A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing
Abstract
:1. Introduction
2. Structure and Properties of g-C3N4
3. g-C3N4 Characterizations
4. g-C3N4 Preparation
5. g-C3N4 Modifications
5.1. Doping
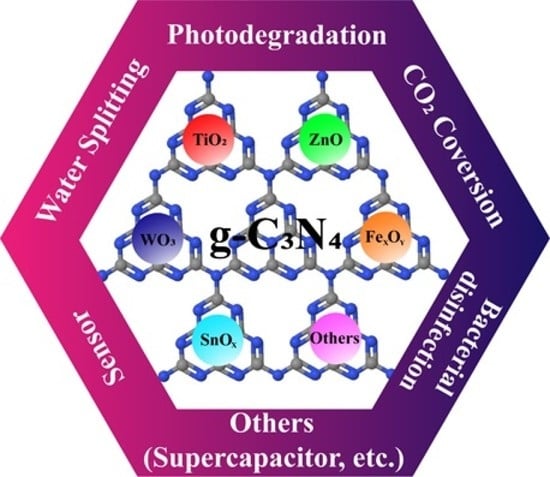
5.2. Metal Oxide-Based g-C3N4 Nanocomposite
- (1)
- Type I heterojunction,
- (2)
- Type II heterojunction,
- (3)
- Z-scheme heterojunction
- (4)
- p-n heterojunction,
- (5)
- Schottky junction.
5.2.1. TiO2-g-C3N4
5.2.2. ZnO-g-C3N4
5.2.3. Iron Oxide-g-C3N4
5.2.4. WO3-g-C3N4
5.2.5. Tin Oxide-g-C3N4
5.2.6. Other Metal Oxides
6. Application of Metal Oxide-Based g-C3N4 Nanocomposites
6.1. Photocatalysts
6.1.1. H2 Generation via Water Splitting
6.1.2. CO2 Reduction
6.1.3. Photodegradation of Organic Pollutants
6.2. Sensors
6.3. Bacterial Disinfection
6.4. Other Applications
7. Conclusions, Limitations and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Metal-Free Catalysis of Sustainable Friedel–Crafts Reactions: Direct Activation of Benzene by Carbon Nitrides to Avoid the Use of Metal Chlorides and Halogenated Compounds. Chem. Commun. 2006, 43, 4530–4532. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent Developments of Doped g-C3N4 Photocatalysts for the Degradation of Organic Pollutants. Crit. Rev. Environ. Sci. Technol. 2020, 51, 751–790. [Google Scholar] [CrossRef]
- Taha, M.M.; Ghanem, L.G.; Hamza, M.A.; Allam, N.K. Highly Stable Supercapacitor Devices Based on Three-Dimensional Bioderived Carbon Encapsulated g-C3N4 Nanosheets. ACS Appl. Energy Mater. 2021, 4, 10344–10355. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Wang, D.; Hong, X.; Liang, B. Recent Advances in Heteroatom Doped Graphitic Carbon Nitride (g-C3N4) and g-C3N4/Metal Oxide Composite Photocatalysts. Curr. Org. Chem. 2020, 24, 673–693. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Hu, X.; Xue, H.; Pang, H. Metal/Graphitic Carbon Nitride Composites: Synthesis, Structures, and Applications. Chem. Asian J. 2016, 11, 3305–3328. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zeng, D.; Ong, W.-J. Interfacial Engineering of Graphitic Carbon Nitride (g-C3N4)-Based Metal Sulfide Heterojunction Photocatalysts for Energy Conversion: A Review. Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Ismael, M. A Review on Graphitic Carbon Nitride (g-C3N4) Based Nanocomposites: Synthesis, Categories, and Their Application in Photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Lin, T.-J.; Lin, Y.-R.; Chiu, W.-L.; Nguyen, B.-S.; Hu, C. Influence of P, S, O-Doping on g-C3N4 for Hydrogel Formation and Photocatalysis: An Experimental and Theoretical Study. Carbon 2020, 169, 338–348. [Google Scholar] [CrossRef]
- Wei, T.; Xu, J.; Kan, C.; Zhang, L.; Zhu, X. Au Tailored on g-C3N4/TiO2 Heterostructure for Enhanced Photocatalytic Performance. J. Alloys Compd. 2022, 894, 162338. [Google Scholar] [CrossRef]
- Gotipamul, P.P.; Vattikondala, G.; Rajan, K.D.; Khanna, S.; Rathinam, M.; Chidambaram, S. Impact of Piezoelectric Effect on the Heterogeneous Visible Photocatalysis of g-C3N4/Ag/ZnO Tricomponent. Chemosphere 2022, 287, 132298. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Zou, W.; Deng, B.; Hu, X.; Zhou, Y.; Pu, Y.; Yu, S.; Ma, K.; Sun, J.; Wan, H.; Dong, L. Crystal-Plane-Dependent Metal Oxide-Support Interaction in CeO2/g-C3N4 for Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2018, 238, 111–118. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Zhu, Z. Improved Photoelectrochemical Performance of Z-Scheme g-C3N4/Bi2O3/BiPO4 Heterostructure and Degradation Property. Appl. Surf. Sci. 2016, 385, 34–41. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Zhang, J.; Liang, Q.; Mitsuzaki, N.; Chen, Z. Construction of CdS Quantum Dots Modified g-C3N4/ZnO Heterostructured Photoanode for Efficient Photoelectrochemical Water Splitting. J. Photochem. Photobiol. A Chem. 2019, 371, 109–117. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of Contact Interface between TiO2 and g-C3N4 on the Photoreactivity of g-C3N4/TiO2 Photocatalyst:(0 0 1) vs (1 0 1) Facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar]
- Liang, B.; Han, D.; Sun, C.; Zhang, W.; Qin, Q. Synthesis of SnO/g-C3N4 Visible Light Driven Photocatalysts via Grinding Assisted Ultrasonic Route. Ceram. Int. 2018, 44, 7315–7318. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, J.; Wei, Z.; Zhang, P.; Li, H.; Wang, Y. Combination of Carbon Nitride and Carbon Nanotubes: Synergistic Catalysts for Energy Conversion. ChemSusChem 2014, 7, 2303–2309. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, J.; Yang, Y.; Wang, D.; Bie, L.; Fahlman, B.D. Novel g-C3N4/CoO Nanocomposites with Significantly Enhanced Visible-Light Photocatalytic Activity for H2 Evolution. ACS Appl. Mater. Interfaces 2017, 9, 12427–12435. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.-W.; Ma, D.; Fan, Z.; Lu, L.; Niu, C. In Situ Synthesis of C-Doped TiO2@ g-C3N4 Core-Shell Hollow Nanospheres with Enhanced Visible-Light Photocatalytic Activity for H2 Evolution. Chem. Eng. J. 2017, 322, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Hasanvandian, F.; Moradi, M.; Samani, S.A.; Kakavandi, B.; Setayesh, S.R.; Noorisepehr, M. Effective Promotion of g-C3N4 Photocatalytic Performance via Surface Oxygen Vacancy and Coupling with Bismuth-Based Semiconductors towards Antibiotics Degradation. Chemosphere 2022, 287, 132273. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual Optimization Approach to Mo Single Atom Dispersed g-C3N4 Photocatalyst: Morphology and Defect Evolution. Appl. Catal. B Environ. 2022, 303, 120904. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent Developments in Fabrication and Structure Regulation of Visible-Light-Driven g-C3N4-Based Photocatalysts towards Water Purification: A Critical Review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-Free Catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of Nonmetals (B, O, P, and S) Doped with Porous g-C3N4 for Improved Electron Transfer towards Photocatalytic CO2 Reduction with Water into CH4. Chemosphere 2022, 286, 131765. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, Q.; Han, W.; Shen, Y.; Ni, Z.; Zhang, S.; Chen, L.; Zhang, L.; Cao, J.; Zheng, H. A Simple and High Efficient Composite Based on g-C3N4 for Super Rapid Removal of Multiple Organic Dyes in Water under Sunlight. Catal. Sci. Technol. 2022, in press.
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Water Disinfection and Microbial Control: A Review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic Carbon Nitride: Synthesis, Characterization and Photocatalytic Decomposition of Nitrous Oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the Energy Band Gap and Edges’ Potentials of g-C3N4/TiO2 Composite Photocatalysts for NOx Removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at Different Temperatures for Superior Visible/UV Photocatalytic Performance and Photoelectrochemical Sensing of MB Solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of Calcination Temperature, PH and Catalyst Loading on Photodegradation Efficiency of Urea Derived Graphitic Carbon Nitride towards Methylene Blue Dye Solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Xing, Z.; Shu, Z.; Zhang, L.; Wang, L.; Shi, J. Improved Photocatalytic Activity of g-C3N4 Derived from Cyanamide–Urea Solution. RSC Adv. 2015, 5, 8323–8328. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yu, D.; Sun, T.; Du, H.; Jiang, W.; Muhammad, Y.; Huang, L. Fabrication of Surface Alkalinized g-C3N4 and TiO2 Composite for the Synergistic Adsorption-Photocatalytic Degradation of Methylene Blue. Appl. Surf. Sci. 2019, 473, 855–863. [Google Scholar] [CrossRef]
- Li, F.; Zhao, R.; Yang, B.; Wang, W.; Liu, Y.; Gao, J.; Gong, Y. Facial Synthesis of Dandelion-like g-C3N4/Ag with High Performance of Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 30185–30195. [Google Scholar] [CrossRef]
- Hu, C.; Hung, W.-Z.; Wang, M.-S.; Lu, P.-J. Phosphorus and Sulfur Codoped g-C3N4 as an Efficient Metal-Free Photocatalyst. Carbon 2018, 127, 374–383. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Lin, S.; Zhang, Y.; Wang, X. Activation of N→ Π* Transitions in Two-Dimensional Conjugated Polymers for Visible Light Photocatalysis. J. Phys. Chem. C 2014, 118, 29981–29989. [Google Scholar] [CrossRef]
- Dias, E.M.; Christoforidis, K.C.; Francas, L.; Petit, C. Tuning Thermally Treated Graphitic Carbon Nitride for H2 Evolution and CO2 Photoreduction: The Effects of Material Properties and Mid-Gap States. ACS Appl. Energy Mater. 2018, 1, 6524–6534. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, X.; Antonietti, M.; Wang, X. Synthesis of Transition Metal-modified Carbon Nitride Polymers for Selective Hydrocarbon Oxidation. ChemSusChem 2011, 4, 274–281. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A. High-Yield Synthesis and Optical Properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Maeda, K.; Chen, X.; Takanabe, K.; Domen, K.; Hou, Y.; Fu, X.; Antonietti, M. Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.R.; Ho, P. Introduction to Chemical Vapor Deposition (CVD). Chem. Vap. Depos. 2001, 2, 1–22. [Google Scholar]
- Xu, Z.; Guan, L.; Li, H.; Sun, J.; Ying, Z.; Wu, J.; Xu, N. Structure Transition Mechanism of Single-Crystalline Silicon, g-C3N4, and Diamond Nanocone Arrays Synthesized by Plasma Sputtering Reaction Deposition. J. Phys. Chem. C 2015, 119, 29062–29070. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J. Efficient Visible-Light Photocatalytic Hydrogen Evolution and Enhanced Photostability of Core/Shell CdS/g-C3N4 Nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of Graphitic Carbon Nitride (g-C3N4) by Alkaline Hydrothermal Treatment for Photocatalytic NO Oxidation in Gas Phase. J. Mater. Chem. A 2013, 1, 6489–6496. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Fu, X.; Wang, X. Construction of Conjugated Carbon Nitride Nanoarchitectures in Solution at Low Temperatures for Photoredox Catalysis. Angew. Chemie 2012, 124, 11984–11988. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Iqbal, A.; Rahman, N.R.A. Cu2+ Coordinated Graphitic Carbon Nitride (Cu-g-C3N4) Nanosheets from Melamine for the Liquid Phase Hydroxylation of Benzene and VOCs. Appl. Surf. Sci. 2017, 398, 43–55. [Google Scholar] [CrossRef]
- Hu, C.; Chu, Y.-C.; Lin, Y.-R.; Yang, H.-C.; Wang, K.-H. Photocatalytic Dye and Cr (VI) Degradation Using a Metal-Free Polymeric g-C3N4 Synthesized from Solvent-Treated Urea. Polymers. 2019, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Cong, Y.; Wang, F.; Yao, L.; Shi, L. Hydrothermal Pre-Treatment Induced Cyanamide to Prepare Porous g-C3N4 with Boosted Photocatalytic Performance. Diam. Relat. Mater. 2019, 98, 107499. [Google Scholar] [CrossRef]
- Cao, J.; Fan, H.; Wang, C.; Ma, J.; Dong, G.; Zhang, M. Facile Synthesis of Carbon Self-Doped g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. Ceram. Int. 2020, 46, 7888–7895. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A Direct One-Step Synthesis of Ultrathin g-C3N4 Nanosheets from Thiourea for Boosting Solar Photocatalytic H2 Evolution. Int. J. Hydrogen Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Wehrstedt, K.-D.; Wildner, W.; Güthner, T.; Holzrichter, K.; Mertschenk, B.; Ulrich, A. Safe Transport of Cyanamide. J. Hazard. Mater. 2009, 170, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-T.; Shin, E.W. Influence of g-C3N4 Precursors in g-C3N4/NiTiO3 Composites on Photocatalytic Behavior and the Interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Chen, X.; Webster, R.D.; Lim, T.-T. Facile Synthesis of Pure g-C3N4 Materials for Peroxymonosulfate Activation to Degrade Bisphenol A: Effects of Precursors and Annealing Ambience on Catalytic Oxidation. Chem. Eng. J. 2020, 387, 123726. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.-T.; Shin, E.W. Effect of g-C3N4 Precursors on the Morphological Structures of g-C3N4/ZnO Composite Photocatalysts. J. Alloys Compd. 2019, 788, 1084–1092. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of Thiourea into Carbon Nitride Semiconductors as Visible Light Photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Wang, Q. Au-Loaded Porous Graphitic C3N4/Graphene Layered Composite as a Ternary Plasmonic Photocatalyst and Its Visible-Light Photocatalytic Performance. Rsc Adv. 2015, 5, 88249–88257. [Google Scholar] [CrossRef]
- Edgar, I.C.; Johnstone, S.M.; Milton, M. Urea to Melamine Process. U.S. Patent 3,095,416, 25 June 1963. [Google Scholar]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on g-C3N4 -Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic Carbon Nitride (g-C3N4)—Based Metal-Free Photocatalysts for Water Splitting: A Review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Teixeira, I.F.; Barbosa, E.C.M.; Tsang, S.C.E.; Camargo, P.H.C. Carbon Nitrides and Metal Nanoparticles: From Controlled Synthesis to Design Principles for Improved Photocatalysis. Chem. Soc. Rev. 2018, 47, 7783–7817. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Ai, Z.; Shao, Y.; Chang, B.; Zhang, L.; Shen, J.; Wu, Y.; Huang, B.; Hao, X. Rational Modulation of Pn Homojunction in P-Doped g-C3N4 Decorated with Ti3C2 for Photocatalytic Overall Water Splitting. Appl. Catal. B Environ. 2019, 259, 118077. [Google Scholar] [CrossRef]
- Pan, Y.; Li, D.; Jiang, H. Sodium-Doped C3N4/MOF Heterojunction Composites with Tunable Band Structures for Photocatalysis: Interplay between Light Harvesting and Electron Transfer. Chem. Eur. J. 2018, 24, 18403–18407. [Google Scholar] [CrossRef] [PubMed]
- Putri, L.K.; Ng, B.-J.; Er, C.-C.; Ong, W.-J.; Chang, W.S.; Mohamed, A.R.; Chai, S.-P. Insights on the Impact of Doping Levels in Oxygen-Doped GC3N4 and Its Effects on Photocatalytic Activity. Appl. Surf. Sci. 2020, 504, 144427. [Google Scholar] [CrossRef]
- Sudrajat, H. A One-Pot, Solid-State Route for Realizing Highly Visible Light Active Na-Doped GC3N4 Photocatalysts. J. Solid State Chem. 2018, 257, 26–33. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Chen, Y.; Ai, S.; Ding, H. Cobalt-Doped g-C3N4 as a Heterogeneous Catalyst for Photo-Assisted Activation of Peroxymonosulfate for the Degradation of Organic Contaminants. Appl. Surf. Sci. 2019, 467, 954–962. [Google Scholar] [CrossRef]
- Li, G.; Wang, R.; Wang, B.; Zhang, J. Sm-Doped Mesoporous g-C3N4 as Efficient Catalyst for Degradation of Tylosin: Influencing Factors and Toxicity Assessment. Appl. Surf. Sci. 2020, 517, 146212. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Krivtsov, I.; García, J.R.; Palmisano, L. Selective Photocatalytic Oxidation of Aromatic Alcohols in Water by Using P-Doped g-C3N4. Appl. Catal. B Environ. 2018, 220, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhang, J.; He, B.; Wang, H.; Wang, R.; Gong, Y. In-Situ Construction of Coral-like Porous P-Doped g-C3N4 Tubes with Hybrid 1D/2D Architecture and High Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 241, 159–166. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Izadyar, M.; Nakhaeipour, A.; Habibi-Yangjeh, A. P-doped g-C3N4 as an Efficient Photocatalyst for CO2 Conversion into Value-added Materials: A Joint Experimental and Theoretical Study. Int. J. Quantum Chem. 2020, 120, e26388. [Google Scholar] [CrossRef]
- Chen, P.; Chen, L.; Ge, S.; Zhang, W.; Wu, M.; Xing, P.; Rotamond, T.B.; Lin, H.; Wu, Y.; He, Y. Microwave Heating Preparation of Phosphorus Doped g-C3N4 and Its Enhanced Performance for Photocatalytic H2 Evolution in the Help of Ag3PO4 Nanoparticles. Int. J. Hydrogen Energy 2020, 45, 14354–14367. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-Doped g-C3N4 Nanosheets with Carbon Vacancies: General Synthesis and Improved Activity for Simulated Solar-Light Photocatalytic Nitrogen Fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Jiang, D.; Men, K.; Li, Z.; Li, L.; Sun, S.; Li, J.; Huang, Z.-H.; Wang, L.-N. Facile Synthesis of Bimodal Macroporous g-C3N4/SnO2 Nanohybrids with Enhanced Photocatalytic Activity. Sci. Bull. 2019, 64, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Long, D.; Chen, Z.; Rao, X.; Zhang, Y. Sulfur-Doped g-C3N4 and BiPO4 Nanorod Hybrid Architectures for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. ACS Appl. Energy Mater. 2020, 3, 5024–5030. [Google Scholar]
- Oh, W.-D.; Lok, L.-W.; Veksha, A.; Giannis, A.; Lim, T.-T. Enhanced Photocatalytic Degradation of Bisphenol A with Ag-Decorated S-Doped g-C3N4 under Solar Irradiation: Performance and Mechanistic Studies. Chem. Eng. J. 2018, 333, 739–749. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Liu, H.; Zheng, X.; Zou, W.; Dong, L.; Qi, L. Enhanced Activity of Visible-Light Photocatalytic H2 Evolution of Sulfur-Doped g-C3N4 Photocatalyst via Nanoparticle Metal Ni as Cocatalyst. Appl. Catal. B Environ. 2018, 235, 66–74. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, Y.; Hu, S.; Pei, Y.; Ma, W.; Fan, Z. Hydrothermal Synthesis of Band Gap-Tunable Oxygen-Doped g-C3N4 with Outstanding “Two-Channel” Photocatalytic H2O2 Production Ability Assisted by Dissolution–Precipitation Process. Nano 2019, 14, 1950023. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable One-Step Production of Porous Oxygen-Doped g-C3N4 Nanorods with Effective Electron Separation for Excellent Visible-Light Photocatalytic Activity. Appl. Catal. B Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Li, J.; Lu, Z.; Wang, X. Self-Assembled Synthesis of Oxygen-Doped g-C3N4 Nanotubes in Enhancement of Visible-Light Photocatalytic Hydrogen. J. Energy Chem. 2021, 54, 36–44. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, Bifunctional Granules of Carbon-Doped g-C3N4 as Adsorptive Photocatalyst for the Efficient Removal of Tetracycline Antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Serendipitous Assembly of Mixed Phase BiVO4 on B-Doped g-C3N4: An Appropriate p–n Heterojunction for Photocatalytic O2 Evolution and Cr (VI) Reduction. Inorg. Chem. 2019, 58, 12480–12491. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen Self-Doped g-C3N4 Nanosheets with Tunable Band Structures for Enhanced Photocatalytic Tetracycline Degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, Q. Nitrogen Doped g-C3N4 with the Extremely Narrow Band Gap for Excellent Photocatalytic Activities under Visible Light. Appl. Catal. B Environ. 2021, 281, 119474. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, J.; Chen, K.; Ali, A.; Zeng, L.; Zhao, H.; Hu, W.; Zhu, L.; Xu, X. Potassium-Doped g-C3N4 Achieving Efficient Visible-Light-Driven CO2 Reduction. ACS Sustain. Chem. Eng. 2020, 8, 8214–8222. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, S.; Zhang, X.; Jiang, Z.; Zheng, J.; Guo, C. Combination of Experimental and Theoretical Investigation on Ti-Doped g-C3N4 with Improved Photo-Catalytic Activity. Appl. Surf. Sci. 2019, 489, 427–434. [Google Scholar] [CrossRef]
