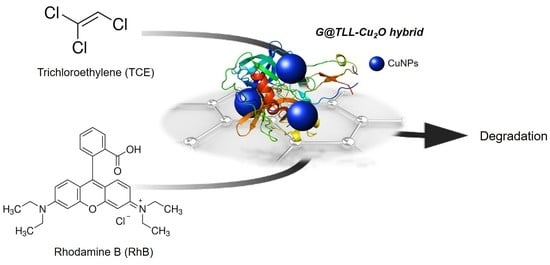
Graphene-TLL-Cu2ONPs Hybrid as Highly Efficient Catalyst for Degradation of Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Structural Characterization
2.3. Synthesis of G@TLL-Cu2O Hybrid
2.4. Experiments of G@TLL-Cu2O Hybrid Enzyme Desorption on Graphene Support
2.5. G@TLL-Cu2O Hybrid Catalysing the Degradation of Trichloroethylene (TCE)
2.6. G@TLL-Cu2O Hybrid Catalysing the Degradation of Rhodamine B (RhB)
3. Results and Discussion
3.1. Preparation and Characterization of G@TLL-Cu2O Hybrid
3.2. Trichloroethylene (TCE) Degradation Catalysed by G@TLL-Cu2O Hybrid
3.3. Rhodamine B (RhB) Degradation Catalysed by G@TLL-Cu2O Hybrid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagheri, S.; Termehyousefi, A.; Do, T.O. Photocatalytic pathway toward degradation of environmental pharmaceutical pollutants: Structure, kinetics and mechanism approach. Catal. Sci. Technol. 2017, 7, 4548–4569. [Google Scholar] [CrossRef]
- Iqbal, M.; Syed, J.H.; Breivik, K.; Chaudhry, M.J.I.; Li, J.; Zhang, G.; Malik, R.N. E-waste driven pollution in Pakistan: The first evidence of environmental and human exposure to flame retardants (FRs) in Karachi City. Environ. Sci. Technol. 2017, 5, 113895–113905. [Google Scholar] [CrossRef] [PubMed]
- Grizzetti, B.; Pistocchi, A.; Liquete, C. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhou, L.; Zhou, W. Decoupling environmental pressure from economic growth on city level: The case study of Chongqing in China. Ecol. Indicat. 2017, 75, 27–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, T.X.; Hua, Y.B. Delta manganese dioxide nanosheets decorated magnesium wire for the degradation of methyl orange. J. Colloid Interface Sci. 2017, 490, 226–232. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Ambigadevi, J.; Kumar, P.S.; Vo, D.V.N.; Haran, S.H.; Raghavan, T.S. Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A review. J. Environ. Chem. Eng. 2021, 9, 104881. [Google Scholar] [CrossRef]
- Lin, Y.T.; Liang, C.J.; Yu, C.W. Trichloroethylene degradation by various forms of iron activated persulfate oxidation with or without the assistance of ascorbic acid. Ind. Eng. Chem. Res. 2016, 55, 2302–2308. [Google Scholar] [CrossRef]
- Wu, X.L.; Gu, X.G.; Lu, S.G.; Qiu, Z.F.; Sui, Q.; Zang, X.K.; Miao, Z.W.; Xu, M.H.; Danish, M. Accelerated degradation of tetrachloroethylene by Fe(II) activated persulfate process with hydroxylamine for enhancing Fe(II) regeneration. J. Chem. Technol. Biot. 2016, 91, 1280–1289. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- US Environmental Protection Agency. Edition of the Drinking Water Standards and Health Advisories; EPA 822-R-18-001; EPA Office of Water: Washington, DC, USA, 2018. [Google Scholar]
- Wang, Z. State-of-the-art on the development of ultrasonic equipment and key problems of ultrasonic oil production technique for EOR in China. Renew. Sustain. Energy Rev. 2018, 82, 2401–2407. [Google Scholar] [CrossRef]
- Wang, Z. Research on removing reservoir core water sensitivity using the method of ultrasound-chemical agent for enhanced oil recovery. Ultrason. Sonochem. 2018, 42, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Birjandi, N.; Younesi, H.; Bahramifar, N.; Ghafari, S.; Zinatizadeh, A.A.; Sethupathi, S. Optimization of coagulation-flocculation treatment on paper-recycling wastewater: Application of response surface methodology. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2013, 48, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.S.; Widjojo, N.; Chung, T.-S.; Weber, M.; Maletzko, C. Positively charged nanofiltration (NF) membranes via UV grafting on sulfonated polyphenylenesulfone (sPPSU) for effective removal of textile dyes from wastewater. J. Membr. Sci. 2012, 417, 52–60. [Google Scholar] [CrossRef]
