydrogen Evolution Reaction. Nanomaterials. 2023; 13(6):1071. https://doi.org/10.3390/nano13061071
Chicago/Turabian StyleMineo, Giacometta, Luca Bruno, Elena Bruno, and Salvo Mirabella. 2023. "WO3 Nanorods Decorated with Very Small Amount of Pt for Effective Hydrogen Evolution Reaction" Nanomaterials 13, no. 6: 1071. https://doi.org/10.3390/nano13061071