- Wang, J.-C.; Cui, C.-X.; Kong, Q.-Q.; Ren, C.-Y.; Li, Z.; Qu, L.; Zhang, Y.; Jiang, K. Mn-Doped g-C3N4 Nanoribbon for Efficient Visible-Light Photocatalytic Water Splitting Coupling with Methylene Blue Degradation. ACS Sustain. Chem. Eng. 2018, 6, 8754–8761. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Harraz, F.A.; Al-Sayari, S.A.; El-Toni, A.M.; Al-Assiri, M.S. Synthesis of Highly Dispersed Silver Doped g-C3N4 Nanocomposites with Enhanced Visible-Light Photocatalytic Activity. Mater. Des. 2016, 98, 223–230. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-Situ Fe-Doped g-C3N4 Heterogeneous Catalyst via Photocatalysis-Fenton Reaction with Enriched Photocatalytic Performance for Removal of Complex Wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Babu, B.; Shim, J.; Yoo, K. Efficient Solar-Light-Driven Photoelectrochemical Water Oxidation of One-Step in-Situ Synthesized Co-Doped g-C3N4 Nanolayers. Ceram. Int. 2020, 46, 16422–16430. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Li, R.; Zhang, Q.; Chen, T.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. An Efficient Metal-Free Phosphorus and Oxygen Co-Doped g-C3N4 Photocatalyst with Enhanced Visible Light Photocatalytic Activity for the Degradation of Fluoroquinolone Antibiotics. Chem. Eng. J. 2019, 374, 242–253. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, X.; Li, L.; Sun, T. P, S Co-Doped g-C3N4 Isotype Heterojunction Composites for High-Efficiency Photocatalytic H2 Evolution. J. Alloys Compd. 2020, 827, 154259. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, H.; Yang, C.; Li, M.; Zhao, Y.; Chen, F. Post-Activation of in Situ BF Codoped g-C3N4 for Enhanced Photocatalytic H2 Evolution. Appl. Surf. Sci. 2018, 441, 621–630. [Google Scholar] [CrossRef]
- Fang, W.; Liu, J.; Yu, L.; Jiang, Z.; Shangguan, W. Novel (Na, O) Co-Doped g-C3N4 with Simultaneously Enhanced Absorption and Narrowed Bandgap for Highly Efficient Hydrogen Evolution. Appl. Catal. B Environ. 2017, 209, 631–636. [Google Scholar] [CrossRef]
- Chen, C.; Xie, M.; Kong, L.; Lu, W.; Feng, Z.; Zhan, J. Mn3O4 Nanodots Loaded g-C3N4 Nanosheets for Catalytic Membrane Degradation of Organic Contaminants. J. Hazard. Mater. 2020, 390, 122146. [Google Scholar] [CrossRef] [PubMed]
- Surikanti, G.R.; Bajaj, P.; Sunkara, M.V. g-C3N4-Mediated Synthesis of Cu2O To Obtain Porous Composites with Improved Visible Light Photocatalytic Degradation of Organic Dyes. ACS Omega 2019, 4, 17301–17316. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Wu, X.; Pei, J.; Wu, C.; Wang, X.; Zhao, H. Construction of g-C3N4/TiO2/Ag Composites with Enhanced Visible-Light Photocatalytic Activity and Antibacterial Properties. Ceram. Ceram. Int. 2020, 46, 696–702. [Google Scholar] [CrossRef]
- Rabani, I.; Zafar, R.; Subalakshmi, K.; Kim, H.-S.; Bathula, C.; Seo, Y.-S. A Facile Mechanochemical Preparation of Co3O4@ g-C3N4 for Application in Supercapacitors and Degradation of Pollutants in Water. J. Hazard. Mater. 2021, 407, 124360. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Alamgholiloo, H.; Gholipour, B.; Rostamnia, S.; Khaksar, S.; Farajzadeh, M.; Shokouhimehr, M. Visible-Light-Driven Photocatalytic Activity of ZnO/g-C3N4 Heterojunction for the Green Synthesis of Biologically Interest Small Molecules of Thiazolidinones. J. Photochem. Photobiol. A Chem. 2020, 402, 112786. [Google Scholar] [CrossRef]
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2 New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, Y.; Mahimwalla, Z.; Johnson, M.B.; Sharma, T.; Brüning, R.; Ghandi, K. Novel Synthesis of Rutile Titanium Dioxide–Polypyrrole Nano Composites and Their Application in Hydrogen Generation. Synth. Met. 2014, 189, 77–85. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Fittipaldi, M.; Jaén, J.J.D.; Fornasiero, P. Photocatalytic Hydrogen Production by Boron Modified TiO2/Carbon Nitride Heterojunctions. ChemCatChem 2019, 11, 6408–6416. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.R.; Petit, C. Titanium Dioxide/Carbon Nitride Nanosheet Nanocomposites for Gas Phase CO2 Photoreduction under UV-Visible Irradiation. Appl. Catal. B Environ. 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A Review on TiO2/g-C3N4 Visible-Light-Responsive Photocatalysts for Sustainable Energy Generation and Environmental Remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Alcudia-Ramos, M.A.; Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Hernández-Como, N.; García-Zaleta, D.S.; Kesarla, M.K.; Torres-Torres, J.G.; Collins-Martínez, V.; Godavarthi, S. Fabrication of g-C3N4/TiO2 Heterojunction Composite for Enhanced Photocatalytic Hydrogen Production. Ceram. Int. 2020, 46, 38–45. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Gao, Y.; An, T. Can Environmental Pharmaceuticals Be Photocatalytically Degraded and Completely Mineralized in Water Using g-C3N4/TiO2 under Visible Light Irradiation?—Implications of Persistent Toxic Intermediates. Appl. Catal. B Environ. 2016, 180, 726–732. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Chen, J.; Jiang, Q.; An, T.; Wong, P.K.; Zhang, H.; Zhao, H.; Yamashita, H. Enhanced Visible-Light-Driven Photocatalytic Inactivation of Escherichia Coli Using g-C3N4/TiO2 Hybrid Photocatalyst Synthesized Using a Hydrothermal-Calcination Approach. Water Res. 2015, 86, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, O.; Fujitsuka, M.; Majima, T. g-C3N4/TiO2 Mesocrystals Composite for H2 Evolution under Visible-Light Irradiation and Its Charge Carrier Dynamics. ACS Appl. Mater. Interfaces 2017, 9, 34844–34854. [Google Scholar] [CrossRef] [PubMed]
- Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Kupková, J.; Kustrowski, P.; Praus, P. Photocatalytic Decomposition of N2O over TiO2/g-C3N4 Photocatalysts Heterojunction. Appl. Surf. Sci. 2017, 396, 1685–1695. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, X.; Wu, Y.; Li, Z.; Han, Y.; Shen, X. A Study of Spherical TiO2/g-C3N4 Photocatalyst: Morphology, Chemical Composition and Photocatalytic Performance in Visible Light. Mol. Catal. 2017, 432, 232–241. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved Photocatalytic Activity of g-C3N4/TiO2 Composites Prepared by a Simple Impregnation Method. J. Photochem. Photobiol. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Xu, X.; Liu, C.; Duan, Y.; Hou, L.; Zhou, Q.; Ma, C.; Yang, X.; Liu, R. Highly Active TiO2/g-C3N4/G Photocatalyst with Extended Spectral Response towards Selective Reduction of Nitrobenzene. Appl. Catal. B Environ. 2017, 203, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D Direct Z-Scheme Heterojunctions of Hierarchical TiO2 Microflowers/g-C3N4 Nanosheets with Enhanced Charge Carrier Separation for Photocatalytic H2 Evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced Visible-Light Photocatalytic Activity of g-C3N4/TiO2 Films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef]
- Li, C.; Lou, Z.; Yang, Y.; Wang, Y.; Lu, Y.; Ye, Z.; Zhu, L. Hollowsphere Nanoheterojunction of g-C3N4@ TiO2 with High Visible Light Photocatalytic Property. Langmuir 2019, 35, 779–786. [Google Scholar] [CrossRef]
- Wei, H.; McMaster, W.A.; Tan, J.Z.Y.; Cao, L.; Chen, D.; Caruso, R.A. Mesoporous TiO2/g-C3N4 Microspheres with Enhanced Visible-Light Photocatalytic Activity. J. Phys. Chem. C 2017, 121, 22114–22122. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, B.; Liu, Y.; Ren, Y.; Ma, J.; Zhou, X.; Cheng, X.; Deng, Y. Controllable Interface-Induced Co-Assembly toward Highly Ordered Mesoporous Pt@ TiO2/g-C3N4 Heterojunctions with Enhanced Photocatalytic Performance. Adv. Funct. Mater. 2018, 28, 1806214. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zeng, X.; Huang, B.; Gao, S.; Lu, J. Synergetic Effect of Ti3+ and Oxygen Doping on Enhancing Photoelectrochemical and Photocatalytic Properties of TiO2/g-C3N4 Heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 11577–11586. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Kmentová, H.; Naldoni, A.; Goswami, A.; Gawande, M.B.; Varma, R.S.; Kment, S.; Zbořil, R. Significant Enhancement of Photoactivity in Hybrid TiO2/g-C3N4 Nanorod Catalysts Modified with Cu–Ni-Based Nanostructures. ACS Appl. Nano Mater. 2018, 1, 2526–2535. [Google Scholar] [CrossRef]
- Liu, C.; Raziq, F.; Li, Z.; Qu, Y.; Zada, A.; Jing, L. Synthesis of TiO2/g-C3N4 Nanocomposites with Phosphate–Oxygen Functional Bridges for Improved Photocatalytic Activity. Chin. J. Catal. 2017, 38, 1072–1078. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Li, H.; Wei, X.; Feng, J.; Liu, K.; Dang, Y.; Zhou, A. Efficient and Stable Photoelectrochemical Seawater Splitting with TiO2@g-C3N4 Nanorod Arrays Decorated by Co-Pi. J. Phys. Chem. C 2015, 119, 20283–20292. [Google Scholar] [CrossRef]
- Kholikov, B.; Hussain, J.; Zeng, H. Gold Modified TiO2/g-C3N4 for Enhanced Photocatalytic Activities to Evolved H2 Fuel. Inorg. Chem. Commun. 2021, 131, 108787. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Xu, H.; Li, Y.; Wei, Y.; Liu, J.; Zhao, Z. Efficient Z-Scheme Photocatalysts of Ultrathin g-C3N4-Wrapped Au/TiO2-Nanocrystals for Enhanced Visible-Light-Driven Conversion of CO2 with H2O. Appl. Catal. B Environ. 2020, 263, 118314. [Google Scholar] [CrossRef]
- Zada, A.; Qu, Y.; Ali, S.; Sun, N.; Lu, H.; Yan, R.; Zhang, X.; Jing, L. Improved Visible-Light Activities for Degrading Pollutants on TiO2/g-C3N4 Nanocomposites by Decorating SPR Au Nanoparticles and 2, 4-Dichlorophenol Decomposition Path. J. Hazard. Mater. 2018, 342, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Rao, Z.; Mahmood, A.; Wang, X.; Wang, Y.; Xie, X.; Sun, J. Improved Photocatalytic Oxidation Performance of Gaseous Acetaldehyde by Ternary g-C3N4/Ag-TiO2 Composites under Visible Light. J. Colloid Interface Sci. 2021, 602, 699–711. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Sun, P.; Ji, Z.; Duan, J. High Efficiency Photocatalytic Degradation of Indoor Formaldehyde by Ag/g-C3N4/TiO2 Composite Catalyst with ZSM-5 as the Carrier. Microporous Mesoporous Mater. 2021, 322, 111134. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Khan, M.W.; Qin, R.; Liu, X. Facile Synthesis of AgNPs Modified TiO2@ g-C3N4 Heterojunction Composites with Enhanced Photocatalytic Activity under Simulated Sunlight. Mater. Res. Bull. 2020, 121, 110641. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of Heterostructured g-C3N4/Ag/TiO2 Microspheres with Enhanced Photocatalysis Performance under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, Y.; Zhang, M.; Chu, B.; Huang, W.; Fan, M.; Dong, L.; Li, B. Enhanced Removal of Toxic Cr (VI) in Wastewater by Synthetic TiO2/g-C3N4 Microspheres/RGO Photocatalyst under Irradiation of Visible Light. Ind. Eng. Chem. Res. 2019, 58, 8979–8989. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure Regulation of ZnS@ g-C3N4/TiO2 Nanospheres for Efficient Photocatalytic H2 Production under Visible-Light Irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Hu, M.; Xing, Z.; Cao, Y.; Li, Z.; Yan, X.; Xiu, Z.; Zhao, T.; Yang, S.; Zhou, W. Ti3+ Self-Doped Mesoporous Black TiO2/SiO2/g-C3N4 Sheets Heterojunctions as Remarkable Visible-Lightdriven Photocatalysts. Appl. Catal. B Environ. 2018, 226, 499–508. [Google Scholar] [CrossRef]
- Obregón, S.; Zhang, Y.; Colón, G. Cascade Charge Separation Mechanism by Ternary Heterostructured BiPO4/TiO2/g-C3N4 Photocatalyst. Appl. Catal. B Environ. 2016, 184, 96–103. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, C.; Mei, J.; Yang, S.; Wong, P.K. Faster Electron Injection and Higher Interface Reactivity in g-C3N4/Fe2O3 Nanohybrid for Efficient Photo-Fenton-like Activity toward Antibiotics Degradation. Environ. Res. 2021, 195, 110842. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Zhang, F.-J.; Kong, C. Porous g-C3N4/WO3 Photocatalyst Prepared by Simple Calcination for Efficient Hydrogen Generation under Visible Light. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 594, 124653. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Wang, Y.; Yang, Y.; Wang, P.; Shi, L.; Feng, L.; Fang, S.; Liu, Q.; Ma, L. Synthesis and Photocatalytic Activity of g-C3N4/ZnO Composite Microspheres under Visible Light Exposure. Ceram. Int. 2022, 48, 3293–3302. [Google Scholar] [CrossRef]
- Moradi, S.; Isari, A.A.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Co-Implanting of TiO2 and Liquid-Phase-Delaminated g-C3N4 on Multi-Functional Graphene Nanobridges for Enhancing Photocatalytic Degradation of Acetaminophen. Chem. Eng. J. 2021, 414, 128618. [Google Scholar] [CrossRef]
- Jo, W.-K.; Natarajan, T.S. Influence of TiO2 Morphology on the Photocatalytic Efficiency of Direct Z-Scheme g-C3N4/TiO2 Photocatalysts for Isoniazid Degradation. Chem. Eng. J. 2015, 281, 549–565. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Mosquera, E.; Gracia, F. Preparation and Characterization of V2O5/ZnO Nanocomposite System for Photocatalytic Application. J. Mol. Liq. 2014, 198, 409–412. [Google Scholar] [CrossRef]
- Landry, C.J.; Burns, F.P.; Baerlocher, F.; Ghandi, K. Novel Solid-State Microbial Sensors Based on ZnO Nanorod Arrays. Adv. Funct. Mater. 2018, 28, 1706309. [Google Scholar] [CrossRef]
- Kumar, S.; Baruah, A.; Tonda, S.; Kumar, B.; Shanker, V.; Sreedhar, B. Cost-Effective and Eco-Friendly Synthesis of Novel and Stable N-Doped ZnO/g-C3N4 Core–Shell Nanoplates with Excellent Visible-Light Responsive Photocatalysis. Nanoscale 2014, 6, 4830–4842. [Google Scholar] [CrossRef] [Green Version]
- Uma, R.; Ravichandran, K.; Sriram, S.; Sakthivel, B. Cost-Effective Fabrication of ZnO/g-C3N4 Composite Thin Films for Enhanced Photocatalytic Activity against Three Different Dyes (MB, MG and RhB). Mater. Chem. Phys. 2017, 201, 147–155. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, J.; Huang, C.; Liu, W.; Tong, P.; Zhang, L. In Situ Hydrothermal Growth of ZnO/g-C3N4 Nanoflowers Coated Solid-Phase Microextraction Fibers Coupled with GC-MS for Determination of Pesticides Residues. Anal. Chim. Acta 2016, 934, 122–131. [Google Scholar] [CrossRef]
- Mohammadi, I.; Zeraatpisheh, F.; Ashiri, E.; Abdi, K. Solvothermal Synthesis of g-C3N4 and ZnO Nanoparticles on TiO2 Nanotube as Photoanode in DSSC. Int. J. Hydrogen Energy 2020, 45, 18831–18839. [Google Scholar] [CrossRef]
- Jang, E.; Kim, D.W.; Hong, S.H.; Park, Y.M.; Park, T.J. Visible Light-Driven g-C3N4@ ZnO Heterojunction Photocatalyst Synthesized via Atomic Layer Deposition with a Specially Designed Rotary Reactor. Appl. Surf. Sci. 2019, 487, 206–210. [Google Scholar] [CrossRef]
- Fageria, P.; Nazir, R.; Gangopadhyay, S.; Barshilia, H.C.; Pande, S. Graphitic-Carbon Nitride Support for the Synthesis of Shape-Dependent ZnO and Their Application in Visible Light Photocatalysts. Rsc Adv. 2015, 5, 80397–80409. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Hang, H.; Zhang, D.; Zhang, N.; Xiao, Z.; Tao, H. Self-Assembly Method Assisted Synthesis of g-C3N4/ZnO Heterostructure Nanocomposites with Enhanced Photocatalytic Performance. Opt. Mater. 2019, 96, 109266. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.-T.; Shin, E.W. Interactions between ZnO Nanoparticles and Amorphous g-C3N4 Nanosheets in Thermal Formation of g-C3N4/ZnO Composite Materials: The Annealing Temperature Effect. Appl. Surf. Sci. 2018, 458, 369–381. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S. Facile Synthesis of g-C3N4/ZnO Composite with Enhanced Visible Light Photooxidation and Photoreduction Properties. Chem. Eng. J. 2012, 209, 386–393. [Google Scholar] [CrossRef]
- Zhang, X.; Ling, S.; Ji, H.; Xu, L.; Huang, Y.; Hua, M.; Xia, J.; Li, H. Metal Ion-Containing Ionic Liquid Assisted Synthesis and Enhanced Photoelectrochemical Performance of g-C3N4/ZnO Composites. Mater. Technol. 2018, 33, 185–192. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, M.; Zhu, Y. Preparation of Visible Light-Driven g-C3N4@ ZnO Hybrid Photocatalyst via Mechanochemistry. Phys. Chem. Chem. Phys. 2014, 16, 17627–17633. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Gautam, S.; Panchal, P.; Nehra, S.P.; Choudhary, P.; Sharma, A. ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application. ACS Omega 2020, 5, 3828–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, P.-Y.; Su, Y.-Z.; Chen, G.-F.; Luo, Z.; Xing, S.-Y.; Li, N.; Liu, Z.-Q. g-C3N4 Decorated ZnO Nanorod Arrays for Enhanced Photoelectrocatalytic Performance. Appl. Surf. Sci. 2015, 358, 296–303. [Google Scholar] [CrossRef]
- Ramachandra, M.; Devi Kalathiparambil Rajendra Pai, S.; Resnik Jaleel UC, J.; Pinheiro, D. Improved Photocatalytic Activity of g-C3N4/ZnO: A Potential Direct Z-Scheme Nanocomposite. ChemistrySelect 2020, 5, 11986–11995. [Google Scholar] [CrossRef]
- Xing, H.; Ma, H.; Fu, Y.; Xue, M.; Zhang, X.; Dong, X.; Zhang, X. Preparation of g-C3N4/ZnO Composites and Their Enhanced Photocatalytic Activity. Mater. Technol. 2015, 30, 122–127. [Google Scholar] [CrossRef]
- Ismael, M. The Photocatalytic Performance of the ZnO/g-C3N4 Composite Photocatalyst toward Degradation of Organic Pollutants and Its Inactivity toward Hydrogen Evolution: The Influence of Light Irradiation and Charge Transfer. Chem. Phys. Lett. 2020, 739, 136992. [Google Scholar] [CrossRef]
- Fang, Q.; Li, B.; Li, Y.-Y.; Huang, W.-Q.; Peng, W.; Fan, X.; Huang, G.-F. 0D/2D Z-Scheme Heterojunctions of g-C3N4 Quantum Dots/ZnO Nanosheets as a Highly Efficient Visible-Light Photocatalyst. Adv. Powder Technol. 2019, 30, 1576–1583. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-Shell g-C3N4@ ZnO Composites as Photoanodes with Double Synergistic Effects for Enhanced Visible-Light Photoelectrocatalytic Activities. Appl. Catal. B Environ. 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Mathialagan, A.; Manavalan, M.; Venkatachalam, K.; Mohammad, F.; Oh, W.C.; Sagadevan, S. Fabrication and Physicochemical Characterization of g-C3N4/ZnO Composite with Enhanced Photocatalytic Activity under Visible Light. Opt. Mater. 2020, 100, 109643. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Significantly Enhanced Visible-Light Photocatalytic Activity of g-C3N4 via ZnO Modification and the Mechanism Study. J. Mol. Catal. A Chem. 2013, 368, 9–15. [Google Scholar] [CrossRef]
