Structural, Spectroscopic, and Biological Characterization of Novel Rubidium(I) and Europium(III) Co-Doped Nano-Hydroxyapatite Materials and Their Potential Use in Regenerative Medicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Method
2.2. Structural Characterization
2.3. Luminescence Properties
2.4. Biological Properties
2.4.1. Preparation of Sample Suspensions
2.4.2. Cell Culture and Viability Assay
2.4.3. Evaluation of Mouse Osteoblast Morphology
2.4.4. Hemolysis Assay
3. Results and Discussion
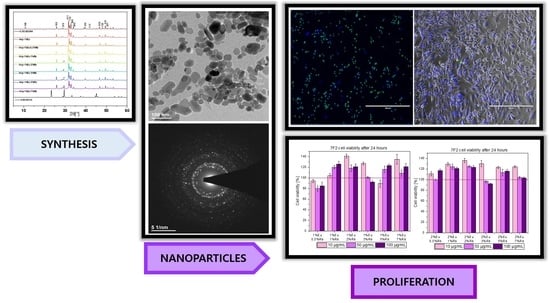
3.1. Characterization of Structure and Morphology
3.2. Investigation of Luminescence Properties
3.3. Evaluation of Biological Properties
3.3.1. Biocompatibility of Obtained Compounds
3.3.2. Evaluation of Hemocompatibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A Review on Hydroxyapatite Coatings for the Biomedical Applications: Experimental and Theoretical Perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Majhooll, A.A.; Zainol, I.; Jaafar, C.N.A.; Alsailawi, H.A.; Hassan, M.Z.; Mudhafar, M.; Majhool, A.A.; Asaad, A. A Brief Review on Biomedical Applications of Hydroxyapatite Use as Fillers in Polymer. J. Chem. Chem. Eng. 2019, 13, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of Hydroxyapatite for Biomedical Applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Motamedian, S.R.; Rad, M.R.; Shahriari, M.H.; Nadjmi, N. Polymeric vs Hydroxyapatite-Based Scaffolds on Dental Pulp Stem Cell Proliferation and Differentiation. World World J. Stem Cells 2015, 7, 1215–1221. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Ogden, M.I.; Richmond, W.R.; White, A.H. Lanthanide Ions as Calcium Substitutes: A Structural Comparison of Europium and Calcium Complexes of a Ditopic Calixarene. J. Chem. Soc. Dalton Trans. 1991, 8, 2153–2160. [Google Scholar] [CrossRef]
- Victor, S.P.; Gayathri Devi, M.G.; Paul, W.; Vijayan, V.M.; Muthu, J.; Sharma, C.P. Europium Doped Calcium Deficient Hydroxyapatite as Theranostic Nanoplatforms: Effect of Structure and Aspect Ratio. ACS Biomater. Sci. Eng. 2017, 3, 3588–3595. [Google Scholar] [CrossRef]
- Kandarakis, I.; Cavouras, D.; Panayiotakis, G.S.; Triantis, D.; Nomicos, C.D. Europium-Activated Phosphors for Use in X-Ray Detectors of Medical Imaging Systems. Eur. Radiol. 1998, 8, 313–318. [Google Scholar] [CrossRef]
- Yang, P.; Quan, Z.; Li, C.; Kang, X.; Lian, H.; Lin, J. Bioactive, Luminescent and Mesoporous Europium-Doped Hydroxyapatite as a Drug Carrier. Biomaterials 2008, 29, 4341–4347. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Li, S. Biocompatible Europium Doped Hydroxyapatite Nanoparticles as a Biological Fluorescent Probe. Curr. Nanosci. 2010, 6, 178–183. [Google Scholar] [CrossRef]
- Escudero, A.; Calvo, M.E.; Rivera-ferna, S. Microwave-Assisted Synthesis of Biocompatible Europium-Doped Calcium Hydroxyapatite and Fluoroapatite Luminescent Nanospindles Functionalized with Poly(acrylic acid). Langmuir 2013, 29, 1985–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, N.; Hassanipour, M.; Yarmohammadi, F.; Faghir-Ghanesefat, H.; Pourshadi, N.; Bahramnejad, E.; Dehpour, A.R. Nitric Oxide and Glutamate Are Contributors of Anti-Seizure Activity of Rubidium Chloride: A Comparison with Lithium. Neurosci. Lett. 2019, 708, 134349. [Google Scholar] [CrossRef] [PubMed]
- Kordjazy, N.; Haj-Mirzaian, A.; Amiri, S.; Ostadhadi, S.; Kordjazy, M.; Sharifzadeh, M.; Dehpour, A.R. Elevated Level of Nitric Oxide Mediates the Anti-Depressant Effect of Rubidium Chloride in Mice. Eur. J. Pharmacol. 2015, 762, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Huang, Q.; Liu, B.; Wu, H.; Liu, T.; Liu, Y. Rubidium Chloride Targets JNK/P38-Mediated NF-ΚB Activation to Attenuate Osteoclastogenesis and Facilitate Osteoblastogenesis. Front. Pharmacol. 2019, 10, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I.; Bianco, A.; Pezzotti, G.; Gusmano, G. Synthesis, Thermal Behaviour and Luminescence Properties of Rare Earth-Doped Titania Nanofibers. Chem. Eng. J. 2011, 166, 751–764. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The Emission Spectrum and the Radiative Lifetime of Eu3+ in Luminescent Lanthanide Complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Gaydamaka, A.A.; Ponomareva, V.G.; Bagryantseva, I.N. Phase Composition, Thermal and Transport Properties of the System Based on the Mono- and Dihydrogen Phosphates of Rubidium. Solid State Ionics 2019, 329, 124–130. [Google Scholar] [CrossRef]
