Foliar Fertilization by the Sol-Gel Particles Containing Cu and Zn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
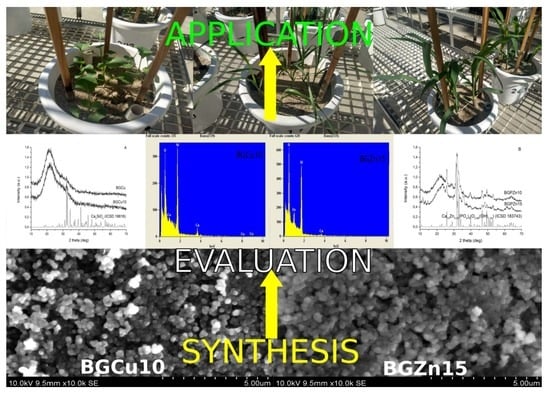
2.2. Particle Synthesis
2.3. Plant and Soil Materials
2.4. Foliar Application
2.5. Statistical Analysis
3. Results
3.1. Sol-Gel Particles Characterization
3.1.1. DLS Analysis
3.1.2. SEM and Energy-Dispersive X-ray (EDX) Analysis
3.1.3. X-ray Diffraction (XRD)
3.2. Foliar Fertilizer Application
3.2.1. Foliar Fertilizer Applied on Wheat (Triticum sativum)
3.2.2. Foliar Fertilizer Applied on Maize (Zea mays)
3.2.3. Foliar Fertilizer Applied on Rape Brassica napus L. var napus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Kumar, V.; Lee, S.S.; Raza, N.; Kim, K.H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Int. 2018, 119, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, R.M.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2020, 21, 104–118. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Shweta Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Ma, X.; Yan, J. Plant Uptake and Accumulation of Engineered Metallic Nanoparticles from Lab to Field Conditions. Curr. Opin. Environ. Sci. Health 2018, 6, 16–20. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes Through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 31, 289. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Sotiropoulos, T.; Brown, P. Foliar Fertilization: Scientific Principles and Field Practices, 1st ed.; IFA: Paris, France, 2013; Available online: https://www.fertilizer.org/images/Library_Downloads/2013_foliar_fertilization_HR.pdf (accessed on 4 November 2022).
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, T.; Sarkar, D.; Mashayekhi, H.; Xing, B.S. Growth and enzymatic activity of maize (Zea mays L.) plant: Solution culture test for copper dioxide nano particles. J. Plant Nutr. 2016, 39, 102–118. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets: Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Arshad, R.; Gulshad, L.; Haq, I.-U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef]
- Lukowiak, A.; Lao, J.; Lacroix, J.; Nedelec, J.-M. Bioactive glass nanoparticles obtained through sol–gel chemistry. Chem. Commun. 2013, 49, 6620–6622. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langeluddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Meier, U. (Ed.) BBCH Monograph, Growth Stages of Mono- and Dicotyle-Donous Plants; Julius Kühn-Institut (JKI): Quedlinburg, Germany, 2018. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hong, Z.; Luz, G.M.; Hampel, P.J.; Jin, M.; Liu, A.; Chen, X.; Mano, J.F. Mono-dispersed bioactive glass nanospheres: Preparation and effectson biomechanics of mammalian cells. J. Biomed. Mater. Res. Part A 2010, 95, 747–754. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Preparation and characterization of bioactive glass nanoparticles prepared by sol–gel for biomedical applications. Nanotechnology 2011, 22, 494014. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.F.; Bouchmella, K.; de Almeida Gonçalves, K.; Bettini, J.; Kobarg, J.; Cardoso, M.B. Functionalized Silica Nanoparticles as an Alternative Platform for Targeted Drug-Delivery of Water Insoluble Drugs. Langmuir 2016, 32, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2017, 56, 678–686. [Google Scholar] [CrossRef]