- Wang, L.; Ma, C.; Guo, Z.; Lv, Y.; Chen, W.; Chang, Z.; Yuan, Q.; Ming, H.; Wang, J. In-Situ Growth of g-C3N4 Layer on ZnO Nanoparticles with Enhanced Photocatalytic Performances under Visible Light Irradiation. Mater. Lett. 2017, 188, 347–350. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Lan, H.; Zhang, M.; Zhu, H.; Bu, M. Preparation of Heterostructure g-C3N4/ZnO Nanorods for High Photocatalytic Activity on Different Pollutants (MB, RhB, Cr (VI) and Eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Ahmad, I. Comparative Study of Metal (Al, Mg, Ni, Cu and Ag) Doped ZnO/g-C3N4 Composites: Efficient Photocatalysts for the Degradation of Organic Pollutants. Sep. Purif. Technol. 2020, 251, 117372. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Namgung, G.; Noh, J.-S. Facile Synthesis of Porous Metal-Doped ZnO/g-C3N4 Composites for Highly Efficient Photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 368, 110–119. [Google Scholar]
- Jin, C.; Li, W.; Chen, Y.; Li, R.; Huo, J.; He, Q.; Wang, Y. Efficient Photocatalytic Degradation and Adsorption of Tetracycline over Type-II Heterojunctions Consisting of ZnO Nanorods and K-Doped Exfoliated g-C3N4 Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 2860–2873. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M.B. Highly Efficient g-C3N4/Cr-ZnO Nanocomposites with Superior Photocatalytic and Antibacterial Activity. J. Photochem. Photobiol. A Chem. 2020, 401, 112776. [Google Scholar] [CrossRef]
- Neena, D.; Humayun, M.; Zuo, W.; Liu, C.S.; Gao, W.; Fu, D.J. Hierarchical Hetero-Architectures of in-Situ g-C3N4-Coupled Fe-Doped ZnO Micro-Flowers with Enhanced Visible-Light Photocatalytic Activities. Appl. Surf. Sci. 2020, 506, 145017. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z.; Jiang, X.; Feng, C.; Zeng, R. Optimized Preparation of Co-Pi Decorated g-C3N4 @ ZnO Shell-Core Nanorod Array for Its Improved Photoelectrochemical Performance and Stability. J. Alloys Compd. 2019, 780, 540–551. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, A.; Balaji, R.; Krishnan, V. Highly Efficient Visible Light Active 2D-2D Nanocomposites of N-ZnO-g-C3N4 for Photocatalytic Degradation of Diverse Industrial Pollutants. ChemistrySelect 2018, 3, 1919–1932. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Yao, Z.; Yang, Z.; Xu, H. Enhanced Visible-Light-Driven Photocatalytic Hydrogen Evolution and NO Photo-Oxidation Capacity of ZnO/g-C3N4 with N Dopant. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 599, 124869. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Jaafar, J.; Amin, N.A.S.; Mastuli, M.S.; Wu, H.; Wong, R.J.; Ng, Y.H. Bio-Inspired Hierarchical Hetero-Architectures of in-Situ C-Doped g-C3N4 Grafted on C, N Co-Doped ZnO Micro-Flowers with Booming Solar Photocatalytic Activity. J. Ind. Eng. Chem. 2019, 77, 393–407. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Zhai, H.-F.; Zhang, W.; Wang, S.-S.; Zhao, X.-R.; Li, M.; Li, H.; Li, A.-D.; Wu, D. Visible Light-Driven Photocatalytic Performance of N-Doped ZnO/g-C3N4 Nanocomposites. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Yang, C.; Cao, M.; Zhang, X.; Rajendran, S.; Limpanart, S.; Ma, M.; Liu, R. Two-Dimensional Porous Sheet-like Carbon-Doped ZnO/g-C3N4 Nanocomposite with High Visible-Light Photocatalytic Performance. Mater. Lett. 2017, 189, 156–159. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, M.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Carbon-Doped ZnO Hybridized Homogeneously with Graphitic Carbon Nitride Nanocomposites for Photocatalysis. J. Phys. Chem. C 2014, 118, 10963–10971. [Google Scholar] [CrossRef]
- Xing, Z.; Chen, Y.; Liu, C.; Yang, J.; Xu, J.; Situ, Y.; Huang, H. Synthesis of Core-Shell ZnO/Oxygen Doped g-C3N4 Visible Light Driven Photocatalyst via Hydrothermal Method. J. Alloys Compd. 2017, 708, 853–861. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Pan, Q.-J.; Guo, Y.-R. One-Pot Synthesis of the g-C3N4/S-Doped ZnO Composite with Assistance of Sodium Lignosulfonate and Its Enhanced Photodegradation on Organic Pollutants. Mater. Res. Bull. 2018, 107, 164–170. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Li, Y.; Hao, Y.; Wang, X.; Li, B.; Liu, R. Fabrication of Ternary g-C3N4/Al2O3/ZnO Heterojunctions Based on Cascade Electron Transfer toward Molecular Oxygen Activation. Appl. Catal. B Environ. 2017, 212, 115–128. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Khalid, M.N.; Jailani, M.F.A.M.; Noh, M.F.M.; Arzaee, N.A.; Zhou, D.; Sagu, J.S.; Teridi, M.A.M. Boosting Photocatalytic Activities of BiVO4 by Creation of g-C3N4/ZnO@ BiVO4 Heterojunction. Mater. Res. Bull. 2020, 125, 110779. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Mohapatra, L.; Martha, S.; Parida, K. ZnCr2O4@ ZnO/g-C3N4: A Triple-Junction Nanostructured Material for Effective Hydrogen and Oxygen Evolution under Visible Light. Energy Technol. 2017, 5, 1687–1701. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, Y.; Thirugnanam, N.; Li, G. Double Z-Scheme ZnO/ZnS/g-C3N4 Ternary Structure for Efficient Photocatalytic H2 Production. Appl. Surf. Sci. 2018, 430, 293–300. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Li, Y. A Ternary g-C3N4/Pt/ZnO Photoanode for Efficient Photoelectrochemical Water Splitting. Int. J. Hydrogen Energy 2015, 40, 9080–9087. [Google Scholar] [CrossRef]
- Azimi, E.B.; Badiei, A.; Sadr, M.H. Dramatic Visible Photocatalytic Performance of g-C3N4 -Based Nanocomposite Due to the Synergistic Effect of AgBr and ZnO Semiconductors. J. Phys. Chem. Solids 2018, 122, 174–183. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. One Pot Synthesis and Characterization of Ag-ZnO/g-C3N4 Photocatalyst with Improved Photoactivity and Antibacterial Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 477–484. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Xia, Y.; Wang, P.; Hou, Q.; Zhou, Q. Enhanced Photocatalytic Bactericidal Performance and Mechanism with Novel Ag/ZnO/g-C3N4 Composite under Visible Light. Catal. Today 2019, 330, 179–188. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.N.S. Copyrolysed C3N4-Ag/ZnO Ternary Heterostructure Systems for Enhanced Adsorption and Photocatalytic Degradation of Tetracycline. Eur. J. Inorg. Chem. 2016, 31, 5068–5076. [Google Scholar] [CrossRef]
- Lee, S.J.; Begildayeva, T.; Jung, H.J.; Koutavarapu, R.; Yu, Y.; Choi, M.; Choi, M.Y. Plasmonic ZnO/Au/g-C3N4 Nanocomposites as Solar Light Active Photocatalysts for Degradation of Organic Contaminants in Wastewater. Chemosphere 2021, 263, 128262. [Google Scholar] [CrossRef]
- Thang, N.Q.; Sabbah, A.; Chen, L.-C.; Chen, K.-H.; Thi, C.M.; Van Viet, P. Localized Surface Plasmonic Resonance Role of Silver Nanoparticles in the Enhancement of Long-Chain Hydrocarbons of the CO2 Reduction over Ag-GC3N4/ZnO Nanorods Photocatalysts. Chem. Eng. Sci. 2021, 229, 116049. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Chen, H.; Shao, G.; Zhang, R.; Lu, H. Synthesis and Properties of Ag/ZnO/g-C3N4 Ternary Micro/Nano Composites by Microwave-Assisted Method. Mater. Res. Express 2018, 5, 15021. [Google Scholar] [CrossRef]
- Wei, X.; Liu, H.; Li, T.; Jiang, Z.; Hu, W.; Niu, Q.; Chen, J. Three-Dimensional Flower Heterojunction g-C3N4/Ag/ZnO Composed of Ultrathin Nanosheets with Enhanced Photocatalytic Performance. J. Photochem. Photobiol. A Chem. 2020, 390, 112342. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Jiang, Z.; Rong, J.; Pan, J.; Zhang, T.; Yang, D. Preparation of Ternary Combined ZnO-Ag2O/Porous g-C3N4 Composite Photocatalyst and Enhanced Visible-Light Photocatalytic Activity for Degradation of Ciprofloxacin. Chem. Eng. Res. Des. 2016, 111, 253–261. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, M.; Duan, M.; Xie, L.; Zhao, M. High Visible Light Response Z-Scheme Ag3PO4/g-C3N4/ZnO Composite Photocatalyst for Efficient Degradation of Tetracycline Hydrochloride: Preparation, Properties and Mechanism. J. Alloys Compd. 2020, 840, 155714. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Li, M.; Lv, S.; Li, W.; Li, Z. Novel Visible Light Induced Ag2S/g-C3N4/ZnO Nanoarrays Heterojunction for Efficient Photocatalytic Performance. Appl. Surf. Sci. 2018, 462, 896–903. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.M.A.; Kumar, M.P.; Ravichandran, S.; Babu, R.R.; Sethurman, K.; Ramamurthi, K. Investigation on the g-C3N4 Encapsulated ZnO Nanorods Heterojunction Coupled with GO for Effective Photocatalytic Activity under Visible Light Irradiation. Arab. J. Chem. 2020, 13, 2826–2843. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Javed, M.; Hakami, O.; Irfan, R.M.; Ahmad, Z.; AlObaid, A.; Al-Anazy, M.M.; Baghdadi, H.B.; Abd-Rabboh, H.S.M. Design Ag-Doped ZnO Heterostructure Photocatalyst with Sulfurized Graphitic C3N4 Showing Enhanced Photocatalytic Activity. Mater. Sci. Eng. B 2021, 272, 115320. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhou, Q.; Li, Z.; Cui, Z.; Liu, X.; Liang, Y.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. A Z-Scheme Heterojunction of ZnO/CDots/C3N4 for Strengthened Photoresponsive Bacteria-Killing and Acceleration of Wound Healing. J. Mater. Sci. Technol. 2020, 57, 1–11. [Google Scholar] [CrossRef]
- Zhao, S.-W.; Zheng, M.; Sun, H.-L.; Li, S.-J.; Pan, Q.-J.; Guo, Y.-R. Construction of Heterostructured g-C3N4/ZnO/Cellulose and Its Antibacterial Activity: Experimental and Theoretical Investigations. Dalt. Trans. 2020, 49, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Faraz, M. Novel g-C3N4/Fe-ZnO/RGO Nanocomposites with Boosting Visible Light Photocatalytic Activity for MB, Cr (VI), and Outstanding Catalytic Activity toward Para-Nitrophenol Reduction. Nanotechnology 2020, 31, 325603. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chang, Y.; Qin, W.; Yuan, Z.; Zhao, J.; Zhang, J.; Han, E.; Wang, S.; Yang, M.; Shen, Y. ZnO-Reduced Graphene Oxide Composites Sensitized with Graphitic Carbon Nitride Nanosheets for Ethanol Sensing. ACS Appl. Nano Mater. 2019, 2, 2734–2742. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Guan, W.; Huang, H.; Liu, Y. Carbon Dots/g-C3N4/ZnO Nanocomposite as Efficient Visible-Light Driven Photocatalyst for Tetracycline Total Degradation. Sep. Purif. Technol. 2017, 173, 295–303. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Z.; Song, Y.; Zheng, X.; Qu, L.; Qian, J.; Wu, Y.; Wu, X.; Wu, Z. Remediation of Phenanthrene Contaminated Soil by g-C3N4/Fe3O4 Composites and Its Phytotoxicity Evaluation. Chemosphere 2019, 221, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, Y.; Dong, H.; Liu, Z.; Li, C.; Huo, P.; Yan, Y. Intercalation Effect of Attapulgite in g-C3N4 Modified with Fe3O4 Quantum Dots to Enhance Photocatalytic Activity for Removing 2-Mercaptobenzothiazole under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 10614–10623. [Google Scholar] [CrossRef]
- Lima, M.J.; Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Magnetically Recoverable Fe3O4/g-C3N4 Composite for Photocatalytic Production of Benzaldehyde under UV-Led Radiation. Catal. Today 2019, 328, 293–299. [Google Scholar] [CrossRef]
- Wang, M.; Cui, S.; Yang, X.; Bi, W. Synthesis of g-C3N4/Fe3O4 Nanocomposites and Application as a New Sorbent for Solid Phase Extraction of Polycyclic Aromatic Hydrocarbons in Water Samples. Talanta 2015, 132, 922–928. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Constructing 2D/2D Fe2O3/g-C3N4 Direct Z-scheme Photocatalysts with Enhanced H2 Generation Performance. Sol. Rrl 2018, 2, 1800006. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, L.-G.; Yuan, Y.-P.; Zhu, Y.-J.; Xia, J.; Zhu, J.-F. Facile Fabrication of Magnetically Separable Graphitic Carbon Nitride Photocatalysts with Enhanced Photocatalytic Activity under Visible Light. J. Mater. Chem. A 2013, 1, 3008–3015. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Lamba, B.Y. A Review on Synthesis, Characterisation and Surface Modification of Magnetic Nanoparticle and Its Composite for Removal of Heavy Metals from Wastewater. Int. J. Environ. Anal. Chem. 2021, 1–16. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hosseini, H.R.M. An Investigation on the Optimum Conditions of Synthesizing a Magnetite Based Ferrofluid as MRI Contrast Agent Using Taguchi Method. Mater. Sci. Pol. 2013, 31, 253–258. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A. Recent Advances in the Modification of Carbon-Based Quantum Dots for Biomedical Applications. Mater. Sci. Eng. C 2020, 120, 111756. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Madaah Hosseini, H.R. A Facile, Two-Step Synthesis and Characterization of Fe3O4–LCysteine–Graphene Quantum Dots as a Multifunctional Nanocomposite. Appl. Nanosci. 2021, 11, 849–860. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Jiang, Y.; Zhao, Z.; Jiang, G.; Sun, Z. Synthesis of Fe2O3 Loaded Porous g-C3N4 Photocatalyst for Photocatalytic Reduction of Dinitrogen to Ammonia. Chem. Eng. J. 2019, 373, 572–579. [Google Scholar] [CrossRef]
- Ding, Q.; Lam, F.L.Y.; Hu, X. Complete Degradation of Ciprofloxacin over g-C3N4 -Iron Oxide Composite via Heterogeneous Dark Fenton Reaction. J. Environ. Manag. 2019, 244, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Ciprofloxacin Pharmacokinetics in Clinical Canine Patients. J. Vet. Intern. Med. 2017, 31, 1508–1513. [Google Scholar] [CrossRef]
- Duan, B.; Mei, L. A Z-Scheme Fe2O3/g-C3N4 Heterojunction for Carbon Dioxide to Hydrocarbon Fuel under Visible Illuminance. J. Colloid Interface Sci. 2020, 575, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Z-Scheme 2D/2D α-Fe2O3/g-C3N4 Heterojunction for Photocatalytic Oxidation of Nitric Oxide. Appl. Catal. B Environ. 2021, 280, 119409. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, L.; Fan, X.; Wang, M.; Li, M.; Shi, J. One-Step Construction of FeOx Modified g-C3N4 for Largely Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Carbon 2016, 101, 62–70. [Google Scholar] [CrossRef]
- Wang, J.; Qin, C.; Wang, H.; Chu, M.; Zada, A.; Zhang, X.; Li, J.; Raziq, F.; Qu, Y.; Jing, L. Exceptional Photocatalytic Activities for CO2 Conversion on AlO Bridged g-C3N4/α-Fe2O3 z-Scheme Nanocomposites and Mechanism Insight with IsotopesZ. Appl. Catal. B Environ. 2018, 221, 459–466. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Bontempi, E.; Zafeiratos, S.; Jaén, J.J.D.; Fornasiero, P. Synthesis and Photocatalytic Application of Visible-Light Active β-Fe2O3/g-C3N4 Hybrid Nanocomposites. Appl. Catal. B Environ. 2016, 187, 171–180. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Nguyen, C.-C.; Do, T.-O. Synthesis of Fe2O3/Pt/Au Nanocomposite Immobilized on g-C3N4 for Localized Plasmon Photocatalytic Hydrogen Evolution. Appl. Surf. Sci. 2019, 489, 741–754. [Google Scholar] [CrossRef]
- Ghandi, K.; Wang, F.; Landry, C.; Mostafavi, M. Naked Gold Nanoparticles and Hot Electrons in Water. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Li, X.; Zhang, Y.; Yan, Y.; Huo, P.; Wang, H. g-C3N4 Quantum Dots and Au Nano Particles Co-Modified CeO2/Fe3O4 Micro-Flowers Photocatalyst for Enhanced CO2 Photoreduction. Renew. Energy 2021, 179, 756–765. [Google Scholar]
- Liu, L.; Wang, M.; Wang, C. In-Situ Synthesis of Graphitic Carbon Nitride/Iron Oxide−Copper Composites and Their Application in the Electrochemical Detection of Glucose. Electrochim. Acta 2018, 265, 275–283. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, G.; Huang, J.; Chen, W. Three-Dimensional Multi-Walled Carbon Nanotubes@ g-C3N4@ Fe3O4nanocomposites-Based Magnetic Solid Phase Extraction for the Determination of Polycyclic Aromatic Hydrocarbons in Water Samples. Microchem. J. 2018, 142, 385–393. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Q.; Dou, Y.; Zhang, Z.; Yan, T.; Li, Y. Fabricating a Novel Ternary Recyclable Fe3O4/Graphene/Sulfur-Doped g-C3N4 Composite Catalyst for Enhanced Removal of Ranitidine under Visible-Light Irradiation and Reducing of Its N-Nitrosodimethylamine Formation Potential. J. Hazard. Mater. 2021, 413, 125288. [Google Scholar] [CrossRef]
- Rashid, J.; Parveen, N.; Haq, T.U.; Iqbal, A.; Talib, S.H.; Awan, S.U.; Hussain, N.; Zaheer, M. g-C3N4/CeO2/Fe3O4Ternary Composite as an Efficient Bifunctional Catalyst for Overall Water Splitting. ChemCatChem 2018, 10, 5587–5592. [Google Scholar] [CrossRef]
- Zhu, Z.; Huo, P.; Lu, Z.; Yan, Y.; Liu, Z.; Shi, W.; Li, C.; Dong, H. Fabrication of Magnetically Recoverable Photocatalysts Using g-C3N4 for Effective Separation of Charge Carriers through like-Z-Scheme Mechanism with Fe3O4 Mediator. Chem. Eng. J. 2018, 331, 615–625. [Google Scholar] [CrossRef]
- Mishra, P.; Behera, A.; Kandi, D.; Ratha, S.; Parida, K. Novel Magnetic Retrievable Visible-Light-Driven Ternary Fe3O4@ NiFe2O4/Phosphorus-Doped g-C3N4 Nanocomposite Photocatalyst with Significantly Enhanced Activity through a Double-Z-Scheme System. Inorg. Chem. 2020, 59, 4255–4272. [Google Scholar] [CrossRef]
- Ji, H.; Jing, X.; Xu, Y.; Yan, J.; Li, H.; Li, Y.; Huang, L.; Zhang, Q.; Xu, H.; Li, H. Magnetic g-C3N4/NiFe2O4 Hybrids with Enhanced Photocatalytic Activity. RSC Adv. 2015, 5, 57960–57967. [Google Scholar] [CrossRef]
- Palanivel, B.; Ayappan, C.; Jayaraman, V.; Chidambaram, S.; Maheswaran, R.; Mani, A. Inverse Spinel NiFe2O4 Deposited g-C3N4 Nanosheet for Enhanced Visible Light Photocatalytic Activity. Mater. Sci. Semicond. Process. 2019, 100, 87–97. [Google Scholar] [CrossRef]
- Patnaik, S.; Das, K.K.; Mohanty, A.; Parida, K. Enhanced Photo Catalytic Reduction of Cr (VI) over Polymer-Sensitized g-C3N4/ZnFe2O4 and Its Synergism with Phenol Oxidation under Visible Light Irradiation. Catal. Today 2018, 315, 52–66. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A Facile Synthesis Method for Fabrication of LaFeO3/g-C3N4 Nanocomposite as Efficient Visible-Light-Driven Photocatalyst for Photodegradation of RhB and 4-CP. New J. Chem. 2019, 43, 13783–13793. [Google Scholar] [CrossRef]
- Khasevani, S.G.; Gholami, M.R. Synthesis of BiOI/ZnFe2O4–Metal–Organic Framework and g-C3N4-Based Nanocomposites for Applications in Photocatalysis. Ind. Eng. Chem. Res. 2019, 58, 9806–9818. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Shang, T.; Chen, L.; Gu, L.; Peng, T. Direct Z-Scheme g-C3N4/WO3 Photocatalyst with Atomically Defined Junction for H2 Production. Appl. Catal. B Environ. 2017, 219, 693–704. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Z.; Zhu, Y.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2, 5-Diformylfuran on WO3/g-C3N4 Composite under Irradiation of Visible Light. J. Photochem. Photobiol. A Chem. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon Nitride Based Nanocomposites: A Review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, J.; Hu, Y.; Guo, L. WO3/g-C3N4 Composites: One-Pot Preparation and Enhanced Photocatalytic H2 Production under Visible-Light Irradiation. Nanotechnology 2017, 28, 164002. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhu, Z.; Long, Y.; Li, W. Single-Source-Precursor-Assisted Synthesis of Porous WO3/g-C3N4 with Enhanced Photocatalytic Property. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 582, 123857. [Google Scholar] [CrossRef]