- Komornikov, V.A.; Grebenev, V.V.; Andreev, P.V.; Dmitricheva, E.V. Study of Phase Equilibria in the Rb3H(SO4)2-RbH2PO4-H2O System. Crystallogr. Rep. 2015, 60, 431–437. [Google Scholar] [CrossRef]
- Hoy, R.F.; Chambers, D.C. Silica-Related Diseases in the Modern World. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2805–2817. [Google Scholar] [CrossRef]
- Szyszka, K.; Targońska, S.; Lewińska, A.; Watras, A.; Wiglusz, R.J. Quenching of the Eu3+ Luminescence by Cu2+ Ions in the Nanosized Hydroxyapatite Designed for Future Bio-Detection. Nanomaterials 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic Substituted Hydroxyapatite for Bone Regeneration Applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Odusote, J.K.; Danyuo, Y.; Baruwa, A.D.; Azeez, A.A. Synthesis and Characterization of Hydroxyapatite from Bovine Bone for Production of Dental Implants. Appl. Biomater. Funct. Mater. 2019, 17. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Bioceramic Hydroxyapatite-Based Scaffold with a Porous Structure Using Honeycomb as a Natural Polymeric Porogen for Bone Tissue Engineering. Biomater. Res. 2021, 25, 2. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A Novel Hydroxyapatite-Hardystonite Nanocomposite Ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Hanifi, A.; Fathi, M.H. Bioresorbability Evaluation of Hydroxyapatite Nanopowders in a Stimulated Body Fluid Medium. Iran. J. Pharm. Sci. 2008, 4, 141–148. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Massuyeau, F.; Constantin, L.V.; Costescu, A.; Predoi, D. Synthesis, Structure, and Luminescent Properties of Europium-Doped Hydroxyapatite Nanocrystalline Powders. J. Nanomater. 2012, 2012, 942801. [Google Scholar] [CrossRef] [Green Version]
- Saville, A.I.; Creuziger, A.; Mitchell, E.B.; Vogel, S.C.; Benzing, J.T.; Klemm-Toole, J.; Clarke, K.D.; Clarke, A.J. MAUD Rietveld Refinement Software for Neutron Diffraction Texture Studies of Single- and Dual-Phase Materials. Integr. Mater. Manuf. Innov. 2021, 10, 461–487. [Google Scholar] [CrossRef]
- Longo, E.; de Almeida La Porta, F. Recent Advances in Complex Functional Materials: From Design to Application; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319538983. [Google Scholar]
- Jaggi, N. Handbook of Applied Solid State Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387375902. [Google Scholar]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Suvorova, E.I.; Buffat, P.A. Electron Diffraction from Micro- and Nanoparticles of Hydroxyapatite. J. Microsc. 1999, 196, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Miki, T.; Yumoto, M.; Konishi, T.; Aizawa, M. Ultrastructural Observation of Hydroxyapatite Ceramics with Preferred Orientation to A-Plane Using High-Resolution Transmission Electron Microscopy. Procedia Eng. 2012, 36, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Maciejewska, K.; Poźniak, B.; Tikhomirov, M.; Kobylińska, A.; Marciniak, L. Synthesis, Cytotoxicity Assessment and Optical Properties Characterization of Colloidal Gdpo4:Mn2+, Eu3+ for High Sensitivity Luminescent Nanothermometers Operating in the Physiological Temperature Range. Nanomaterials 2020, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyszka, K.; Targonska, S.; Gazinska, M.; Szustakiewicz, K.; Wiglusz, R.J. The Comprehensive Approach to Preparation and Investigation of the Eu3+ Doped Hydroxyapatite/Poly(L-Lactide) Nanocomposites: Promising Materials for Theranostics Application. Nanomaterials 2019, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- García Domínguez, G.; Garrido Hernández, A.; Cerón Montes, G.; Morales Ramírez, A.J.; de la Torre, S.D. Structural and Luminescent Properties of Europium-Doped and Undoped Hydroxyapatite Powders Sintered by Spark Plasma. Ceram. Silik. 2019, 63, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Liu, G.K. The Standard and Anomalous Crystal-Field Spectra of Eu3+. J. Solid State Chem. 2005, 178, 419–428. [Google Scholar] [CrossRef]