- Charumathi, D.; Das, N. Packed bed column studies for the removal of synthetic dyes from textile wastewater using immobilised dead C. tropicalis. Desalination 2012, 285, 22–30. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhang, J.; Li, W.; Zhou, J.; Shao, L.; Qian, G. Efficient removal of dyes by a novel magnetic Fe3O4/ZnCr-layered double hydroxide adsorbent from heavy metal wastewater. J. Hazard. Mater. 2012, 243, 152–160. [Google Scholar] [CrossRef]
- Kangralkar, M.V.; Kangralkar, V.A.; Manjanna, J. Adsorption of Cr (VI) and photodegradation of rhodamine b, rose bengal and methyl red on Cu2O nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100417. [Google Scholar] [CrossRef]
- Dinda, G.; Halder, D.; Vazquez-Vazquez, C.; Lopez-Quintela, M.A.; Mitra, A. Green synthesis of copper nanoparticles and their antibacterial property. J. Surf. Sci. Technol. 2015, 31, 117–122. [Google Scholar]
- Li, H.X.; Zhao, J.X.; Shi, R.N.; Hao, P.P.; Liu, S.S.; Li, Z.; Ren, J. Remarkable activity of nitrogen-doped hollow carbon spheres encapsulated Cu on synthesis of dimethyl carbonate: Role of effective nitrogen. Appl. Surf. Sci. 2018, 436, 803–813. [Google Scholar] [CrossRef]
- Shi, R.N.; Wang, J.; Zhao, J.X.; Liu, S.S.; Hao, P.P.; Li, Z.; Ren, J. Cu nanoparticles encapsulated with hollow carbon spheres for methanol oxidative carbonylation: Tuning of the catalytic properties by particle size control. Appl. Surf. Sci. 2018, 459, 707–715. [Google Scholar] [CrossRef]
- Liang, A.D.; Serrano-Plana, J.; Peterson, R.L.; Ward, T.R. Artificial Metalloenzymes Based on the Biotin-Streptavidin Technology: Enzymatic Cascades and Directed Evolution. Acc. Chem. Res. 2019, 52, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhai, S.; Wang, M.; Ji, H.; He, L.; Ye, C.; Chuanbin, W.; Shaoming, F.; Zhang, H. Photocatalytic degradation of rhodamine B by using a nanocomposite of cuprous oxide, three-dimensional reduced graphene oxide, and nanochitosan prepared via one-pot synthesis. J. Alloys Compd. 2016, 659, 101–111. [Google Scholar] [CrossRef]
- Shayegan Mehr, E.; Sorbiun, M.; Ramazani, A.; Taghavi Fardood, S. Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using ferulago angulata (schlecht) boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 1333–1340. [Google Scholar] [CrossRef]
- Garcia-Sanz, C.; Andreu, A.; de las Rivas, B.; Jiménez, A.I.; Pop, A.; Silvestru, C.; Urriolabeitia, E.P.; Palomo, J.M. Pd-oxazolone complexes conjugated to an engineered enzyme: Improving fluorescence and catalytic properties. Org. Biomol. Chem. 2021, 19, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fu, Z.; Chen, G.; Wang, Z.; Jian, Y.; Zhang, Y.; Jiang, G.; Lu, D.; Wu, J.; Liu, Z. Graphene oxide enabled long-term enzymatic transesterification in an anhydrous gas flux. Nat. Commun. 2019, 10, 2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomo, J.M. Nanobiohybrids: A new concept for metal nanoparticles synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [Green Version]