- Huang, S.; Long, Y.; Ruan, S.; Zeng, Y.-J. Enhanced Photocatalytic CO2 Reduction in Defect-Engineered Z-Scheme WO3–x/g-C3N4 Heterostructures. ACS Omega 2019, 4, 15593–15599. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; He, S. WOx/g-C3N4 Layered Heterostructures with Controlled Crystallinity towards Superior Photocatalytic Degradation and H2 Generation. Carbon 2020, 156, 488–498. [Google Scholar] [CrossRef]
- Han, X.; Xu, D.; An, L.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. WO3/g-C3N4 Two-Dimensional Composites for Visible-Light Driven Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 4845–4855. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vadivel, S. Fiber Optic Ethanol Gas Sensor Based WO3 and WO3/GC3N4 Nanocomposites by a Novel Microwave Technique. Opt. Laser Technol. 2019, 118, 44–51. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Su, J.; Gao, X. Preparation of WO3/g-C3N4 Composites and Their Application in Oxidative Desulfurization. Appl. Surf. Sci. 2017, 392, 810–816. [Google Scholar] [CrossRef]
- Cadan, F.M.; Ribeiro, C.; Azevedo, E.B. Improving g-C3N4: WO3 Z-Scheme Photocatalytic Performance under Visible Light by Multivariate Optimization of g-C3N4 Synthesis. Appl. Surf. Sci. 2021, 537, 147904. [Google Scholar] [CrossRef]
- Singh, J.; Arora, A.; Basu, S. Synthesis of Coral like WO3/g-C3N4 Nanocomposites for the Removal of Hazardous Dyes under Visible Light. J. Alloys Compd. 2019, 808, 151734. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Ma, C.; Cheng, Y.; Shen, D.; Zhang, S.; Liu, W.; Huo, P.; Wang, H. Direct Z-Scheme WO3/Graphitic Carbon Nitride Nanocomposites for the Photoreduction of CO2. ACS Appl. Nano Mater. 2020, 3, 1298–1306. [Google Scholar] [CrossRef]
- Chang, F.; Zheng, J.; Wu, F.; Wang, X.; Deng, B. Binary Composites WO3/g-C3N4 in Porous Morphology: Facile Construction, Characterization, and Reinforced Visible Light Photocatalytic Activity. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 563, 11–21. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 Step-Scheme H2-Production Photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Karimi-Nazarabad, M.; Goharshadi, E.K. Highly Efficient Photocatalytic and Photoelectrocatalytic Activity of Solar Light Driven WO3/g-C3N4 Nanocomposite. Sol. Energy Mater. Sol. Cells 2017, 160, 484–493. [Google Scholar] [CrossRef]
- Chai, B.; Liu, C.; Yan, J.; Ren, Z.; Wang, Z. In-Situ Synthesis of WO3 Nanoplates Anchored on g-C3N4 Z-Scheme Photocatalysts for Significantly Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2018, 448, 1–8. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Nawawi, M.G.M. Highly STable 3D/2D WO3/g-C3N4 Z-Scheme Heterojunction for Stimulating Photocatalytic CO2 Reduction by H2O/H2 to CO and CH4 under Visible Light. J. CO2 Util. 2020, 41, 101270. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Meng, J.; Liu, Y.; Ren, M.; Guo, Y.; Yang, Y. Robust Z-Scheme g-C3N4/WO3 Heterojunction Photocatalysts with Morphology Control of WO3 for Efficient Degradation of Phenolic Pollutants. Sep. Purif. Technol. 2021, 255, 117693. [Google Scholar] [CrossRef]
- Borthakur, S.; Basyach, P.; Kalita, L.; Sonowal, K.; Tiwari, A.; Chetia, P.; Saikia, L. Sunlight Assisted Degradation of a Pollutant Dye in Water by a WO3@ g-C3N4 Nanocomposite Catalyst. New J. Chem. 2020, 44, 2947–2960. [Google Scholar] [CrossRef]
- Jing, H.; Ou, R.; Yu, H.; Zhao, Y.; Lu, Y.; Huo, M.; Huo, H.; Wang, X. Engineering of g-C3N4 Nanoparticles/WO3 Hollow Microspheres Photocatalyst with Z-Scheme Heterostructure for Boosting Tetracycline Hydrochloride Degradation. Sep. Purif. Technol. 2021, 255, 117646. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In Situ Construction of Hierarchical WO3/g-C3N4 Composite Hollow Microspheres as a Z-Scheme Photocatalyst for the Degradation of Antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Liu, X.; Jin, A.; Jia, Y.; Xia, T.; Deng, C.; Zhu, M.; Chen, C.; Chen, X. Synergy of Adsorption and Visible-Light Photocatalytic Degradation of Methylene Blue by a Bifunctional Z-Scheme Heterojunction of WO3/g-C3N4. Appl. Surf. Sci. 2017, 405, 359–371. [Google Scholar] [CrossRef]
- Zhuang, H.; Cai, Z.; Xu, W.; Huang, M.; Liu, X. In Situ Construction of WO3/g-C3N4 Composite Photocatalyst with 2D–2D Heterostructure for Enhanced Visible Light Photocatalytic Performance. New J. Chem. 2019, 43, 17416–17422. [Google Scholar] [CrossRef]
- Meng, J.; Pei, J.; He, Z.; Wu, S.; Lin, Q.; Wei, X.; Li, J.; Zhang, Z. Facile Synthesis of g-C3N4 Nanosheets Loaded with WO3 Nanoparticles with Enhanced Photocatalytic Performance under Visible Light Irradiation. RSC Adv. 2017, 7, 24097–24104. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Lu, N.; Su, Y.; Liu, N.; Yu, H.; Li, J.; Wu, Y. Fabrication of WO3@ g-C3N4 with Core@ Shell Nanostructure for Enhanced Photocatalytic Degradation Activity under Visible Light. Appl. Surf. Sci. 2017, 423, 197–204. [Google Scholar] [CrossRef]
- Zhao, C.; Ran, F.; Dai, L.; Li, C.; Zheng, C.; Si, C. Cellulose-Assisted Construction of High Surface Area Z-Scheme C-Doped g-C3N4/WO3 for Improved Tetracycline Degradation. Carbohydr. Polym. 2021, 255, 117343. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Kumar, M.K.; Kim, H. Synthesis of Oxygen-Doped-g-C3N4/WO3 Porous Structures for Visible Driven Photocatalytic H2 Production. Phys. E Low-dimensional Syst. Nanostructures 2021, 126, 114428. [Google Scholar]
- Sun, L.; Li, B.; Chu, X.; Sun, N.; Qu, Y.; Zhang, X.; Khan, I.; Bai, L.; Jing, L. Synthesis of Si–O-Bridged g-C3N4/WO3 2D-Heterojunctional Nanocomposites as Efficient Photocatalysts for Aerobic Alcohol Oxidation and Mechanism Insight. ACS Sustain. Chem. Eng. 2019, 7, 9916–9927. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Uncovering the Key Role of the Fermi Level of the Electron Mediator in a Z-Scheme Photocatalyst by Detecting the Charge Transfer Process of WO3-Metal-GC3N4 (Metal = Cu, Ag, Au). ACS Appl. Mater. Interfaces 2016, 8, 2111–2119. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X.; Wang, Y.; Ye, Z. Ag Nanoparticles Decorated WO3/g-C3N4 2D/2D Heterostructure with Enhanced Photocatalytic Activity for Organic Pollutants Degradation. Appl. Surf. Sci. 2019, 467, 1000–1010. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Yan, Z.; Luo, J.; Du, B.; Zhan, J.; Liu, L.; Zhang, J. Double Photoelectron-Transfer Mechanism in Ag− AgCl/WO3/g-C3N4 Photocatalyst with Enhanced Visible-Light Photocatalytic Activity for Trimethoprim Degradation. J. Hazard. Mater. 2021, 403, 123964. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Pham, T.-D.; Van Thuan, D.; Tran, D.T.; Nguyen, M.V.; Dang, N.M.; Trang, H.T. Superior Activity of Cu-NiWO4/g-C3N4 Z Direct System for Photocatalytic Decomposition of VOCs in Aerosol under Visible Light. J. Alloys Compd. 2019, 798, 12–18. [Google Scholar] [CrossRef]
- Ahmed, K.E.; Kuo, D.-H.; Zeleke, M.A.; Zelekew, O.A.; Abay, A.K. Synthesis of Sn-WO3/g-C3N4 Composites with Surface Activated Oxygen for Visible Light Degradation of Dyes. J. Photochem. Photobiol. A Chem. 2019, 369, 133–141. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Mohd Nawawi, M.G. Well-Designed 3D/2D/2D WO3/Bt/g-C3N4 Z-Scheme Heterojunction for Tailoring Photocatalytic CO2 Methanation with 2D-Layered Bentonite-Clay as the Electron Moderator under Visible Light. Energy Fuels 2020, 34, 14400–14418. [Google Scholar]
- Ouyang, K.; Xu, B.; Yang, C.; Wang, H.; Zhan, P.; Xie, S. Synthesis of a Novel Z-Scheme Ag/WO3/g-C3N4 Nanophotocatalyst for Degradation of Oxytetracycline Hydrochloride under Visible Light. Mater. Sci. Semicond. Process. 2022, 137, 106168. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, J.; Meng, F.; Lin, X.; Feng, Y.; Yan, Y.; Meng, M. Rationally Constructing of a Novel 2D/2D WO3/Pt/g-C3N4 Schottky-Ohmic Junction towards Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution and Mechanism Insight. J. Colloid Interface Sci. 2021, 586, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Feng, S.; Wu, Y.; Lv, X.; Wang, H.; Chen, S.-M.; Hao, Q.; Cao, Y.; Lei, W.; Tong, Z. Label-Free Photoelectrochemical Immunosensor for Aflatoxin B1 Detection Based on the Z-Scheme Heterojunction of g-C3N4/Au/WO3. Biosens. Bioelectron. 2021, 189, 113373. [Google Scholar]
- Li, J.; Hao, H.; Zhu, Z. Construction of g-C3N4-WO3-Bi2WO6 Double Z-Scheme System with Enhanced Photoelectrochemical Performance. Mater. Lett. 2016, 168, 180–183. [Google Scholar] [CrossRef]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. Synthesis of BaWO4/NRGO–g-C3N4 Nanocomposites with Excellent Multifunctional Catalytic Performance via Microwave Approach. Front. Mater. Sci. 2018, 12, 247–263. [Google Scholar] [CrossRef]
- Huang, R.; Gu, X.; Sun, W.; Chen, L.; Du, Q.; Guo, X.; Li, J.; Zhang, M.; Li, C. In Situ Synthesis of Cu+ Self-Doped CuWO4/g-C3N4 Heterogeneous Fenton-like Catalysts: The Key Role of Cu+ in Enhancing Catalytic Performance. Sep. Purif. Technol. 2020, 250, 117174. [Google Scholar] [CrossRef]
- Jia, J.; Jiang, C.; Zhang, X.; Li, P.; Xiong, J.; Zhang, Z.; Wu, T.; Wang, Y. Urea-Modified Carbon Quantum Dots as Electron Mediator Decorated g-C3N4/WO3 with Enhanced Visible-Light Photocatalytic Activity and Mechanism Insight. Appl. Surf. Sci. 2019, 495, 143524. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Luo, X.; Wen, J.; Ma, S.; Li, X.; Fang, Y.; Zhang, X. Enhanced Visible Light Photocatalytic H2 Production over Z-Scheme g-C3N4 Nansheets/WO3 Nanorods Nanocomposites Loaded with Ni (OH) x Cocatalysts. Chin. J. Catal. 2017, 38, 240–252. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Chen, X.; Yu, H.; Wang, H.; Wu, Z.; Zhang, J.; Xiong, T. In-Situ Synthesis of Direct Solid-State Dual Z-Scheme WO3/g-C3N4/Bi2O3 Photocatalyst for the Degradation of Refractory Pollutant. Appl. Catal. B Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Ali, S.; Humayun, M.; Pi, W.; Yuan, Y.; Wang, M.; Khan, A.; Yue, P.; Shu, L.; Zheng, Z.; Fu, Q. Fabrication of BiFeO3-g-C3N4–WO3 Z-Scheme Heterojunction as Highly Efficient Visible-Light Photocatalyst for Water Reduction and 2, 4-Dichlorophenol Degradation: Insight Mechanism. J. Hazard. Mater. 2020, 397, 122708. [Google Scholar] [CrossRef]
- Bilal Tahir, M.; Nadeem Riaz, K.; Asiri, A.M. Boosting the Performance of Visible Light-driven WO3/g-C3N4 Anchored with BiVO4 Nanoparticles for Photocatalytic Hydrogen Evolution. Int. J. Energy Res. 2019, 43, 5747–5758. [Google Scholar] [CrossRef]
- Beyhaqi, A.; Zeng, Q.; Chang, S.; Wang, M.; Azimi, S.M.T.; Hu, C. Construction of g-C3N4/WO3/MoS2 Ternary Nanocomposite with Enhanced Charge Separation and Collection for Efficient Wastewater Treatment under Visible Light. Chemosphere 2020, 247, 125784. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, L.; Quan, X.; Zhao, Z.; Shi, Y.; Puma, G.L. Acetate Production from Inorganic Carbon (HCO3−) in Photo-Assisted Biocathode Microbial Electrosynthesis Systems Using WO3/MoO3/g-C3N4 Heterojunctions and Serratia Marcescens Species. Appl. Catal. B Environ. 2020, 267, 118611. [Google Scholar] [CrossRef]
- Ibrahim, Y.O.; Gondal, M.A. Visible-Light-Driven Photocatalytic Performance of a Z-Scheme Based TiO2/WO3/g-C3N4 Ternary Heterojunctions. Mol. Catal. 2021, 505, 111494. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, Y.; Yu, S.; Chen, L.; Cui, W.; Dong, F.; Zhou, Y. SnO2 Quantum Dots Anchored on g-C3N4 for Enhanced Visible-Light Photocatalytic Removal of NO and Toxic NO2 Inhibition. Appl. Surf. Sci. 2019, 496, 143630. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, J.; Wei, L.; Liu, Y.; Yang, C. Z-Scheme Heterostructure of Fe-Doped SnO2 Decorated Layered g-C3N4 with Enhanced Photocatalytic Activity under Simulated Solar Light Irradiation. Opt. Mater. 2020, 101, 109769. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Fan, M.; Wang, X.; Walbridge, M.L.; Nong, Q.; Wu, Y.; Zhao, L. Z-Scheme SnO2−x/g-C3N4 Composite as an Efficient Photocatalyst for Dye Degradation and Photocatalytic CO2 Reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Zhang, B.; Gong, Y.; Wang, X.; Sun, G.; Bala, H.; Zhang, Z. Synthesis of g-C3N4 Nanosheet Modified SnO2 Composites with Improved Performance for Ethanol Gas Sensing. RSC Adv. 2017, 7, 25504–25511. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; He, Y.; Xu, L.; Xia, Y.; Gang, R. SnO2 Particles as Efficient Photocatalysts for Organic Dye Degradation Grown In-Situ on g-C3N4 Nanosheets by Microwave-Assisted Hydrothermal Method. Mater. Sci. Semicond. Process. 2021, 121, 105298. [Google Scholar] [CrossRef]
- Zhu, K.; Lv, Y.; Liu, J.; Wang, W.; Wang, C.; Li, S.; Wang, P.; Zhang, M.; Meng, A.; Li, Z. Facile Fabrication of g-C3N4/SnO2 Composites and Ball Milling Treatment for Enhanced Photocatalytic Performance. J. Alloys Compd. 2019, 802, 13–18. [Google Scholar] [CrossRef]
- Seza, A.; Soleimani, F.; Naseri, N.; Soltaninejad, M.; Montazeri, S.M.; Sadrnezhaad, S.K.; Mohammadi, M.R.; Moghadam, H.A.; Forouzandeh, M.; Amin, M.H. Novel Microwave-Assisted Synthesis of Porous g-C3N4/SnO2 Nanocomposite for Solar Water-Splitting. Appl. Surf. Sci. 2018, 440, 153–161. [Google Scholar] [CrossRef]
- Bian, H.; Wang, A.; Li, Z.; Li, Z.; Diao, Y.; Lu, J.; Li, Y.Y. g-C3N4-Modified Water-Crystallized Mesoporous SnO2 for Enhanced Photoelectrochemical Properties. Part. Part. Syst. Charact. 2018, 35, 1800155. [Google Scholar] [CrossRef]
- Ji, H.; Fan, Y.; Yan, J.; Xu, Y.; She, X.; Gu, J.; Fei, T.; Xu, H.; Li, H. Construction of SnO2/Graphene-like g-C3N4 with Enhanced Visible Light Photocatalytic Activity. RSC Adv. 2017, 7, 36101–36111. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Li, L.; Li, X.; Lin, R.; Li, G. Synergistic Collaboration of g-C3N4/SnO2 Composites for Enhanced Visible-Light Photocatalytic Activity. Chem. Eng. J. 2014, 246, 277–286. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, F.; Qi, Y.; Zhang, X.; Yang, J.; Liu, X.; Li, S. A Facile One-Pot and Alkali-Free Synthetic Procedure for Binary SnO2/g-C3N4 Composites with Enhanced Photocatalytic Behavior. Mater. Sci. Semicond. Process. 2020, 115, 105112. [Google Scholar] [CrossRef]
- Peng, F.; Ni, Y.; Zhou, Q.; Kou, J.; Lu, C.; Xu, Z. New g-C3N4 Based Photocatalytic Cement with Enhanced Visible-Light Photocatalytic Activity by Constructing Muscovite Sheet/SnO2 Structures. Constr. Build. Mater. 2018, 179, 315–325. [Google Scholar] [CrossRef]
- Babu, B.; Cho, M.; Byon, C.; Shim, J. Sunlight-Driven Photocatalytic Activity of SnO2 QDs-g-C3N4 Nanolayers. Mater. Lett. 2018, 212, 327–331. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.; Duan, L.; Liu, R.; Li, H. Enhanced Visible Light Photocatalytic Activity in SnO2@ g-C3N4 Core-Shell Structures. Mater. Sci. Eng. B 2017, 218, 23–30. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P. Flower-like SnO2/g-C3N4 Heterojunctions: The Face-to-Face Contact Interface and Improved Photocatalytic Properties. Adv. Powder Technol. 2018, 29, 1153–1157. [Google Scholar] [CrossRef]
- Singh, J.; Kumari, P.; Basu, S. Degradation of Toxic Industrial Dyes Using SnO2/g-C3N4 Nanocomposites: Role of Mass Ratio on Photocatalytic Activity. J. Photochem. Photobiol. A Chem. 2019, 371, 136–143. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, Y.; Wu, L.; Pan, J.; Xiong, X. In Situ Construction of an SnO2/g-C3N4 Heterojunction for Enhanced Visible-Light Photocatalytic Activity. Rsc Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Shi, L.; Cao, L.; Liu, H.; Liu, Y.; Li, Y.; Song, H.; Jie, Y.; Ye, J. Sb Doped SnO2-Decorated Porous g-C3N4 Nanosheet Heterostructures with Enhanced Photocatalytic Activities under Visible Light Irradiation. Appl. Catal. B Environ. 2018, 221, 670–680. [Google Scholar] [CrossRef]
- Raziq, F.; Qu, Y.; Humayun, M.; Zada, A.; Yu, H.; Jing, L. Synthesis of SnO2/BP Codoped g-C3N4 Nanocomposites as Efficient Cocatalyst-Free Visible-Light Photocatalysts for CO2 Conversion and Pollutant Degradation. Appl. Catal. B Environ. 2017, 201, 486–494. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. In Situ Fabrication of SnO2/S-Doped g-C3N4 Nanocomposites and Improved Visible Light Driven Photodegradation of Methylene Blue. J. Mol. Liq. 2017, 248, 688–702. [Google Scholar] [CrossRef]
- Guo, W.; Huang, L.; Zhang, J.; He, Y.; Zeng, W. Ni-Doped SnO2/g-C3N4 Nanocomposite with Enhanced Gas Sensing Performance for the Eff; Ective Detection of Acetone in Diabetes Diagnosis. Sens. Actuators B Chem. 2021, 334, 129666. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Yuvakkumar, R.; Velauthapillai, D.; Ahamad, T.; Khan, M.A.M.; Mohammed, M.K.A.; Vijayaprabhu, N.; Ravi, G. The Bifunctional Performance Analysis of Synthesized Ce Doped SnO2/g-C3N4 Composites for Asymmetric Supercapacitor and Visible Light Photocatalytic Applications. J. Alloys Compd. 2021, 866, 158807. [Google Scholar] [CrossRef]
- Zada, A.; Humayun, M.; Raziq, F.; Zhang, X.; Qu, Y.; Bai, L.; Qin, C.; Jing, L.; Fu, H. Exceptional Visible-light-driven Cocatalyst-free Photocatalytic Activity of g-C3N4 by Well Designed Nanocomposites with Plasmonic Au and SnO2. Adv. Energy Mater. 2016, 6, 1601190. [Google Scholar] [CrossRef]
- Yuan, F.; Sun, Z.; Li, C.; Tan, Y.; Zhang, X.; Zheng, S. Multi-Component Design and in-Situ Synthesis of Visible-Light-Driven SnO2/g-C3N4/Diatomite Composite for High-Efficient Photoreduction of Cr (VI) with the Aid of Citric Acid. J. Hazard. Mater. 2020, 396, 122694. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced Visible-Light-Driven Photoelectrochemical and Photocatalytic Performance of Au-SnO2 Quantum Dot-Anchored g-C3N4 Nanosheets. Sep. Purif. Technol. 2020, 240, 116652. [Google Scholar] [CrossRef]
- Peng, L.; Zheng, R.; Feng, D.; Yu, H.; Dong, X. Synthesis of Eco-Friendly Porous g-C3N4/SiO2/SnO2 Composite with Excellent Visible-Light Responsive Photocatalysis. Arab. J. Chem. 2020, 13, 4275–4285. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Zhou, J.; Wang, K.; Zheng, Y.-Z.; Tao, X. Uniformly Assembling N-Type Metal Oxide Nanostructures (TiO2 Nanoparticles and SnO2 Nanowires) onto P Doped g-C3N4 Nanosheets for Efficient Photocatalytic Water Splitting. Appl. Catal. B Environ. 2020, 278, 119301. [Google Scholar] [CrossRef]
- Faraji, M.; Mohaghegh, N.; Abedini, A. Ternary Composite of TiO2 Nanotubes/Ti Plates Modified by g-C3N4 and SnO2 with Enhanced Photocatalytic Activity for Enhancing Antibacterial and Photocatalytic Activity. J. Photochem. Photobiol. B Biol. 2018, 178, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-P.; Huang, B.; Mao, C.-J.; Chen, J.-S.; Jin, B.-K. Electrochemiluminescence Aptasensor for Lincomycin Antigen Detection by Using a SnO2/Chitosan/g-C3N4 Nanocomposite. Talanta 2021, 233, 122546. [Google Scholar] [CrossRef]