- Souza, A.S.; Oliveira, Y.A.R.; Couto Dos Santos, M.A. Enhanced Approach to the Eu3+ Ion 5D0 → 7F0 Transition Intensity. Opt. Mater. 2013, 35, 1633–1635. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Hua, X.; Dong, Y.; Li, H.; Feng, C.; Wang, Y.; Xia, M. Engineering Mixed Polyanion Red-Emitting Rb2Bi(PO4)(WO4):Eu3+ Phosphors with Negligible Thermal Quenching and High Quantum Yield. J. Alloy. Compd. 2020, 844, 155875. [Google Scholar] [CrossRef]
- Matsunaga, K.; Murata, H.; Mizoguchi, T. Atsushi Nakahira Mechanism of Incorporation of Zinc into Hydroxyapatite. Acta Biomater. 2010, 6, 2289–2293. [Google Scholar] [CrossRef] [Green Version]
- Bunzli, J.C.G.; Plancherel, D.; Pradervand, G.O. Eu(III) Ion as Luminescent Probe: Structural Investigation of Complexes between Europium Nitrate and Two Polyethers. J. Phys. Chem. 1989, 93, 980–984. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Ezerskyte, E.; Minderyte, A.; Stanionyte, S.; Juskenas, R.; Sakirzanovas, S.; Katelnikovas, A. Optical Properties of Red-Emitting Rb2Bi(PO4)(MoO4)Eu3+ Powders and Ceramics with High Quantum Efficiency for White LEDs. Materials 2019, 12, 3275. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhu, R.; Huang, Y.; Taob, Y.; Seo, H.J. Luminescence and Microstructures of Eu3+-Doped Ca9LiGd2/3(PO4)7. Dalton Trans. 2011, 40, 11433–11440. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhao, W.; Zhou, G.; Dou, X.; Yi, C.; Zhou, M. Luminescence Properties of Red Phosphors Ca10Li(PO4)7:Eu3+. J. Rare Earths 2011, 29, 440–443. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S.Y. Structural and Spectroscopic Properties of New Noncentrosymmetric Self-Activated Borate Rb3EuB6O12 with B5O10 Units. Mater. Des. 2018, 140, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tan, Y.; Wu, J. Rubidium Doped Nano-Hydroxyapatite with Cytocompatibility and Antibacterial. J. Asian Ceram. Soc. 2021, 9, 300–310. [Google Scholar] [CrossRef]
- Tan, Y.N.; Chen, W.J.; Wei, W.; Huang, Q.L.; He, X. Rubidium-Modified Bioactive Glass-Ceramics with Hydroxyapatite Crystals for Bone Regeneration. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2021, 31, 521–532. [Google Scholar] [CrossRef]
- Guan, R.G.; Johnson, I.; Cui, T.; Zhao, T.; Zhao, Z.Y.; Li, X.; Liu, H. Electrodeposition of Hydroxyapatite Coating on Mg-4.0Zn-1.0Ca-0.6Zr Alloy and in Vitro Evaluation of Degradation, Hemolysis, and Cytotoxicity. J. Biomed. Mater. Res. A 2012, 100A, 999–1015. [Google Scholar] [CrossRef]
- Slota, D.; Gląb, M.; Tyliszczak, B.; Dogulas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-upiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef]
- Reda, H. Sedimentation Rate in Sheep. Br. Vet. J. 1950, 106, 235–239. [Google Scholar] [CrossRef]












| Sample | ICP OES Technique Results | |||
|---|---|---|---|---|
| n Ca [mol] | n Eu [mol] | n Rb [mol] | n P [mol] | |
| Ca9.7Eu0.2Rb0.1(PO4)6(OH)2 | 9.66 | 0.20 | 0.14 | 7.01 |
| Ca9.5Eu0.2Rb0.3(PO4)6(OH)2 | 9.43 | 0.20 | 0.37 | 7.17 |
| Ca9.3Eu0.2Rb0.5(PO4)6(OH)2 | 9.20 | 0.20 | 0.60 | 7.27 |
| Ca9.1Eu0.2Rb0.7(PO4)6(OH)2 | 9.00 | 0.20 | 0.81 | 7.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, N.; Czekanowska, D.; Reeks, J.M.; Wiglusz, R.J. Structural, Spectroscopic, and Biological Characterization of Novel Rubidium(I) and Europium(III) Co-Doped Nano-Hydroxyapatite Materials and Their Potential Use in Regenerative Medicine. Nanomaterials 2022, 12, 4475. https://doi.org/10.3390/nano12244475
Nowak N, Czekanowska D, Reeks JM, Wiglusz RJ. Structural, Spectroscopic, and Biological Characterization of Novel Rubidium(I) and Europium(III) Co-Doped Nano-Hydroxyapatite Materials and Their Potential Use in Regenerative Medicine. Nanomaterials. 2022; 12(24):4475. https://doi.org/10.3390/nano12244475
Chicago/Turabian StyleNowak, Nicole, Dominika Czekanowska, John M. Reeks, and Rafal J. Wiglusz. 2022. "Structural, Spectroscopic, and Biological Characterization of Novel Rubidium(I) and Europium(III) Co-Doped Nano-Hydroxyapatite Materials and Their Potential Use in Regenerative Medicine" Nanomaterials 12, no. 24: 4475. https://doi.org/10.3390/nano12244475