- Losada-Garcia, N.; Rodriguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme–copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Losada-Garcia, N.; Jimenez-Alesanco, A.; Velazquez-Campoy, A.; Abian, O.; Palomo, J.M. Enzyme/nanocopper hybrid nanozymes: Modulating enzyme-like activity by the protein structure for biosensing and tumor catalytic therapy. ACS Appl. Mat. Inter. 2021, 13, 5111–5124. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Berenguer-Murcia, A.; Cazorla-Amorós, D.; Palomo, J.M. Efficient production of multi-layer graphene from graphite flakes in water by lipase-graphene sheets conjugation. Nanomaterials 2019, 9, 1344. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Seelajaroen, H.; Bakandritsos, A.; Otyepka, M.; Zboril, R.; Sariciftci, N.S. Immobilized Enzymes on Graphene as Nanobiocatalyst. ACS Appl. Mater. Inter. 2020, 12, 250–259. [Google Scholar] [CrossRef]
- Wang, D.; Zou, J.; Cai, H.; Huang, Y.; Li, F.; Cheng, Q. Effective degradation of Orange G and Rhodamine B by alkali-activated hydrogen peroxide: Roles of HO2− and O2·−. Environ. Sci. Pollut. Res. 2019, 26, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Mei, L.; Wang, X. Enhanced photocatalytic degradation of rhodamine B by Cu2O coated silicon nanowire arrays in presence of H2O2. J. Mater. Sci. Technol. 2014, 30, 1124–1129. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Peng, F.; Wang, A.; Cai, X.; Fu, L. Growth of Cu2O nanoparticle on reduced graphene sheets with high photocatalytic activity for degradation of Rhodamine, B. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 149–153. [Google Scholar] [CrossRef]
- Tariq, M.; Muhammad, M.; Khan, J.; Raziq, A.; Uddin, M.K.; Niaz, A.; Ahmed, S.S.; Rahim, A. Removal of Rhodamine B dye from aqueous solutions using photo-Fenton processes and novel Ni-Cu@ MWCNTs photocatalyst. J. Mol. Liq. 2020, 312, 113399. [Google Scholar] [CrossRef]






| Catalyst | Cu (%w/w) | Method | [RhB] (mM) | [H2O2] (mM) | Catalyst (g/L) | Time (min) | RhB Removal (%) | Ref |
|---|---|---|---|---|---|---|---|---|
| G@TLL-Cu2O hybrid | 6.4 | Natural light | 0.1 | 250 | 2.5 | 50 | 100 | This work |
| Cu2O@3D-rGO@NCS nanocomposite | nd | AM 1.5G filter, 500 W Xe lamp | 0.01 | - | 0.2 | 150 | 90 | [23] |
| SiNWAs/Cu2O heterojunctions | 65.11 | Xe lamp irradiation with a cut-off filter (l > 420 nm) | 0.02 | 160 | nd | 60 | 100 | [34] |
| Cu2O/RGO-3 | nd | 500-W high-pressure Hg arc lamp | 0.01 | - | 0.4 | 120 | 90 | [35] |
| Cu2O NPs | nd | UV light (250 W) | 0.01 | - | 20 | 220 | 100 | [18] |
| CuONPs | nd | Fluorescent lamp | 0.01 | - | 1 | 150 | 83 | [24] |
| Ni-Cu@MWCNTs | nd | UV light | 0.04 | 0.12 + [Fe2+] | 0.2 | 50 | 98 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada-Garcia, N.; Carranza, J.; Palomo, J.M. Graphene-TLL-Cu2ONPs Hybrid as Highly Efficient Catalyst for Degradation of Organic Compounds. Nanomaterials 2023, 13, 449. https://doi.org/10.3390/nano13030449
Losada-Garcia N, Carranza J, Palomo JM. Graphene-TLL-Cu2ONPs Hybrid as Highly Efficient Catalyst for Degradation of Organic Compounds. Nanomaterials. 2023; 13(3):449. https://doi.org/10.3390/nano13030449
Chicago/Turabian StyleLosada-Garcia, Noelia, Jannier Carranza, and Jose M. Palomo. 2023. "Graphene-TLL-Cu2ONPs Hybrid as Highly Efficient Catalyst for Degradation of Organic Compounds" Nanomaterials 13, no. 3: 449. https://doi.org/10.3390/nano13030449