- Ali, G.; Zaidi, S.J.A.; Basit, M.A.; Park, T.J. Synergetic Performance of Systematically Designed g-C3N4/RGO/SnO2 Nanocomposite for Photodegradation of Rhodamine-B Dye. Appl. Surf. Sci. 2021, 570, 151140. [Google Scholar] [CrossRef]
- Seo, Y.J.; Das, P.K.; Arunachalam, M.; Ahn, K.-S.; Ha, J.-S.; Kang, S.H. Drawing the Distinguished Graphite Carbon Nitride (g-C3N4) on SnO2 Nanoflake Film for Solar Water Oxidation. Int. J. Hydrogen Energy 2020, 45, 22567–22575. [Google Scholar] [CrossRef]
- Wu, H.; Yu, S.; Wang, Y.; Han, J.; Wang, L.; Song, N.; Dong, H.; Li, C. A Facile One-Step Strategy to Construct 0D/2D SnO2/g-C3N4 Heterojunction Photocatalyst for High-Efficiency Hydrogen Production Performance from Water Splitting. Int. J. Hydrogen Energy 2020, 45, 30142–30152. [Google Scholar] [CrossRef]
- Versaci, D.; Amici, J.; Francia, C.; Bodoardo, S. Simple Approach Using g-C3N4 to Enable SnO2 Anode High Rate Performance for Li Ion Battery. Solid State Ionics 2020, 346, 115210. [Google Scholar] [CrossRef]
- Tran, H.H.; Nguyen, P.H.; Le, M.L.P.; Kim, S.-J.; Vo, V. SnO2 Nanosheets/Graphite Oxide/g-C3N4 Composite as Enhanced Performance Anode Material for Lithium Ion Batteries. Chem. Phys. Lett. 2019, 715, 284–292. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials 2017, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Akhundi, A.; Habibi-Yangjeh, A. A Simple Large-Scale Method for Preparation of g-C3N4/SnO2 Nanocomposite as Visible-Light-Driven Photocatalyst for Degradation of an Organic Pollutant. Mater. Express 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X. SnO2 Nanocrystallines Decorated g-C3N4 Composites with Enhanced Visible-Light Photocatalytic Activity. Integr. Ferroelectr. 2019, 197, 121–132. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Peng, R. One-Step Synthesis of SnO 2 Nanoparticles-Loaded Graphitic Carbon Nitride and Their Application in Thermal Decomposition of Ammonium Perchlorate. New J. Chem. 2015, 39, 8703–8707. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, C.; Tang, H.; Sun, X.; Yang, J.; Cheng, X. One-Pot Synthesis of g-C3N4/V2O5 Composites for Visible Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2015, 358, 188–195. [Google Scholar] [CrossRef]
- Jayaraman, T.; Raja, S.A.; Priya, A.; Jagannathan, M.; Ashokkumar, M. Synthesis of a Visible-Light Active V2O5–g-C3N4 Heterojunction as an Efficient Photocatalytic and Photoelectrochemical Material. New J. Chem. 2015, 39, 1367–1374. [Google Scholar] [CrossRef]
- Jin, C.; Wang, M.; Li, Z.; Kang, J.; Zhao, Y.; Han, J.; Wu, Z. Two Dimensional Co3O4/g-C3N4 Z-Scheme Heterojunction: Mechanism Insight into Enhanced Peroxymonosulfate-Mediated Visible Light Photocatalytic Performance. Chem. Eng. J. 2020, 398, 125569. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Che, H.; Hu, H.; Hu, W.; Liu, C.; Ai, J.; Dong, H. Decoration of Mesoporous Co3O4 Nanospheres Assembled by Monocrystal Nanodots on g-C3N4 to Construct Z-Scheme System for Improving Photocatalytic Performance. Appl. Surf. Sci. 2018, 440, 308–319. [Google Scholar] [CrossRef]
- Qiao, Q.; Yang, K.; Ma, L.-L.; Huang, W.-Q.; Zhou, B.-X.; Pan, A.; Hu, W.; Fan, X.; Huang, G.-F. Facile in Situ Construction of Mediator-Free Direct Z-Scheme g-C3N4/CeO2 Heterojunctions with Highly Efficient Photocatalytic Activity. J. Phys. D Appl. Phys. 2018, 51, 275302. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Xu, H.; Xu, Y.; Xia, J.; Wang, K.; Li, H.; Cheng, X. Synthesis and Characterization of CeO2/g-C3N4 Composites with Enhanced Visible-Light Photocatatalytic Activity. Rsc Adv. 2013, 3, 22269–22279. [Google Scholar] [CrossRef]
- Zou, W.; Shao, Y.; Pu, Y.; Luo, Y.; Sun, J.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Enhanced Visible Light Photocatalytic Hydrogen Evolution via Cubic CeO2 Hybridized g-C3N4 Composite. Appl. Catal. B Environ. 2017, 218, 51–59. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Huang, L.; Xu, H.; Zhang, R.; Qu, M.; Gao, Q.; Li, H. g-C3N4 Modified Bi2O3 Composites with Enhanced Visible-Light Photocatalytic Activity. J. Phys. Chem. Solids 2015, 76, 112–119. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a Direct Z-Scheme Photocatalyst: Preparation and Characterization of Bi2O3/g-C3N4 with High Visible Light Activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, X.; Feng, Q.; Lin, X.; Huang, Y.; Huang, X.; Li, X.; Chen, K.; Zhao, B.; Zhang, Z. In-Situ Growth of β-Bi2O3 Nanosheets on g-C3N4 to Construct Direct Z-Scheme Heterojunction with Enhanced Photocatalytic Activities. J. Alloys Compd. 2021, 859, 157795. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Li, X.; Li, F.; Li, Y.; Zhao, J.; Liu, R. Construction of g-C3N4/Al2O3 Hybrids via in-Situ Acidification and Exfoliation with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2017, 394, 340–350. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Zhang, Z.-Y.; Fan, W.-L.; Hu, C.; Zhang, L.; Qi, J.-J. High-Performance g-C3N4 Added Carbon-Based Perovskite Solar Cells Insulated by Al2O3 Layer. Sol. Energy 2019, 193, 859–865. [Google Scholar]
- Shi, J.-W.; Zou, Y.; Cheng, L.; Ma, D.; Sun, D.; Mao, S.; Sun, L.; He, C.; Wang, Z. In-Situ Phosphating to Synthesize Ni2P Decorated NiO/g-C3N4 Pn Junction for Enhanced Photocatalytic Hydrogen Production. Chem. Eng. J. 2019, 378, 122161. [Google Scholar] [CrossRef]
- Li, F.; Zhao, Y.; Wang, Q.; Wang, X.; Hao, Y.; Liu, R.; Zhao, D. Enhanced Visible-Light Photocatalytic Activity of Active Al2O3/g-C3N4 Heterojunctions Synthesized via Surface Hydroxyl Modification. J. Hazard. Mater. 2015, 283, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, G.; Tsvetkov, M.; Spassov, T. Ammonia-Evaporation-Induced Construction of Three-Dimensional NiO/g-C3N4 Composite with Enhanced Adsorption and Visible Light-Driven Photocatalytic Performance. Superlattices Microstruct. 2018, 119, 122–133. [Google Scholar] [CrossRef]
- Sumathi, M.; Prakasam, A.; Anbarasan, P.M. Fabrication of Hexagonal Disc Shaped Nanoparticles g-C3N4/NiO Heterostructured Nanocomposites for Efficient Visible Light Photocatalytic Performance. J. Clust. Sci. 2019, 30, 757–766. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Zhang, R.; Cheng, X.; Xia, J.; Xu, Y.; Li, H. Synthesis and Characterization of g-C3N4/MoO3 Photocatalyst with Improved Visible-Light Photoactivity. Appl. Surf. Sci. 2013, 283, 25–32. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, J.; Chen, H.; Mao, M.; Liu, L.; She, X.; Ji, H.; Wu, X.; Yuan, S.; Xu, H. Construction of a Few-Layer g-C3N4/α-MoO3 Nanoneedles All-Solid-State Z-Scheme Photocatalytic System for Photocatalytic Degradation. J. Energy Chem. 2019, 29, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Qi, Y.; Hu, J.; An, W.; Lin, S.; Liang, Y.; Cui, W. Stable Cu2O@ g-C3N4 Core@ Shell Nanostructures: Efficient Visible-Light Photocatalytic Hydrogen Evolution. Mater. Lett. 2015, 158, 278–281. [Google Scholar] [CrossRef]
- Han, S.; Hu, X.; Yang, W.; Qian, Q.; Fang, X.; Zhu, Y. Constructing the Band Alignment of Graphitic Carbon Nitride (g-C3N4)/Copper (I) Oxide (Cu2O) Composites by Adjusting the Contact Facet for Superior Photocatalytic Activity. ACS Appl. Energy Mater. 2018, 2, 1803–1811. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, X.; Han, R.; Dai, Y.; Sun, Y.; Lin, Y.; Gao, D.; Wang, X.; Luo, C. Study on the Internal Electric Field in the Cu2O/g-C3N4 p–n Heterojunction Structure for Enhancing Visible Light Photocatalytic Activity. New J. Chem. 2020, 44, 1795–1805. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhardiya, S.R.; Asati, A.; Rai, V.K.; Singh, M.; Rai, A. Cu/Cu2O@ g-C3N4: Recyclable Photocatalyst under Visible Light to Access 2-Aryl-/Benzimidazoles/Benzothiazoles in Water. ChemistrySelect 2020, 5, 14270–14275. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Y.; Cai, Z.; She, C. Preparation of Porous g-C3N4/Ag/Cu2O: A New Composite with Enhanced Visible-light Photocatalytic Activity. Appl. Organomet. Chem. 2016, 30, 932–938. [Google Scholar] [CrossRef]
- Chaudhary, P.; Ingole, P.P. In-Situ Solid-State Synthesis of 2D/2D Interface between Ni/NiO Hexagonal Nanosheets Supported on g-C3N4 for Enhanced Photo-Electrochemical Water Splitting. Int. J. Hydrogen Energy 2020, 45, 16060–16070. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Fang, L.; Guo, Y.; Feng, Y.; Yang, M. Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite. Catalysts 2020, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Chen, K. A Novel Z-Scheme Visible Light Driven Cu2O/Cu/g-C3N4 Photocatalyst Using Metallic Copper as a Charge Transfer Mediator. Mol. Catal. 2017, 432, 187–195. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Zeng, H. Design of Cu-Cu2O/g-C3N4 Nanocomponent Photocatalysts for Hydrogen Evolution under Visible Light Irradiation Using Water-Soluble Erythrosin B Dye Sensitization. Appl. Surf. Sci. 2017, 391, 404–414. [Google Scholar] [CrossRef]
- Liu, C.; Mao, D.; Pan, J.; Qian, J.; Zhang, W.; Chen, F.; Chen, Z.; Song, Y. Fabrication of Highly Efficient Heterostructured Ag-CeO2/g-C3N4 Hybrid Photocatalyst with Enhanced Visible-Light Photocatalytic Activity. J. Rare Earths 2019, 37, 1269–1278. [Google Scholar] [CrossRef]
- Pinheiro, D.; Devi, K.R.S.; Jose, A.; Karthik, K.; Sugunan, S.; Mohan, M.K. Experimental Design for Optimization of 4-Nitrophenol Reduction by Green Synthesized CeO2/g-C3N4/Ag Catalyst Using Response Surface Methodology. J. Rare Earths 2020, 38, 1171–1177. [Google Scholar] [CrossRef]
- Yin, H.Y.; Zheng, Y.F.; Wang, L. Au/CeO2/g-C3N4 Nanocomposite Modified Electrode as Electrochemical Sensor for the Determination of Phenol. J. Nanosci. Nanotechnol. 2020, 20, 5539–5545. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Guo, Y.; He, Y.; Zhang, Y. A g-C3N4/Bi2O2CO3 Composite with High Visible-Light-Driven Photocatalytic Activity for Rhodamine B Degradation. Appl. Surf. Sci. 2014, 322, 249–254. [Google Scholar] [CrossRef]
- Mohammad, A.; Karim, M.R.; Khan, M.E.; Khan, M.M.; Cho, M.H. Biofilm-Assisted Fabrication of Ag@ SnO2-g-C3N4 Nanostructures for Visible Light-Induced Photocatalysis and Photoelectrochemical Performance. J. Phys. Chem. C 2019, 123, 20936–20948. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Karthik, R.; Chen, S.-M.; Kumar, J.V.; Prakash, K.; Muthuraj, V. Construction of Novel Pd/CeO2/g-C3N4 Nanocomposites as Efficient Visible-Light Photocatalysts for Hexavalent Chromium Detoxification. J. Colloid Interface Sci. 2017, 504, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-Scheme g-C3N4/Bi/BiVO4 Hybrid Photocatalysts toward Artificial Carbon Cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Han, C.; Zhang, R.; Ye, Y.; Wang, L.; Ma, Z.; Su, F.; Xie, H.; Zhou, Y.; Wong, P.K.; Ye, L. Chainmail Co-Catalyst of NiO Shell-Encapsulated Ni for Improving Photocatalytic CO2 Reduction over GC3N4. J. Mater. Chem. A 2019, 7, 9726–9735. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.; Li, J.; Li, X.; Wang, H.; Huo, P.; Yan, Y. CeO2/3D g-C3N4 Heterojunction Deposited with Pt Cocatalyst for Enhanced Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2021, 537, 147891. [Google Scholar] [CrossRef]
- Karimi, M.A.; Atashkadi, M.; Ranjbar, M.; Habibi-Yangjeh, A. Novel Visible-Light-Driven Photocatalyst of NiO/Cd/g-C3N4 for Enhanced Degradation of Methylene Blue. Arab. J. Chem. 2020, 13, 5810–5820. [Google Scholar] [CrossRef]
- Jia, Z.; Lyu, F.; Zhang, L.C.; Zeng, S.; Liang, S.X.; Li, Y.Y.; Lu, J. Pt Nanoparticles Decorated Heterostructured g-C3N4/Bi2MoO6 Microplates with Highly Enhanced Photocatalytic Activities under Visible Light. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhang, C.; Zhang, C.; Xin, S.; Zhang, P.; Shi, Q.; Zhang, D.; You, Y. Co3O4 Modified Ag/g-C3N4 Composite as a Bifunctional Cathode for Lithium-Oxygen Battery. J. Energy Chem. 2020, 41, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of Carbon Dots Modified MoO3/g-C3N4 Z-Scheme Photocatalyst with Enhanced Visible-Light Photocatalytic Activity for the Degradation of Tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Shi, W.; Wang, J.; Yang, S.; Lin, X.; Guo, F.; Shi, J. Fabrication of a Ternary Carbon Dots/CoO/g-C3N4 Nanocomposite Photocatalyst with Enhanced Visible-light-driven Photocatalytic Hydrogen Production. J. Chem. Technol. Biotechnol. 2020, 95, 2129–2138. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Naushad, M.; Stadler, F.J. CeO2/g-C3N4/V2O5 Ternary Nano Hetero-Structures Decorated with CQDs for Enhanced Photo-Reduction Capabilities under Different Light Sources: Dual Z-Scheme Mechanism. J. Alloys Compd. 2020, 838, 155692. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Qu, T.; Kang, Z.; Wang, D. Co3O4 and CDots Nanocrystals on g-C3N4 as a Synergetic Catalyst for Oxygen Reduction Reaction. Green Process. Synth. 2015, 4, 411–419. [Google Scholar] [CrossRef]
- Shekardasht, M.B.; Givianrad, M.H.; Gharbani, P.; Mirjafary, Z.; Mehrizad, A. Preparation of a Novel Z-Scheme g-C3N4/RGO/Bi2Fe4O9 Nanophotocatalyst for Degradation of Congo Red Dye under Visible Light. Diam. Relat. Mater. 2020, 109, 108008. [Google Scholar] [CrossRef]
- Gong, L.; Li, X.; Zhang, Q.; Huang, B.; Yang, Q.; Yang, G.; Liu, Y. Ultrafast and Large-Scale Synthesis of Co3O4 Quantum Dots-C3N4/RGO as an Excellent ORR Electrocatalyst via a Controllable Deflagration Strategy. Appl. Surf. Sci. 2020, 525, 146624. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, T.; Wu, T.; Zhang, N.; Yang, Q.; Sun, M.; Yan, L.; Du, B.; Wei, Q. A Label-Free Photoelectrochemical Aptasensing Platform Base on Plasmon Au Coupling with MOF-Derived In2O3@ g-C3N4 Nanoarchitectures for Tetracycline Detection. Sensors Actuators B Chem. 2019, 298, 126817. [Google Scholar] [CrossRef]
- Cui, Y.; Nengzi, L.; Gou, J.; Huang, Y.; Li, B.; Cheng, X. Fabrication of Dual Z-Scheme MIL-53 (Fe)/α-Bi2O3/g-C3N4 Ternary Composite with Enhanced Visible Light Photocatalytic Performance. Sep. Purif. Technol. 2020, 232, 115959. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Synthesis and Characterization of Novel Sm2O3/S-Doped g-C3N4 Nanocomposites with Enhanced Photocatalytic Activities under Visible Light Irradiation. Appl. Surf. Sci. 2018, 427, 375–387. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Z.; Xie, W.; Li, D.; Yan, G.; Guo, S.; Pan, C.; Wu, J. In Situ Fabrication of I-Doped Bi2O2CO3/g-C3N4 Heterojunctions for Enhanced Photodegradation Activity under Visible Light. J. Hazard. Mater. 2020, 385, 121622. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Hou, X.; Xie, W.; Wei, X.; He, D. Dramatic Improvement of Photocatalytic Activity for N-Doped Bi2O3/g-C3N4 Composites. Mater. Lett. 2015, 161, 640–643. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, X.; Sun, T. Facile Fabrication of In2O3/S-Doped g-C3N4 Heterojunction Hybrids for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Mater. Lett. 2020, 261, 127159. [Google Scholar] [CrossRef]
- Jin, X.; Guan, Q.; Tian, T.; Li, H.; Han, Y.; Hao, F.; Cui, Y.; Li, W.; Zhu, Y.; Zhang, Y. In2O3/Boron Doped g-C3N4 Heterojunction Catalysts with Remarkably Enhanced Visible-Light Photocatalytic Efficiencies. Appl. Surf. Sci. 2020, 504, 144241. [Google Scholar] [CrossRef]
- Liu, J.-L.; Jiang, J.; Zhang, J.-Q.; Chai, Y.-Q.; Xiao, Q.; Yuan, R. The Combination of Ternary Electrochemiluminescence System of g-C3N4 Nanosheet/TEA/Cu@ Cu2O and G-Quadruplex-Driven Regeneration Strategy for Ultrasensitive Bioanalysis. Biosens. Bioelectron. 2020, 152, 112006. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, W.; Naraginti, S.; Sivakumar, A.; Zhang, C. Fabrication of Novel Tetrahedral Ag3PO4/g-C3N4/BiVO4 Ternary Composite for Efficient Detoxification of Sulfamethoxazole. Process Saf. Environ. Prot. 2020, 143, 340–347. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Wang, Z.-W.; Song, Y.; Wang, M.; Zhang, Z.; Liu, C.-S. Bimetallic-Organic Framework Derived Porous Co3O4/Fe3O4/C-Loaded g-C3N4 Nanocomposites as Non-Enzymic Electrocatalysis Oxidization toward Ascorbic Acid, Dopamine Acid, and Uric Acid. Appl. Surf. Sci. 2018, 441, 694–707. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Wang, W.; Gao, P.; Zhong, S.; Zhang, L.; Zhao, Q.; Liu, B. Synthesizing Co3O4-BiVO4/g-C3N4 Heterojunction Composites for Superior Photocatalytic Redox Activity. Sep. Purif. Technol. 2020, 239, 116562. [Google Scholar] [CrossRef]
- Sudrajat, H.; Hartuti, S. Structural Properties and Catalytic Activity of a Novel Ternary CuO/GC3N4/Bi2O3 Photocatalyst. J. Colloid Interface Sci. 2018, 524, 227–235. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, J.; Zheng, H. Facile Preparation of Dual Z-Scheme Bi2O3/g-C3N4/Ag6Si2O7 Photocatalyst for Highly Efficient Visible-Light Photocatalytic Degradation of Organic Pollutants. Appl. Surf. Sci. 2020, 527, 146901. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Police, A.K.R.; Shim, J.; Byon, C. In Situ Fabrication of the Bi2O3–V2O5 Hybrid Embedded with Graphitic Carbon Nitride Nanosheets: Oxygen Vacancies Mediated Enhanced Visible-Light–Driven Photocatalytic Degradation of Organic Pollutants and Hydrogen Evolution. Appl. Surf. Sci. 2018, 447, 740–756. [Google Scholar] [CrossRef]
- Wu, S.; Guo, J.; Wang, Y. Bi2O2CO3–Bi2O2(OH)NO3/g-C3N4 Heterojunction as a Visible-Light-Driven Photocatalyst with Enhanced Photogenerated Charge Separation. J. Alloys Compd. 2020, 818, 152852. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, H.; Shen, M.; Yao, Y.; Gao, Q. Rationally Designed a g-C3N4/BiOI/Bi2O2CO3 Composite with Promoted Photocatalytic Activity. J. Alloys Compd. 2021, 853, 157307. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Ala’a, H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-Templated g-C3N4/Bi2O2CO3/CoFe2O4 Nano-Assembly for Visible and Solar Assisted Photo-Degradation of Paraquat, Nitrophenol Reduction and CO2 Conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Min, Z.; Wang, X.; Li, Y.; Jiang, J.; Li, J.; Qian, D.; Li, J. A Highly Efficient Visible-Light-Responding Cu2O∓TiO2/g-C3N4 Photocatalyst for Instantaneous Discolorations of Organic Dyes. Mater. Lett. 2017, 193, 18–21. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Nasalevich, M.A.; Savenije, T.J.; Kapteijn, F.; Gascon, J.; Kubacka, A.; Fernández-García, M. Enhancing Promoting Effects in g-C3N4-Mn+/CeO2-TiO2 Ternary Composites: Photo-Handling of Charge Carriers. Appl. Catal. B Environ. 2015, 176, 687–698. [Google Scholar] [CrossRef]