- Ma, X.; Geisler-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Q.; Huang, Y.; Keller, A.A. Response at Genetic, Metabolic, and Physiological Levels of Maize (Zea mays) Exposed to a Cu(OH)2 Nanopesticide. ACS Sustain. Chem. Eng. 2017, 5, 8294–8301. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yassen, A.; Abdallah, E.; Gaballah, M.; Zaghloul, S. Role of Silicon Dioxide Nano Fertilizer in Mitigating Salt Stress on Growth, Yield and Chemical Composition of Cucumber (Cucumis sativus L.). Int. J. Agric. Res. 2017, 12, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomášková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. Pap. 2015, 76, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Behboudi, F.; Sarvestani, Z.T.; Kassaee, M.Z.; Sanavi, S.A.M.M.; Sorooshzadeh, A. Improving Growth and Yield of Wheat under Drought Stress via Application of SiO2 Nanoparticles. J. Agric. Sci. Technol. 2018, 20, 1479–1492. [Google Scholar]
- Kang, H.; Elmer, W.; Shen, Y.; Zuverza-Mena, N.; Ma, C.; Botella, P.; White, J.C.; Haynes, C.L. Silica Nanoparticle Dissolution Rate Controls the Suppression of Fusarium wilt of Watermelon (Citrullus lanatus). Environ. Sci. Technol. 2021, 55, 13513–13522. [Google Scholar] [CrossRef]
- Zarafshar, M.; Akbarinia, M.; Askari, H.; Hosseini, S.M.; Rahaie, M.; Struve, D. Toxicity assessment of SiO2 nanoparticles to pear seedlings. Int. J. Nanosci. Nanotechnol. 2015, 11, 13–22. [Google Scholar]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N.S.; Prabu, P. Influence of Nanosilica Powder on the Growth of Maize Crop (Zea mays L.). Int. J. Green Nanotechnol. 2011, 3, 180–190. [Google Scholar] [CrossRef]
- Neethirajan, S.; Gordon, R.; Wang, L. Potential of silica bodies (phytoliths) for nanotechnology. Trends Biotechnol. 2009, 27, 461–467. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica Nanoparticle Phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]












| Sample | Ca g/kg | Cu g/kg | Zn g/kg |
|---|---|---|---|
| BGCu | 55.40 | 41.62 | - |
| BGCu10 | 131.21 | 103.26 | - |
| BGPCu15 | 97.86 | 153.05 | - |
| SiO2/Cu2+ | - | 150.64 | - |
| BGZn10 | 131.29 | - | 105.81 |
| BGZn15 | 99.76 | - | 155.06 |
| BGPZn10 | 129.01 | - | 107.68 |
| BGPZn15 | 97.64 | - | 158.99 |
| Particles with Cu | Size (nm) | PdI | Particles with Zn | Size (nm) | PdI |
|---|---|---|---|---|---|
| BGCu | 203 | 0.137 | BGZn10 | 180 | 0.109 |
| BGCu10 | 178 | 0.081 | BGZn15 | 153 | 0.095 |
| BGPCu15 | 179 | 0.112 | BGPZn15 | 175 | 0.116 |
| SiO2/Cu2+ | 202 | 0.065 | BGPZn10 | 149 | 0.112 |
| Atom % | |||||
|---|---|---|---|---|---|
| Sample | O | Si | Ca | P | Cu |
| BGCu | 74.40 | 20.54 | 2.14 | - | 2.92 |
| BGCu10 | 73.72 | 22.40 | 0.93 | - | 2.95 |
| BGPCu15 | 74.62 | 16.09 | 3.60 | 2.44 | 3.25 |
| SiO2/Cu2+ | 75.59 | 19.92 | - | - | 4.49 |
| Atom % | |||||
|---|---|---|---|---|---|
| Sample | O | Si | Ca | P | Cu |
| BGZn10 | 72.32 | 22.60 | 2.87 | - | 2.21 |
| BGZn15 | 72.71 | 22.77 | 1.52 | - | 3.00 |
| BGPZn15 | 64.02 | 23.22 | 5.18 | 2.79 | 4.79 |
| BGPZn10 | 67.86 | 15.99 | 8.83 | 4.16 | 3.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borak, B.; Gediga, K.; Piszcz, U.; Sacała, E. Foliar Fertilization by the Sol-Gel Particles Containing Cu and Zn. Nanomaterials 2023, 13, 165. https://doi.org/10.3390/nano13010165
Borak B, Gediga K, Piszcz U, Sacała E. Foliar Fertilization by the Sol-Gel Particles Containing Cu and Zn. Nanomaterials. 2023; 13(1):165. https://doi.org/10.3390/nano13010165
Chicago/Turabian StyleBorak, Beata, Krzysztof Gediga, Urszula Piszcz, and Elżbieta Sacała. 2023. "Foliar Fertilization by the Sol-Gel Particles Containing Cu and Zn" Nanomaterials 13, no. 1: 165. https://doi.org/10.3390/nano13010165