- da Silva Veras, T.; Mozer, T.S.; da Silva César, A. Hydrogen: Trends, Production and Characterization of the Main Process Worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- He, R.; Liang, H.; Li, C.; Bai, J. Enhanced Photocatalytic Hydrogen Production over Co3O4@ g-C3N4 Pn Junction Adhering on One-Dimensional Carbon Fiber. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124200. [Google Scholar] [CrossRef]
- Anandan, S.; Wu, J.J.; Bahnemann, D.; Emeline, A.; Ashokkumar, M. Crumpled Cu2O-g-C3N4 Nanosheets for Hydrogen Evolution Catalysis. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 527, 34–41. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, B.; Mao, L.; Cheng, C.; Hu, Y.; Wang, H.; Li, G.; Jing, D.; Liang, X. MoO3/g-C3N4 Z-Scheme (S-Scheme) System Derived from MoS2/Melamine Dual Precursors for Enhanced Photocatalytic H2 Evolution Driven by Visible Light. Int. J. Hydrogen Energy 2021, 46, 2927–2935. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Lobinsky, A.A.; Nevedomskiy, V.N.; Popkov, V.I. NiO-Decorated Graphitic Carbon Nitride toward Electrocatalytic Hydrogen Production from Ethanol. Dalt. Trans. 2020, 49, 12088–12097. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, J.; Wang, B.; Li, Q.; Chu, S. Hierarchical Cu2O Foam/g-C3N4 Photocathode for Photoelectrochemical Hydrogen Production. Appl. Surf. Sci. 2018, 427, 907–916. [Google Scholar] [CrossRef]
- Paul, A.M.; Sajeev, A.; Nivetha, R.; Gothandapani, K.; Bhardwaj, P.; Govardhan, K.; Raghavan, V.; Jacob, G.; Sellapan, R.; Jeong, S.K. Cuprous Oxide (Cu2O)/Graphitic Carbon Nitride (g-C3N4) Nanocomposites for Electrocatalytic Hydrogen Evolution Reaction. Diam. Relat. Mater. 2020, 107, 107899. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, J.; Rajendran, S.; Zhang, X.; Liu, R. Heterostructured D-Ti3C2/TiO2/g-C3N4 Nanocomposites with Enhanced Visible-light Photocatalytic Hydrogen Production Activity. ChemSusChem 2018, 11, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Sun, Y.; Li, H.; Liang, Z.; Wu, H.; Zhang, J.; Zeng, H.; Geyer, S.M.; Jiang, L. A Highly Active Three-Dimensional Z-Scheme ZnO/Au/g-C3N4 Photocathode for Efficient Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2020, 263, 118180. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Pu, Y.; Liu, A.; Chen, C.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y. Facile Ball-Milling Synthesis of CeO2/g-C3N4 Z-Scheme Heterojunction for Synergistic Adsorption and Photodegradation of Methylene Blue: Characteristics, Kinetics, Models, and Mechanisms. Chem. Eng. J. 2020, 470, 127719. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, J.; Yang, S.; Xu, Y.; Cai, X.; Li, X.; Zhang, X.; Peng, F.; Fang, Y. Electrodeposition of Cu2O/g-C3N4 Heterojunction Film on an FTO Substrate for Enhancing Visible Light Photoelectrochemical Water Splitting. Chin. J. Catal. 2017, 38, 365–371. [Google Scholar] [CrossRef]
- Ji, C.; Yin, S.-N.; Sun, S.; Yang, S. An in Situ Mediator-Free Route to Fabricate Cu2O/g-C3N4 Type-II Heterojunctions for Enhanced Visible-Light Photocatalytic H2 Generation. Appl. Surf. Sci. 2018, 434, 1224–1231. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.; Hu, J.; Liang, Y.; Cui, W. Efficient Visible-Light Photocatalytic Hydrogen Evolution and Enhanced Photostability of Core@ Shell Cu2O@ g-C3N4 Octahedra. Appl. Surf. Sci. 2015, 351, 1146–1154. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H. TiO2–g-C3N4 Composite Materials for Photocatalytic H2 Evolution under Visible Light Irradiation. J. Alloys Compd. 2011, 509, L26–L29. [Google Scholar] [CrossRef]
- Marchal, C.; Cottineau, T.; Méndez-Medrano, M.G.; Colbeau-Justin, C.; Caps, V.; Keller, V. Au/TiO2/GC3N4 Nanocomposites for Enhanced Photocatalytic H2 Production from Water under Visible Light Irradiation with Very Low Quantities of Sacrificial Agents. Adv. Energy Mater. 2018, 8, 1702142. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; You, M.; Chi, C.; Dong, Z.; Wang, B.; Zhu, M.; Zhao, W.; Song, C.; Zheng, Y.; Li, C. The Two Dimension Carbon Quantum Dots Modified Porous g-C3N4/TiO2 Nano-Heterojunctions for Visible Light Hydrogen Production Enhancement. Int. J. Hydrogen Energy 2018, 43, 6586–6593. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. ZnO Nanosheets Decorated with Graphite-like Carbon Nitride Quantum Dots as Photoanodes in Photoelectrochemical Water Splitting. ACS Appl. Nano Mater. 2020, 3, 1999–2007. [Google Scholar] [CrossRef]
- Qin, H.; Zuo, Y.; Jin, J.; Wang, W.; Xu, Y.; Cui, L.; Dang, H. ZnO Nanorod Arrays Grown on g-C3N4 Micro-Sheets for Enhanced Visible Light Photocatalytic H2 Evolution. RSC Adv. 2019, 9, 24483–24488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2017, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Gismondi, P.; Kolasinski, K.W.; Shumlas, S.L.; Rangan, S.; Eslami, B.; McConnell, A.; Bui, T.; Cunfer, K. Fabrication of Electrospun Nanofiber Composite of g-C3N4 and Au Nanoparticles as Plasmonic Photocatalyst. Surfaces Interfaces 2021, 26, 101367. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic Photocatalysis. Reports Prog. Phys. 2013, 76, 46401. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Dong, Q.; Sun, C.; Xia, D.; Huang, H.; Yang, G.; Wang, G.; Leung, D.Y.C. A Novel Au/g-C3N4 Nanosheets/CeO2 Hollow Nanospheres Plasmonic Heterojunction Photocatalysts for the Photocatalytic Reduction of Hexavalent Chromium and Oxidation of Oxytetracycline Hydrochloride. Chem. Eng. J. 2021, 409, 128185. [Google Scholar] [CrossRef]
- Bashiri, R.; Mohamed, N.M.; Suhaimi, N.A.; Shahid, M.U.; Kait, C.F.; Sufian, S.; Khatani, M.; Mumtaz, A. Photoelectrochemical Water Splitting with Tailored TiO2/SrTiO3@ g-C3N4 Heterostructure Nanorod in Photoelectrochemical Cell. Diam. Relat. Mater. 2018, 85, 5–12. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, F.; Weng, Y.; Yuan, G.; Fang, L.; You, L. Enhanced Photoelectrochemical Performance by Interface Engineering in Ternary g-C3N4/TiO2/PbTiO3 Films. Adv. Mater. Interfaces 2020, 7, 2000185. [Google Scholar] [CrossRef]
- Qu, A.; Xu, X.; Xie, H.; Zhang, Y.; Li, Y.; Wang, J. Effects of Calcining Temperature on Photocatalysis of g-C3N4/TiO2 Composites for Hydrogen Evolution from Water. Mater. Res. Bull. 2016, 80, 167–176. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Chen, Q.; Cao, C.; Zhao, Y.; Yang, W.; Zeng, L.; Huang, L. Fabrication of 0D/2D TiO2 Nanodots/g-C3N4 S-Scheme Heterojunction Photocatalyst for Efficient Photocatalytic Overall Water Splitting. Appl. Surf. Sci. 2021, 571, 151287. [Google Scholar] [CrossRef]
- Liu, J.; Yan, X.-T.; Qin, X.-S.; Wu, S.-J.; Zhao, H.; Yu, W.-B.; Chen, L.-H.; Li, Y.; Su, B.-L. Light-Assisted Preparation of Heterostructured g-C3N4/ZnO Nanorods Arrays for Enhanced Photocatalytic Hydrogen Performance. Catal. Today 2019, 355, 932–936. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhou, H.; Li, T.; Zhang, L. A Z-Scheme Mechanism of N-ZnO/g-C3N4 for Enhanced H2 Evolution and Photocatalytic Degradation. Appl. Surf. Sci. 2019, 466, 133–140. [Google Scholar] [CrossRef]
- Gu, Y.; Bao, A.; Zhang, X.; Yan, J.; Du, Q.; Zhang, M.; Qi, X. Facile Fabrication of Sulfur-Doped Cu2O and g-C3N4 with Z-Scheme Structure for Enhanced Photocatalytic Water Splitting Performance. Mater. Chem. Phys. 2021, 266, 124542. [Google Scholar] [CrossRef]
- Alhaddad, M.; Navarro, R.M.; Hussein, M.A.; Mohamed, R.M. Bi2O3/g-C3N4 Nanocomposites as Proficient Photocatalysts for Hydrogen Generation from Aqueous Glycerol Solutions beneath Visible Light. Ceram. Int. 2020, 46, 24873–24881. [Google Scholar] [CrossRef]
- Ma, R.; Dong, L.; Li, B.; Su, T.; Luo, X.; Qin, Z.; Ji, H. g-C3N4/BiYO3 Composite for Photocatalytic Hydrogen Evolution. ChemistrySelect 2018, 3, 5891–5899. [Google Scholar] [CrossRef]
- Xu, J.; Yu, H.; Guo, H. Synthesis and Behaviors of g-C3N4 Coupled with LaxCo3-XO4 Nanocomposite for Improved Photocatalytic Activeity and Stability under Visible Light. Mater. Res. Bull. 2018, 105, 342–348. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Kong, X.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. In Situ Synthesis of a Novel Mn3O4/g-C3N4 Pn Heterostructure Photocatalyst for Water Splitting. J. Colloid Interface Sci. 2021, 586, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, X.; Yang, P. Bifunctional Nitrogen-Doped Carbon Dots in g-C3N4/WOx Heterojunction for Enhanced Photocatalytic Water-Splitting Performance. Langmuir 2021, 37, 4236–4247. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, C.; Wang, H.; Dou, Y.; Shi, W.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. High-Performance NiO/g-C3N4 Composites for Visible-Light-Driven Photocatalytic Overall Water Splitting. Inorg. Chem. Front. 2018, 5, 1646–1652. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.-Y.; Liao, Y.-S.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-Fuels Conversion over In2O3/g-C3N4 Hybrid Photocatalysts. Appl. Catal. B Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, B.; Wen, T.; Zhang, S.; Zeng, M.; Hu, N.; Su, Y.; Yang, Z.; Yang, B. A Z-Scheme Photocatalyst for Enhanced Photocatalytic H2 Evolution, Constructed by Growth of 2D Plasmonic MoO3−x Nanoplates onto 2D g-C3N4 Nanosheets. J. Colloid Interface Sci. 2020, 567, 213–223. [Google Scholar] [CrossRef]
- Hou, H.L.; Gao, F.; Wang, L.; Shang, M.; Yang, Z.; Zheng, J.; Yang, W. Superior Ternary Hybrid Photocatalysts of TiO2/WO3/GC3N4 Thoroughly Mesoporous Nanofibers for Visible-Lightdriven Hydrogen Evolution. J. Mater. Chem. A 2016, 4, 6276–6281. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Lam, T.C.; Khan, A.; Vo, T.-T.T.; Nguyen, T.-Q.; Doan, V.D.; Tran, V.A. TiO2/Ti3C2/g-C3N4 Ternary Heterojunction for Photocatalytic Hydrogen Evolution. Chemosphere 2021, 285, 131429. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-Assembled Hierarchical Direct Z-Scheme g-C3N4/ZnO Microspheres with Enhanced Photocatalytic CO2 Reduction Performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Mulik, B.B.; Bankar, B.D.; Munde, A.V.; Chavan, P.P.; Biradar, A.V.; Sathe, B.R. Electrocatalytic and Catalytic CO2 Hydrogenation on ZnO/g-C3N4 Hybrid Nanoelectrodes. Appl. Surf. Sci. 2021, 538, 148120. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Chen, Z.; Li, J.; Li, X.; Huo, P.; Wang, Q. TiO2 Modified g-C3N4 with Enhanced Photocatalytic CO2 Reduction Performance. Solid State Sci. 2020, 100, 106099. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Bach, L.G.; Hanh, N.T.; Pham, T.-D.; Le Chi, N.T.P.; Tran, D.T.; Nguyen, M.V. The Superior Photocatalytic Activity of Nb Doped TiO2/g-C3N4 Direct Z-Scheme System for Efficient Conversion of CO2 into Valuable Fuels. J. Colloid Interface Sci. 2019, 540, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fu, L.; Yan, T.; Tian, W.; Ma, D.; Li, J.; Jiang, Y.; Wang, X. Improved Photocatalytic Activity of Porous ZnO Nanosheets by Thermal Deposition Graphene-like g-C3N4 for CO2 Reduction with H2O Vapor. Appl. Surf. Sci. 2020, 509, 144773. [Google Scholar] [CrossRef]
- Shen, D.; Li, X.; Ma, C.; Zhou, Y.; Sun, L.; Yin, S.; Huo, P.; Wang, H. Synthesized Z-Scheme Photocatalyst ZnO/g-C3N4 for Enhanced Photocatalytic Reduction of CO2. New J. Chem. 2020, 44, 16390–16399. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, B.; Liu, H.; Guo, H. Controlled Assemble of Hollow Heterostructured g-C3N4@ CeO2 with Rich Oxygen Vacancies for Enhanced Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2019, 243, 566–575. [Google Scholar] [CrossRef]
- Tang, J.; Guo, R.; Zhou, W.; Huang, C.; Pan, W. Ball-Flower like NiO/g-C3N4 Heterojunction for Efficient Visible Light Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2018, 237, 802–810. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, W.; Zhao, L.; Chen, H.; He, X.; Li, W.; Tian, P.; Huang, Z. g-C3N4 Foam/Cu2O QDs with Excellent CO2 Adsorption and Synergistic Catalytic Effect for Photocatalytic CO2 Reduction. Environ. Int. 2019, 130, 104898. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Pham, T.-D.; Nguyen, M.V.; Van Thuan, D.; Thao, P.; Trang, H.T.; Tran, D.T.; Minh, D.N.; Hanh, N.T.; Ngoc, H.M. Advanced NiMoO4/g-C3N4 Z-Scheme Heterojunction Photocatalyst for Efficient Conversion of CO2 to Valuable Products. J. Alloys Compd. 2020, 842, 155860. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Wu, M.; Du, Y.; Fan, X.; Wang, M.; Zhang, L.; Kong, Q.; Shi, J. Mesostructured CeO2/g-C3N4 Nanocomposites: Remarkably Enhanced Photocatalytic Activity for CO2 Reduction by Mutual Component Activations. Nano Energy 2016, 19, 145–155. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Ma, C.; Zhu, Z.; Song, X.; Wang, H.; Huo, P.; Li, X. Local Surface Plasma Resonance Effect Enhanced Z-Scheme ZnO/Au/g-C3N4 Film Photocatalyst for Reduction of CO2 to CO. Appl. Catal. B Environ. 2021, 283, 119638. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-Efficiency Conversion of CO2 to Fuel over ZnO/g-C3N4 Photocatalyst. Appl. Catal. B Environ. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Reli, M.; Huo, P.; Šihor, M.; Ambrozova, N.; Troppová, I.; Matejova, L.; Lang, J.; Svoboda, L.; Kuśtrowski, P.; Ritz, M. Novel TiO2/C3N4 Photocatalysts for Photocatalytic Reduction of CO2 and for Photocatalytic Decomposition of N2O. J. Phys. Chem. A 2016, 120, 8564–8573. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, C.; He, W.; Wei, Y.; Zhao, Z.; Liu, J. The Z-Scheme g-C3N4/3DOM-WO3 Photocatalysts with Enhanced Activity for CO2 Photoreduction into CO. Chin. Chem. Lett. 2021, in press. [CrossRef]
- Guo, H.; Wan, S.; Wang, Y.; Ma, W.; Zhong, Q.; Ding, J. Enhanced Photocatalytic CO2 Reduction over Direct Z-Scheme NiTiO3/g-C3N4 Nanocomposite Promoted by Efficient Interfacial Charge Transfer. Chem. Eng. J. 2021, 412, 128646. [Google Scholar] [CrossRef]
- Carmen, Z.; Daniela, S. Textile Organic Dyes-Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents-a Critical Overview; IntechOpen: Rijekta, Croatia, 2012. [Google Scholar]
- Clarke, E.A.; Anliker, R. Organic Dyes and Pigments. In Anthropogenic Compounds; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals. 2020, 10, 1604. [Google Scholar] [CrossRef]
- Devi, K.R.S.; Mathew, S.; Rajan, R.; Georgekutty, J.; Kasinathan, K.; Pinheiro, D.; Sugunan, S. Biogenic Synthesis of g-C3N4/Bi2O3 Heterojunction with Enhanced Photocatalytic Activity and Statistical Optimization of Reaction Parameters. Appl. Surf. Sci. 2019, 494, 465–476. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Z.; Li, D.; Xie, W.; Yan, G.; Guo, S. Visible-Light Responsive Z-Scheme Bi@ β-Bi2O3/g-C3N4 Heterojunction for Efficient Photocatalytic Degradation of 2, 3-Dihydroxynaphthalene. Chem. Eng. J. 2020, 392, 123686. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Bafaqeer, A. Constructing a STable 2D Layered Ti3C2 MXene Cocatalyst-Assisted TiO2/g-C3N4/Ti3C2 Heterojunction for Tailoring Photocatalytic Bireforming of Methane under Visible Light. Energy Fuels 2020, 34, 9810–9828. [Google Scholar]
- Ma, J.; Tan, X.; Yu, T.; Li, X. Fabrication of g-C3N4/TiO2 Hierarchical Spheres with Reactive {001} TiO2 Crystal Facets and Its Visible-Light Photocatalytic Activity. Int. J. Hydrogen Energy 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- Rasheed, H.U.; Lv, X.; Wei, W.; Yaseen, W.; Ullah, N.; Xie, J.; Zhu, W. Synthesis and Studies of ZnO Doped with g-C3N4 Nanocomposites for the Degradation of Tetracycline Hydrochloride under the Visible Light Irradiation. J. Environ. Chem. Eng. 2019, 7, 103152. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, F.; Goei, R.; Zhou, Y. Construction of WO3–g-C3N4 Composites as Efficient Photocatalysts for Pharmaceutical Degradation under Visible Light. Catal. Sci. Technol. 2017, 7, 2591–2600. [Google Scholar] [CrossRef]
- Lu, N.; Wang, P.; Su, Y.; Yu, H.; Liu, N.; Quan, X. Construction of Z-Scheme g-C3N4/RGO/WO3 with in Situ Photoreduced Graphene Oxide as Electron Mediator for Efficient Photocatalytic Degradation of Ciprofloxacin. Chemosphere 2019, 215, 444–453. [Google Scholar] [CrossRef]
- Truong, H.B.; Huy, B.T.; Ray, S.K.; Lee, Y.-I.; Cho, J.; Hur, J. H2O2-Assisted Photocatalysis for Removal of Natural Organic Matter Using Nanosheet C3N4-WO3 Composite under Visible Light and the Hybrid System with Ultrafiltration. Chem. Eng. J. 2020, 399, 125733. [Google Scholar] [CrossRef]
- Shafawi, A.N.; Mahmud, R.A.; Ali, K.A.; Putri, L.K.; Rosli, N.I.M.; Mohamed, A.R. Bi2O3 Particles Decorated on Porous g-C3N4 Sheets: Enhanced Photocatalytic Activity through a Direct Z-Scheme Mechanism for Degradation of Reactive Black 5 under UV–Vis Light. J. Photochem. Photobiol. A Chem. 2020, 389, 112289. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Li, L.; Gu, P.; Wen, T.; Khan, A.; Li, S.; Li, B.; Wang, S.; Wang, X. Enhanced Visible-Light-Induced Photoactivity of Type-II CeO2/g-C3N4 Nanosheet toward Organic Pollutants Degradation. ACS Sustain. Chem. Eng. 2019, 7, 9699–9708. [Google Scholar]
- Barathi, D.; Rajalakshmi, N.; Ranjith, R.; Sangeetha, R.; Meyvel, S. Controllable Synthesis of CeO2/g-C3N4 Hybrid Catalysts and Its Structural, Optical and Visible Light Photocatalytic Activity. Diam. Relat. Mater. 2021, 111, 108161. [Google Scholar] [CrossRef]
- Cui, S.; Xie, B.; Li, R.; Pei, J.; Tian, Y.; Zhang, J.; Xing, X. g-C3N4/CeO2 Binary Composite Prepared and Its Application in Automobile Exhaust Degradation. Materials. 2020, 13, 1274. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Xu, J.; Zhang, X.; Hang, Z.; Jia, Y.; Wang, S. Synthesis of g-C3N4/CeO2 Nanocomposites with Improved Catalytic Activity on the Thermal Decomposition of Ammonium Perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Chen, G.; Bian, S.; Guo, C.-Y.; Wu, X. Insight into the Z-Scheme Heterostructure WO3/g-C3N4 for Enhanced Photocatalytic Degradation of Methyl Orange. Mater. Lett. 2019, 236, 596–599. [Google Scholar] [CrossRef]
- Guan, R.; Li, J.; Zhang, J.; Zhao, Z.; Wang, D.; Zhai, H.; Sun, D. Photocatalytic Performance and Mechanistic Research of ZnO/g-C3N4 on Degradation of Methyl Orange. ACS omega 2019, 4, 20742–20747. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Zhang, X.; Liang, T.; Xu, X.; Qiu, L.; Li, P.; Duo, S. Facile In-Situ Synthesis of 2D/3D g-C3N4/Cu2O Heterojunction for High-Performance Photocatalytic Dye Degradation. Mater. Res. Express 2020, 7, 15524. [Google Scholar] [CrossRef]
- Tian, Q.; Shen, X.; Wang, Z.; Zhu, B.; Osotsi, M.I.; Xie, X.; Jin, Y.; Chen, Z.; Zhang, L. Growth of Cu2O Spherical Superstructures on g-C3N4 as Efficient Visible-Light-Driven p–n Heterojunction Photocatalysts for Degrading Various Organic Pollutants. J. Nanosci. Nanotechnol. 2018, 18, 7355–7363. [Google Scholar] [CrossRef]
- Rabé, K.; Liu, L.; Nahyoon, N.A.; Zhang, Y.; Idris, A.M. Enhanced Rhodamine B and Coking Wastewater Degradation and Simultaneous Electricity Generation via Anodic g-C3N4/Fe0 (1%)/TiO2 and Cathodic WO3 in Photocatalytic Fuel Cell System under Visible Light Irradiation. Electrochim. Acta 2019, 298, 430–439. [Google Scholar] [CrossRef]
- Yu, T.; Liu, L.; Yang, F. Heterojunction between Anodic TiO2/g-C3N4 and Cathodic WO3/W Nano-Catalysts for Coupled Pollutant Removal in a Self-Biased System. Chin. J. Catal. 2017, 38, 270–277. [Google Scholar] [CrossRef]
- Tripathi, A.; Narayanan, S. Impact of TiO2 and TiO2/g-C3N4 Nanocomposite to Treat Industrial Wastewater. Environ. Nanotechnol. Monit. Manag. 2018, 10, 280–291. [Google Scholar] [CrossRef]
- Pawar, R.C.; Son, Y.; Kim, J.; Ahn, S.H.; Lee, C.S. Integration of ZnO with g-C3N4 Structures in Core–Shell Approach via Sintering Process for Rapid Detoxification of Water under Visible Irradiation. Curr. Appl. Phys. 2016, 16, 101–108. [Google Scholar] [CrossRef]
- Zhang, S.; Su, C.; Ren, H.; Li, M.; Zhu, L.; Ge, S.; Wang, M.; Zhang, Z.; Li, L.; Cao, X. In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter. Nanomaterials 2019, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, M.; Yang, J.; Li, X.; Hu, T.; Wang, J.; Sui, Y.; Wu, X.; Kong, L. Synergistic Effect of Efficient Adsorption g-C3N4/ZnO Composite for Photocatalytic Property. J. Phys. Chem. Solids 2014, 75, 441–446. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.M.A.; Sarathbavan, M.; Ramamurthi, K.; Sethuraman, K.; Babu, R.R. Synergetic Effect of g-C3N4/ZnO Binary Nanocomposites Heterojunction on Improving Charge Carrier Separation through 2D/1D Nanostructures for Effective Photocatalytic Activity under the Sunlight Irradiation. Sep. Purif. Technol. 2020, 244, 116356. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.; Li, Y.; Xu, F.; Gao, Z.; Zhang, X.; Song, Y.; Xu, H.; Li, H. Facile Synthesis of Few-Layer g-C3N4/ZnO Composite Photocatalyst for Enhancing Visible Light Photocatalytic Performance of Pollutants Removal. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 537, 516–523. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, H.; Zhang, D.; Hu, S.; Min, T.; Liu, B.; Yang, C.; Pu, W.; Hu, J.; Yang, J. Enhanced Visible-Light Driven Photocatalytic Activity of Hybrid ZnO/g-C3N4 by High Performance Ball Milling. J. Photochem. Photobiol. A Chem. 2018, 350, 1–9. [Google Scholar] [CrossRef]
- Tang, Q.; Meng, X.; Wang, Z.; Zhou, J.; Tang, H. One-Step Electrospinning Synthesis of TiO2/g-C3N4 Nanofibers with Enhanced Photocatalytic Properties. Appl. Surf. Sci. 2018, 430, 253–262. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.-G.; He, H.-W.; Wang, X.-X.; Zhang, J.; Zhang, Q.-Q.; Tong, Y.-F.; Liu, H.-L.; Ramakrishna, S.; Yan, S.-Y. One-Step Synthesis Heterostructured g-C3N4/TiO2 Composite for Rapid Degradation of Pollutants in Utilizing Visible Light. Nanomaterials 2018, 8, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-Assisted Surface Modification Enhances the Visible Light Photocatalytic Performance of g-C3N4@ C-TiO2 Direct Z-Scheme Heterojunctions. Chin. J. Catal. 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, L.; Peng, J.; Han, J.; Guo, S.; Zhang, L.; Han, Z.; Komarneni, S. TiO2@ g-C3N4 Core/Shell Spheres with Uniform Mesoporous Structures for High Performance Visible-Light Photocatalytic Application. Ceram. Int. 2019, 45, 18844–18851. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Li, F.; Xue, Y.; Liu, R.; Hao, Y. In Situ Microwave-Assisted Synthesis of Porous N-TiO2/g-C3N4 Heterojunctions with Enhanced Visible-Light Photocatalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Song, G.; Chu, Z.; Jin, W.; Sun, H. Enhanced Performance of g-C3N4/TiO2 Photocatalysts for Degradation of Organic Pollutants under Visible Light. Chin. J. Chem. Eng. 2015, 23, 1326–1334. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q. Synthesis of Core-Shell TiO2@ g-C3N4 Hollow Microspheres for Efficient Photocatalytic Degradation of Rhodamine B under Visible Light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gao, Q.; Qin, J.; Hui, X.; Ye, Z.; Li, J.; Ma, Z. A Facile Method for Fabricating TiO2/g-C3N4 Hollow Nanotube Heterojunction and Its Visible Light Photocatalytic Performance. Mater. Lett. 2018, 217, 1–4. [Google Scholar] [CrossRef]
- Christoforidis, K.C. g-C3N4/Ag3PO4 Based Binary and Ternary Heterojunction for Improved Photocatalytic Removal of Organic Pollutants. Int. J. Environ. Anal. Chem. published on line only. 2021. [Google Scholar] [CrossRef]
- Dutta, D.P.; Dagar, D. Efficient Selective Sorption of Cationic Organic Pollutant from Water and Its Photocatalytic Degradation by AlVO4/g-C3N4 Nanocomposite. J. Nanosci. Nanotechnol. 2020, 20, 2179–2194. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-Situ Synthesis of Direct Solid-State Z-Scheme V2O5/g-C3N4 Heterojunctions with Enhanced Visible Light Efficiency in Photocatalytic Degradation of Pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Liu, Y.; Ren, M.; Zhang, X.; Ding, X.; Guo, Y.; Yang, Y. Acid-Induced Molecule Self-Assembly Synthesis of Z-Scheme WO3/g-C3N4 Heterojunctions for Robust Photocatalysis against Phenolic Pollutants. Chem. Eng. J. 2021, 403, 126354. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Zhang, J.; Li, C.; Yang, X.; Chen, X.; Wang, F.; Chen, C. Crystalline Phase Engineering in WO3/g-C3N4 Composites for Improved Photocatalytic Performance under Visible Light. Mater. Res. Express 2020, 7, 65503. [Google Scholar] [CrossRef]
- He, R.; Zhou, J.; Fu, H.; Zhang, S.; Jiang, C. Room-Temperature in Situ Fabrication of Bi2O3/g-C3N4 Direct Z-Scheme Photocatalyst with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2018, 430, 273–282. [Google Scholar] [CrossRef]
- Chen, D.; Wu, S.; Fang, J.; Lu, S.; Zhou, G.; Feng, W.; Yang, F.; Chen, Y.; Fang, Z. A Nanosheet-like α-Bi2O3/g-C3N4 Heterostructure Modified by Plasmonic Metallic Bi and Oxygen Vacancies with High Photodegradation Activity of Organic Pollutants. Sep. Purif. Technol. 2018, 193, 232–241. [Google Scholar] [CrossRef]
- Fan, G.; Ma, Z.; Li, X.; Deng, L. Coupling of Bi2O3 Nanoparticles with g-C3N4 for Enhanced Photocatalytic Degradation of Methylene Blue. Ceram. Int. 2021, 47, 5758–5766. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Fabrication of a Novel Bi2O3 Nanoparticle Impregnated Nitrogen Vacant 2D g-C3N4 Nanosheet Z Scheme Photocatalyst for Improved Degradation of Methylene Blue Dye under LED Light Illumination. Appl. Surf. Sci. 2020, 507, 144965. [Google Scholar] [CrossRef]
- She, X.; Xu, H.; Wang, H.; Xia, J.; Song, Y.; Yan, J.; Xu, Y.; Zhang, Q.; Du, D.; Li, H. Controllable Synthesis of CeO2/g-C3N4 Composites and Their Applications in the Environment. Dalt. Trans. 2015, 44, 7021–7031. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Charkhan, H.; Sabbaghan, M. Synthesis and Application of a Powerful Heterogeneous Photo-Fenton Catalyst Based on RGO/g-C3N4/Fe3O4/TiO2 Nano-Composite for the Removal of Sewage Contaminants. J. Electrochem. Soc. 2020, 167, 67515. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Reddy, P.A.K.; Shim, J.; Byon, C. Visible-Light-Driven Photocatalytic Activity of SnO2–ZnO Quantum Dots Anchored on g-C3N4 Nanosheets for Photocatalytic Pollutant Degradation and H2 Production. ACS omega 2018, 3, 7587–7602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danish, M.; Muneer, M. Novel ZnSQDs-SnO2/g-C3N4 Nanocomposite with Enhanced Photocatalytic Performance for the Degradation of Different Organic Pollutants in Aqueous Suspension under Visible Light. J. Phys. Chem. Solids 2021, 149, 109785. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Liu, D. Co3O4/g-C3N4 Hybrids for Gas-Phase Hg0 Removal at Low Temperature. Processes 2019, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Lu, Z.; Ji, R.; Zhang, M.; Yi, C.; Yan, Y. Biomass Carbon Modified Z-Scheme g-C3N4/Co3O4 Heterojunction with Enhanced Visible-Light Photocatalytic Activity. Catal. Commun. 2018, 112, 49–52. [Google Scholar] [CrossRef]
- Yuan, X.; Duan, S.; Wu, G.; Sun, L.; Cao, G.; Li, D.; Xu, H.; Li, Q.; Xia, D. Enhanced Catalytic Ozonation Performance of Highly Stabilized Mesoporous ZnO Doped g-C3N4 Composite for Efficient Water Decontamination. Appl. Catal. A Gen. 2018, 551, 129–138. [Google Scholar] [CrossRef]
- Zuo, S.; Chen, Y.; Liu, W.; Yao, C.; Li, X.; Li, Z.; Ni, C.; Liu, X. A Facile and Novel Construction of Attapulgite/Cu2O/Cu/g-C3N4 with Enhanced Photocatalytic Activity for Antibiotic Degradation. Ceram. Int. 2017, 43, 3324–3329. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. Enhanced Performance of a Novel Superparamagnetic g-C3N4/NiO/ZnO/Fe3O4 Nanohybrid Photocatalyst for Removal of Esomeprazole: Effects of Reaction Parameters, Co-Existing Substances and Water Matrices. Chem. Eng. J. 2020, 395, 124969. [Google Scholar] [CrossRef]
- Huo, R.; Yang, X.-L.; Yang, J.-Y.; Yang, S.-Y.; Xu, Y.-H. Self-Assembly Synthesis of BiVO4/Polydopamine/g-C3N4 with Enhanced Visible Light Photocatalytic Performance. Mater. Res. Bull. 2018, 98, 225–230. [Google Scholar] [CrossRef]
- Ding, J.; Lu, S.; Shen, L.; Yan, R.; Zhang, Y.; Zhang, H. Enhanced Photocatalytic Reduction for the Dechlorination of 2-Chlorodibenzo-p-Dioxin by High-Performance g-C3N4/NiO Heterojunction Composites under Ultraviolet-Visible Light Illumination. J. Hazard. Mater. 2020, 384, 121255. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, D.; Li, R.; Chen, P.; Zhang, Q.; Liu, H.; Lv, W.; Liu, G.; Feng, Y. One-Step Synthesis of Phosphorus/Oxygen Co-Doped g-C3N4/Anatase TiO2 Z-Scheme Photocatalyst for Significantly Enhanced Visible-Light Photocatalysis Degradation of Enrofloxacin. J. Hazard. Mater. 2020, 386, 121634. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, S.; Ren, F.; Wang, Z.; Lei, J.; Wang, X.; Cheng, T.; Li, L. Synthesis of TiO2@ g-C3N4 Core-Shell Nanorod Arrays with Z-Scheme Enhanced Photocatalytic Activity under Visible Light. J. Colloid Interface Sci. 2017, 508, 419–425. [Google Scholar] [CrossRef]
- Karpuraranjith, M.; Chen, Y.; Rajaboopathi, S.; Ramadoss, M.; Srinivas, K.; Yang, D.; Wang, B. Three-Dimensional Porous MoS2 Nanobox Embedded g-C3N4@ TiO2 Architecture for Highly Efficient Photocatalytic Degradation of Organic Pollutant. J. Colloid Interface Sci. 2022, 605, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhao, D.; Sun, Y.; Sheng, X.; Zhao, J.; Guo, J.; Zhou, B. G–C3N4–and Polyaniline-Co-Modified TiO2 Nanotube Arrays for Significantly Enhanced Photocatalytic Degradation of Tetrabromobisphenol A under Visible Light. Chemosphere 2020, 252, 126468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rao, L.; Wang, P.; Shi, Z.; Zhang, L. Photocatalytic Activity of N-TiO2/O-Doped N Vacancy g-C3N4 and the Intermediates Toxicity Evaluation under Tetracycline Hydrochloride and Cr (VI) Coexistence Environment. Appl. Catal. B Environ. 2020, 262, 118308. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and Uniform TiO2@ g-C3N4 Core-Shell Quantum Heterojunction for Photocatalytic Degradation of Tetracycline Antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P. Visible-Light Activation of Persulfate by TiO2/g-C3N4 Photocatalyst toward Efficient Degradation of Micropollutants. Chem. Eng. J. 2020, 384, 123245. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Lian, J.; Xu, H.; She, X.; Li, H. g-C3N4/TiO2 Nanocomposites for Degradation of Ciprofloxacin under Visible Light Irradiation. ChemistrySelect 2016, 1, 5679–5685. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and Characterization of Efficient ZnO/g-C3N4 Nanocomposites Photocatalyst for Photocatalytic Degradation of Methylene Blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-Scheme 2D/3D g-C3N4@ ZnO with Enhanced Photocatalytic Activity for Cephalexin Oxidation under Solar Light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Prabhu, S.; Pudukudy, M.; Harish, S.; Navaneethan, M.; Sohila, S.; Murugesan, K.; Ramesh, R. Facile Construction of Djembe-like ZnO and Its Composite with g-C3N4 as a Visible-Light-Driven Heterojunction Photocatalyst for the Degradation of Organic Dyes. Mater. Sci. Semicond. Process. 2020, 106, 104754. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic Polyacrylamide-Assisted Construction of Thin 2D-2D WO3/g-C3N4 Step-Scheme Heterojunction for Enhanced Tetracycline Degradation under Visible Light Irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Senthil, R.A.; Selvi, A.; Arunachalam, P.; Kumar, C.K.S.; Madhavan, J.; Boddula, R.; Pothu, R.; Al-Mayouf, A.M. A Study of Photocatalytic and Photoelectrochemical Activity of As-Synthesized WO3/g-C3N4 Composite Photocatalysts for AO7 Degradation. Mater. Sci. Energy Technol. 2020, 3, 43–50. [Google Scholar] [CrossRef]
- Navarro-Aguilar, A.I.; Obregón, S.; Sanchez-Martinez, D.; Hernández-Uresti, D.B. An Efficient and Stable WO3/g-C3N4 Photocatalyst for Ciprofloxacin and Orange G Degradation. J. Photochem. Photobiol. A Chem. 2019, 384, 112010. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Harish, S.; Archana, J.; Navaneethan, M. Fabrication of Novel Hybrid Z-Scheme WO3@g-C3N4@ MWCNT Nanostructure for Photocatalytic Degradation of Tetracycline and the Evaluation of Antimicrobial Activity. Chemosphere 2021, 287, 132050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Y.; Zhou, K.; Ba, D.; Ao, Y.; Wang, P. In-Situ Construction of Z-Scheme g-C3N4/WO3 Composite with Enhanced Visible-Light Responsive Performance for Nitenpyram Degradation. Chin. Chem. Lett. 2021, 32, 2179–2182. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, Z.; Tang, H.; Jiang, C. Fabrication of Flower-like Direct Z-Scheme β-Bi2O3/g-C3N4 Photocatalyst with Enhanced Visible Light Photoactivity for Rhodamine B Degradation. Appl. Surf. Sci. 2018, 436, 162–171. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Yin, B.; Li, D.; Zhang, Z.; Mao, B.; Fan, W.; Gu, W.; Shi, W. Promoting Visible-Light-Induced Photocatalytic Degradation of Tetracycline by an Efficient and Stable Beta-Bi2O3@ g-C3N4 Core/Shell Nanocomposite. Chem. Eng. J. 2018, 338, 137–146. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Hu, Z. Nano-Sized g-C3N4 Thin Layer@ CeO2 Sphere Core-Shell Photocatalyst Combined with H2O2 to Degrade Doxycycline in Water under Visible Light Irradiation. Sep. Purif. Technol. 2019, 227, 115665. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Yao, J. Shuttle-like CeO2/g-C3N4 Composite Combined with Persulfate for the Enhanced Photocatalytic Degradation of Norfloxacin under Visible Light. Ecotoxicol. Environ. Saf. 2020, 190, 110062. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, L.; Li, Z.; Li, H.; Wu, J. Synthesis of Novel Kaolin-Supported g-C3N4/CeO2 Composites with Enhanced Photocatalytic Removal of Ciprofloxacin. Materials. 2020, 13, 3811. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Long, J.; Jiang, T.; Shao, L.; Li, D.; Xie, X.; Xu, F. Magnetic Fe3O4/CeO2/g-C3N4 Composites with a Visible-Light Response as a High Efficiency Fenton Photocatalyst to Synergistically Degrade Tetracycline. Sep. Purif. Technol. 2021, 278, 119609. [Google Scholar] [CrossRef]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Xiao, X.; Zhang, Y.; Guo, L. Novel Visible Light Induced Co3O4-g-C3N4 Heterojunction Photocatalysts for Efficient Degradation of Methyl Orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Qiu, L.-G.; Xiao, J.-D.; Ye, S.; Jiang, X.; Yuan, Y.-P. Inorganic–Organic Hybrid NiO–g-C3N4 Photocatalyst for Efficient Methylene Blue Degradation Using Visible Light. RSC Adv. 2014, 4, 22491–22496. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Annalakshmi, M.; Chen, S.-M.; Chen, T.-W. Sonochemical Synthesis of Molybdenum Oxide (MoO3) Microspheres Anchored Graphitic Carbon Nitride (g-C3N4) Ultrathin Sheets for Enhanced Electrochemical Sensing of Furazolidone. Ultrason. Sonochem. 2019, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kim, D.-H. Heterojunction C3N4/MoO3 Microcomposite for Highly Efficient Photocatalytic Oxidation of Rhodamine B. Appl. Surf. Sci. 2020, 511, 145595. [Google Scholar] [CrossRef]
- Xue, S.; Wu, C.; Pu, S.; Hou, Y.; Tong, T.; Yang, G.; Qin, Z.; Wang, Z.; Bao, J. Direct Z-Scheme Charge Transfer in Heterostructured MoO3/g-C3N4 Photocatalysts and the Generation of Active Radicals in Photocatalytic Dye Degradations. Environ. Pollut. 2019, 250, 338–345. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Yu, H.; Wan, J.; Liu, L.; Yi, K.; Zhang, W.; Zhang, C. Construction of MoO3 Nanopaticles/g-C3N4 Nanosheets 0D/2D Heterojuntion Photocatalysts for Enhanced Photocatalytic Degradation of Antibiotic Pollutant. Chemosphere 2021, 282, 131049. [Google Scholar] [CrossRef] [PubMed]
- Adorna Jr, J.; Annadurai, T.; Bui, T.A.N.; Tran, H.L.; Lin, L.-Y.; Doong, R.-A. Indirect Z-Scheme Nitrogen-Doped Carbon Dot Decorated Bi2MoO6/g-C3N4 Photocatalyst for Enhanced Visible-Light-Driven Degradation of Ciprofloxacin. Chem. Eng. J. 2021, 422, 130103. [Google Scholar]
- Van Pham, V.; Mai, D.-Q.; Bui, D.-P.; Van Man, T.; Zhu, B.; Zhang, L.; Sangkaworn, J.; Tantirungrotechai, J.; Reutrakul, V.; Cao, T.M. Emerging 2D/0D g-C3N4/SnO2 S-Scheme Photocatalyst: New Generation Architectural Structure of Heterojunctions toward Visible-Light-Driven NO Degradation. Environ. Pollut. 2021, 286, 117510. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Liu, Y.; Yu, J.; Wang, F.; Tong, J.; Su, B.; Wang, Q. Fabricating a g-C3N4/CuO x Heterostructure with Tunable Valence Transition for Enhanced Photocatalytic Activity. RSC Adv. 2016, 6, 39774–39783. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jing, Q.; Tang, Q.; Mu, Y.; Du, A. Facile Synthesis of g-C3N4 Nanosheets/ZnO Nanocomposites with Enhanced Photocatalytic Activity in Reduction of Aqueous Chromium (VI) under Visible Light. Nanomaterials 2016, 6, 173. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, I.M.; Kalimuthu, S.; Ponniah, G. Highly Active ZnO Modified g-C3N4 Nanocomposite for Dye Degradation under UV and Visible Light with Enhanced Stability and Antimicrobial Activity. Compos. Commun. 2017, 5, 64–71. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C. Hydrothermally Synthesized Ternary Heterostructured MoS2/Al2O3/g-C3N4 Photocatalyst. Mater. Res. Bull. 2017, 96, 233–245. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen Defects-Mediated Z-Scheme Charge Separation in g-C3N4/ZnO Photocatalysts for Enhanced Visible-Light Degradation of 4-Chlorophenol and Hydrogen Evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zeng, F.; Sun, C.; Wu, J.; Xie, Y.; Zhang, F.; Rao, S.; Wang, F.; Zhang, J.; Zhao, J. Gd2O3 Nanoparticles Modified g-C3N4 with Enhanced Photocatalysis Activity for the Degradation of Organic Pollutants1. J. Rare Earths 2021, 39, 1353–1361. [Google Scholar] [CrossRef]
- Chen, D.; Xie, Z.; Zeng, Y.; Lv, W.; Zhang, Q.; Wang, F.; Liu, G.; Liu, H. Accelerated Photocatalytic Degradation of Quinolone Antibiotics over Z-Scheme MoO3/g-C3N4 Heterostructure by Peroxydisulfate under Visible Light Irradiation: Mechanism; Kinetic; and Products. J. Taiwan Inst. Chem. Eng. 2019, 104, 250–259. [Google Scholar] [CrossRef]
- Devi KR, S.; Mathew, S.; Rajan, R.; Georgekutty, J.; Pinheiro, D.; Ananthapadmanabhan, U.; Sundararajan, M. Synthesis and Characterization of CeO2/Bi2O3/GC3N4 Ternary Z-scheme Nanocomposite. Int. J. Appl. Ceram. Technol. 2020, 17, 2346–2356. [Google Scholar] [CrossRef]
- Bajiri, M.A.; Hezam, A.; Namratha, K.; Viswanath, R.; Drmosh, Q.A.; Naik, H.S.B.; Byrappa, K. CuO/ZnO/g-C3N4 Heterostructures as Efficient Visible Light-Driven Photocatalysts. J. Environ. Chem. Eng. 2019, 7, 103412. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. Facile Fabrication of g-C3N4 Supported Fe3O4 Nanoparticles/ZnO Nanorods: A Superlative Visible Light Responsive Architecture for Express Degradation of Pantoprazole. Chem. Eng. J. 2020, 387, 123766. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Cui, S. Hydrothermal Synthesis of Fe3O4/TiO2/g-C3N4: Advanced Photocatalytic Application. Appl. Surf. Sci. 2019, 488, 887–895. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, H.; Yu, H. Modified g-C3N4/TiO2 Nanosheets/ZnO Ternary Facet Coupled Heterojunction for Photocatalytic Degradation of p-Toluenesulfonic Acid (p-TSA) under Visible Light. Phys. E Low-dimensional Syst. Nanostruct. 2017, 85, 1–6. [Google Scholar]
- Tahir, M.B.; Sagir, M.; Shahzad, K. Removal of Acetylsalicylate and Methyl-Theobromine from Aqueous Environment Using Nano-Photocatalyst WO3-TiO2@g-C3N4 Composite. J. Hazard. Mater. 2019, 363, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Meng, F.; Khan, M.W.; Qin, R.; Liu, X. Synthesis of Hollow TiO2@ g-C3N4/Co3O4 Core-Shell Microspheres for Effective Photooxidation Degradation of Tetracycline and MO. Ceram. Int. 2020, 46, 13133–13143. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Hoffmann, M.R.; Wang, Q.; Cong, Y.; Wang, Q.; Jin, H. Synthesis of g-C3N4/Bi2O3/TiO2 Composite Nanotubes: Enhanced Activity under Visible Light Irradiation and Improved Photoelectrochemical Activity. RSC Adv. 2015, 5, 48983–48991. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, R.; Zhang, Z.; Cao, J.; Ma, T. Host–Guest Recognition on 2D Graphitic Carbon Nitride for Nanosensing. Adv. Mater. Interfaces 2019, 6, 1901429. [Google Scholar]
- Liu, J.-W.; Luo, Y.; Wang, Y.-M.; Duan, L.-Y.; Jiang, J.-H.; Yu, R.-Q. Graphitic Carbon Nitride Nanosheets-Based Ratiometric Fluorescent Probe for Highly Sensitive Detection of H2O2 and Glucose. ACS Appl. Mater. Interfaces 2016, 8, 33439–33445. [Google Scholar] [CrossRef]
- Kundu, M.K.; Sadhukhan, M.; Barman, S. Ordered Assemblies of Silver Nanoparticles on Carbon Nitride Sheets and Their Application in the Non-Enzymatic Sensing of Hydrogen Peroxide and Glucose. J. Mater. Chem. B 2015, 3, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Jiang, G.; Huang, Q.; Zhou, H. High-Sensitive Detection of Dopamine Using Graphitic Carbon Nitride by Electrochemical Method. Mater. Res. Bull. 2016, 74, 271–277. [Google Scholar] [CrossRef]
- Zuo, F.; Jin, L.; Fu, X.; Zhang, H.; Yuan, R.; Chen, S. An Electrochemiluminescent Sensor for Dopamine Detection Based on a Dual-Molecule Recognition Strategy and Polyaniline Quenching. Sensors Actuators B Chem. 2017, 244, 282–289. [Google Scholar] [CrossRef]
- Zou, J.; Wu, S.; Liu, Y.; Sun, Y.; Cao, Y.; Hsu, J.-P.; Wee, A.T.S.; Jiang, J. An Ultra-Sensitive Electrochemical Sensor Based on 2D g-C3N4/CuO Nanocomposites for Dopamine Detection. Carbon N. Y. 2018, 130, 652–663. [Google Scholar] [CrossRef]
- Rahman, N.; Yang, J.; Sohail, M.; Khan, R.; Iqbal, A.; Maouche, C.; Khan, A.A.; Husain, M.; Khattak, S.A.; Khan, S.N. Insight into Metallic Oxide Semiconductor (SnO2, ZnO, CuO, α-Fe2O3, WO3)-Carbon Nitride (g-C3N4) Heterojunction for Gas Sensing Application. Sensors Actuators A Phys. 2021, 332, 113128. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Li, P.-P.; Cao, Y.; Mao, C.-J.; Jin, B.-K.; Zhu, J.-J. TiO2/g-C3N4/CdS Nanocomposite-Based Photoelectrochemical Biosensor for Ultrasensitive Evaluation of T4 Polynucleotide Kinase Activity. Anal. Chem. 2018, 91, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, L.; Zhou, Y.; Yin, H.; Ai, S. Enhanced Photoelectrochemical Method for Sensitive Detection of Protein Kinase A Activity Using TiO2/g-C3N4, PAMAM Dendrimer, and Alkaline Phosphatase. Anal. Chem. 2017, 89, 2369–2376. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, T.; Wang, C.; Liu, D. UV-Light-Assisted Ethanol Sensing Characteristics of g-C3N4/ZnO Composites at Room Temperature. Appl. Surf. Sci. 2018, 441, 317–323. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Sun, G.; Luo, N.; Zhang, B.; Zhang, Z. Synthesis of a Flower-like g-C3N4/ZnO Hierarchical Structure with Improved CH4 Sensing Properties. Nanomaterials 2019, 9, 724. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Bai, J.; Dai, M.; Liu, K.; Liu, Y.; Zhou, L.; Liu, F.; Liu, F.; Gao, Y.; Yan, X. Visible Light Activated Excellent NO2 Sensing Based on 2D/2D ZnO/g-C3N4 Heterojunction Composites. Sens. Actuators B Chem. 2020, 304, 127287. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, L.; Wan, X.; Song, H.; Lv, Y. Fabrication of α-Fe2O3/g-C3N4 Composites for Cataluminescence Sensing of H2S. Sensors Actuators B Chem. 2015, 211, 370–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chu, X.; Liang, S.; Kong, L. Preparation of g-C3N4–SnO2 Composites for Application as Acetic Acid Sensor. J. Alloys Compd. 2020, 832, 153355. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V.; Dahiya, M.S.; Sharma, A.; Nehra, S.P.; Duhan, S.; Kailasam, K. An Excellent Humidity Sensor Based on In–SnO2 Loaded Mesoporous Graphitic Carbon Nitride. J. Mater. Chem. A 2017, 5, 14134–14143. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Liu, Y.; Wu, S.; Zou, J. A Facile One-Pot Preparation of Co3O4/g-C3N4 Heterojunctions with Excellent Electrocatalytic Activity for the Detection of Environmental Phenolic Hormones. Appl. Surf. Sci. 2018, 430, 362–370. [Google Scholar] [CrossRef]
- Hassannezhad, M.; Hosseini, M.; Ganjali, M.R.; Arvand, M. A Graphitic Carbon Nitride (g-C3N4/Fe3O4) Nanocomposite: An Efficient Electrode Material for the Electrochemical Determination of Tramadol in Human Biological Fluids. Anal. Methods 2019, 11, 2064–2071. [Google Scholar] [CrossRef]
- Karthika, A.; Suganthi, A.; Rajarajan, M. An In-Situ Synthesis of Novel V2O5/g-C3N4/PVA Nanocomposite for Enhanced Electrocatalytic Activity toward Sensitive and Selective Sensing of Folic Acid in Natural Samples. Arab. J. Chem. 2020, 13, 3639–3652. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. Fabrication of g-C3N4/NiO Heterostructured Nanocomposite Modified Glassy Carbon Electrode for Quercetin Biosensor. Ultrason. Sonochem. 2018, 41, 651–660. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, F.; Han, Q.; Hu, C.; Lv, L.; Chen, N.; Qu, L. Spontaneous Formation of Cu2O–g-C3N4 Core–Shell Nanowires for Photocurrent and Humidity Responses. Nanoscale 2015, 7, 9694–9702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, J.; Sun, Y.; Wu, S.; Cao, Y.; Gong, W.; Zou, J. NiO and Co3O4 Co-Doped g-C3N4 Nanocomposites with Excellent Photoelectrochemical Properties under Visible Light for Detection of Tetrabromobisphenol-A. RSC Adv. 2017, 7, 36015–36020. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Cui, C.; Su, M.; Wang, Y.; Wei, Q.; Tan, W. Construction of Self-Powered Cytosensing Device Based on ZnO Nanodisks@ g-C3N4 Quantum Dots and Application in the Detection of CCRF-CEM Cells. Nano Energy 2018, 46, 101–109. [Google Scholar] [CrossRef]
- Han, Z.; Luo, M.; Weng, Q.; Chen, L.; Chen, J.; Li, C.; Zhou, Y.; Wang, L. ZnO Flower-Rod/g-C3N4-Gold Nanoparticle-Based Photoelectrochemical Aptasensor for Detection of Carcinoembryonic Antigen. Anal. Bioanal. Chem. 2018, 410, 6529–6538. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J.; Zhou, J. Low-Temperature Fabrication of Macroporous Scaffolds through Foaming and Hydration of Tricalcium Silicate Paste and Their Bioactivity. J. Mater. Sci. 2010, 45, 961–968. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Bai, S.; Jiang, J.; Ong, W.-J.; Peng, J.; Xiong, Z.; Liao, G.; Zou, J.; Li, N. Oxygen Vacancy Mediated Step-Scheme Heterojunction of WO2. 9/g-C3N4 for Efficient Electrochemical Sensing of 4-Nitrophenol. Chem. Eng. J. Adv. 2021, 8, 100175. [Google Scholar] [CrossRef]
- Wang, H.; Liang, D.; Xu, Y.; Liang, X.; Qiu, X.; Lin, Z. A Highly Efficient Photoelectrochemical Sensor for Detection of Chlorpyrifos Based on 2D/2D β-Bi2O3/g-C3N4 Heterojunctions. Environ. Sci. Nano 2021, 8, 773–783. [Google Scholar]
- Pu, Y.; Wu, Y.; Yu, Z.; Lu, L.; Wang, X. Simultaneous Determination of Cd2+ and Pb2+ by an Electrochemical Sensor Based on Fe3O4/Bi2O3/C3N4 Nanocomposites. Talanta Open 2021, 3, 100024. [Google Scholar] [CrossRef]
- Bilal, S.; Hassan, M.M.; ur Rehman, M.F.; Nasir, M.; Sami, A.J.; Hayat, A. An Insect Acetylcholinesterase Biosensor Utilizing WO3/g-C3N4 Nanocomposite Modified Pencil Graphite Electrode for Phosmet Detection in Stored Grains. Food Chem. 2021, 346, 128894. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, Z.; Tang, L.; Ouyang, X.; Zhu, X.; Chen, L.; Fan, X.; Zhou, Z.; Wang, J. Self-Powered Photoelectrochemical Aptasensor for Oxytetracycline Cathodic Detection Based on a Dual Z-Scheme WO3/g-C3N4/MnO2 Photoanode. Anal. Chem. 2021, 26, 9129–9138. [Google Scholar] [CrossRef]
- Mao, L.; Xue, X.; Xu, X.; Wen, W.; Chen, M.-M.; Zhang, X.; Wang, S. Heterostructured CuO-g-C3N4 Nanocomposites as a Highly Efficient Photocathode for Photoelectrochemical Aflatoxin B1 Sensing. Sensors Actuators B Chem. 2021, 329, 129146. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical Sterilization of Microbial Cells by Semiconductor Powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Ma, J.; Wang, X.; Wang, J.; Xia, S.; Zhao, J. Removal of Microcystis Aeruginosa and Microcystin-LR Using a Graphitic-C3N4/TiO2 Floating Photocatalyst under Visible Light Irradiation. Chem. Eng. J. 2018, 348, 380–388. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Tian, J.; Liu, Y.; Wang, Y.; Yang, C. A Study on the Photocatalytic Sterilization Performance and Mechanism of Fe-SnO2/g-C3N4 Heterojunction Materials. New J. Chem. 2020, 44, 9456–9465. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Lee, J.H.; Kim, H.-Y.; Park, S.-J. Novel Magnetically Separable Silver-Iron Oxide Nanoparticles Decorated Graphitic Carbon Nitride Nano-Sheets: A Multifunctional Photocatalyst via One-Step Hydrothermal Process. J. Colloid Interface Sci. 2017, 496, 343–352. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Zhang, J.; Han, X.; Shi, H. Visible-Light-Driven g-C3N4/Cu2O Heterostructures with Efficient Photocatalytic Activities for Tetracycline Degradation and Microbial Inactivation. J. Photochem. Photobiol. A Chem. 2019, 378, 1–8. [Google Scholar] [CrossRef]
- Vignesh, S.; Suganthi, S.; Sundar, J.K.; Raj, V. Construction of α-Fe2O3/CeO2 Decorated g-C3N4 Nanosheets for Magnetically Separable Efficient Photocatalytic Performance under Visible Light Exposure and Bacterial Disinfection. Appl. Surf. Sci. 2019, 488, 763–777. [Google Scholar] [CrossRef]
- Shanmugam, V.; Jeyaperumal, K.S.; Mariappan, P.; Muppudathi, A.L. Fabrication of Novel g-C3N4 Based MoS 2 and Bi2O3 Nanorod Embedded Ternary Nanocomposites for Superior Photocatalytic Performance and Destruction of Bacteria. New J. Chem. 2020, 44, 13182–13194. [Google Scholar] [CrossRef]
- Induja, M.; Sivaprakash, K.; Karthikeyan, S. Facile Green Synthesis and Antimicrobial Performance of Cu2O Nanospheres Decorated g-C3N4 Nanocomposite. Mater. Res. Bull. 2019, 112, 331–335. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly Efficient g-C3N4/TiO2/Kaolinite Composite with Novel Three-Dimensional Structure and Enhanced Visible Light Responding Ability towards Ciprofloxacin and S. Aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a Visible-Light-Driven Photocatalytic Membrane by g-C3N4 Quantum Dots and TiO2 Nanotube Array for Enhanced Water Treatment. Sci. Rep. 2017, 7, 1–7. [Google Scholar]
- Xu, J.; Li, Y.; Zhou, X.; Li, Y.; Gao, Z.; Song, Y.; Schmuki, P. Graphitic C3N4-Sensitized TiO2 Nanotube Layers: A Visible-Light Activated Efficient Metal-Free Antimicrobial Platform. Chem. Eur. J. 2016, 22, 3947–3951. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.P.; Awasthi, G.P.; Lee, J.; Park, C.H.; Kim, C.S. Synthesis, Characterization, Organic Compound Degradation Activity and Antimicrobial Performance of g-C3N4 Sheets Customized with Metal Nanoparticles-Decorated TiO2 Nanofibers. RSC Adv. 2016, 6, 55079–55091. [Google Scholar] [CrossRef]
- Jiang, G.; Cao, J.; Chen, M.; Zhang, X.; Dong, F. Photocatalytic NO Oxidation on N-Doped TiO2/g-C3N4 Heterojunction: Enhanced Efficiency, Mechanism and Reaction Pathway. Appl. Surf. Sci. 2018, 458, 77–85. [Google Scholar] [CrossRef]
- Vo, V.; Thi, X.D.N.; Jin, Y.-S.; Thi, G.L.; Nguyen, T.T.; Duong, T.Q.; Kim, S.-J. SnO2 Nanosheets/g-C3N4 Composite with Improved Lithium Storage Capabilities. Chem. Phys. Lett. 2017, 674, 42–47. [Google Scholar] [CrossRef]
- Montazeri, S.M.; Sadrnezhaad, S.K. Kinetics of Sulfur Removal from Tehran Vehicular Gasoline by g-C3N4/SnO2 Nanocomposite. ACS omega 2019, 4, 13180–13188. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Feng, Y.; Li, M.; Li, W.; Wei, H.; Song, D. Smart Hybrids of Zn2GeO4 Nanoparticles and Ultrathin g-C3N4 Layers: Synergistic Lithium Storage and Excellent Electrochemical Performance. Adv. Funct. Mater. 2015, 25, 6858–6866. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Wu, L.; Li, C.-F.; Hu, Z.-Y.; Liu, J.; Deng, Z.; Chen, L.-H.; Li, Y.; Su, B.-L. In-Situ Growing Mesoporous CuO/O-Doped g-C3N4 Nanospheres for Highly Enhanced Lithium Storage. ACS Appl. Mater. Interfaces 2019, 11, 32957–32968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, L.; Chang, X.; Chen, H.; Li, Y.; Fan, Y.; Wang, J.; Cui, D.; Xue, C. Combining In-Situ Sedimentation and Carbon-Assisted Synthesis of Co3O4/g-C3N4 Nanocomposites for Improved Supercapacitor Performance. Diam. Relat. Mater. 2021, 111, 108165. [Google Scholar] [CrossRef]
- Bai, L.; Huang, H.; Zhang, S.; Hao, L.; Zhang, Z.; Li, H.; Sun, L.; Guo, L.; Huang, H.; Zhang, Y. Photocatalysis-Assisted Co3O4/g-C3N4 p–n Junction All-Solid-State Supercapacitors: A Bridge between Energy Storage and Photocatalysis. Adv. Sci. 2020, 7, 2001939. [Google Scholar] [CrossRef] [PubMed]
- Kavil, J.; Anjana, P.M.; Joshy, D.; Babu, A.; Raj, G.; Periyat, P.; Rakhi, R.B. g-C3N4/CuO and g-C3N4/Co3O4 Nanohybrid Structures as Efficient Electrode Materials in Symmetric Supercapacitors. RSC Adv. 2019, 9, 38430–38437. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Sun, L.; Wu, D.; Li, X.; Li, J.; Huo, P.; Wang, H.; Yan, Y. Preparation of 3D Porous g-C3N4@ V2O5 Composite Electrode via Simple Calcination and Chemical Precipitation for Supercapacitors. J. Alloys Compd. 2020, 817, 152707. [Google Scholar] [CrossRef]
- Klose, W.; Rincón, S. Adsorption and Reaction of NO on Activated Carbon in the Presence of Oxygen and Water Vapour. Fuel 2007, 86, 203–209. [Google Scholar] [CrossRef]
- Ou, M.; Zhong, Q.; Zhang, S.; Yu, L. Ultrasound Assisted Synthesis of Heterogeneous g-C3N4/BiVO4 Composites and Their Visible-Light-Induced Photocatalytic Oxidation of NO in Gas Phase. J. Alloys Compd. 2015, 626, 401–409. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Li, Q.; Li, F.; Fan, Z.; Wang, H.; Wang, Y.; Zheng, B.; Wu, G. Fe3+ Doping Promoted N2 Photofixation Ability of Honeycombed Graphitic Carbon Nitride: The Experimental and Density Functional Theory Simulation Analysis. Appl. Catal. B Environ. 2017, 201, 58–69. [Google Scholar] [CrossRef]
- Kong, Y.; Lv, C.; Zhang, C.; Chen, G. Cyano Group Modified g-C3N4: Molten Salt Method Achievement and Promoted Photocatalytic Nitrogen Fixation Activity. Appl. Surf. Sci. 2020, 515, 146009. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Yong, Y.; Xu, Y.; Wang, X.; Yao, X. One-Step Synthesis of Iodine-Doped g-C3N4 with Enhanced Photocatalytic Nitrogen Fixation Performance. Appl. Surf. Sci. 2020, 510, 145413. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, L.; Huang, M.; Tu, H.; Kong, Z.; Liang, Z.; Shao, Y.; Hao, X.; Wu, Y. Energy Band Matching WO3/B-Doped g-C3N4 Z-Scheme Photocatalyst to Fix Nitrogen Effectively. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 633, 127830. [Google Scholar] [CrossRef]
- Liu, X.; Han, X.; Liang, Z.; Xue, Y.; Zhou, Y.; Zhang, X.; Cui, H.; Tian, J. Phosphorous-Doped 1T-MoS2 Decorated Nitrogen-Doped g-C3N4 Nanosheets for Enhanced Photocatalytic Nitrogen Fixation. J. Colloid Interface Sci. 2022, 605, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, X.; Li, X.; Shao, C.; Liu, Y. TiO2/SrTiO3/g-C3N4 Ternary Heterojunction Nanofibers: Gradient Energy Band, Cascade Charge Transfer, Enhanced Photocatalytic Hydrogen Evolution, and Nitrogen Fixation. Nanoscale 2020, 12, 8320–8329. [Google Scholar] [CrossRef]
- Camussi, I.; Mannucci, B.; Speltini, A.; Profumo, A.; Milanese, C.; Malavasi, L.; Quadrelli, P. g-C3N4-Singlet Oxygen Made Easy for Organic Synthesis: Scope and Limitations. ACS Sustain. Chem. Eng. 2019, 7, 8176–8182. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Khosla, A.; Khan, M.; Mishra, P.; Ansari, W.A.; Syed, M.A.; Hahn, Y.-B. Recent Advances in Nanostructured Graphitic Carbon Nitride as a Sensing Material for Heavy Metal Ions. J. Electrochem. Soc. 2019, 167, 37519. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.-M.; Yang, X.-F.; Jiao, M.-G.; Zhou, Z.; Zhang, M.-H.; Wang, D.-H.; Bu, X.-H. Electronic Structure of Heterojunction MoO2/g-C3N4 Catalyst for Oxidative Desulfurization. Appl. Catal. B Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]
- Lei, G.; Zhao, W.; Shen, L.; Liang, S.; Au, C.; Jiang, L. Isolated Iron Sites Embedded in Graphitic Carbon Nitride (g-C3N4) for Efficient Oxidative Desulfurization. Appl. Catal. B Environ. 2020, 267, 118663. [Google Scholar] [CrossRef]
- Ma, R.; Guo, J.; Wang, D.; He, M.; Xun, S.; Gu, J.; Zhu, W.; Li, H. Preparation of Highly Dispersed WO3/Few Layer g-C3N4 and Its Enhancement of Catalytic Oxidative Desulfurization Activity. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 572, 250–258. [Google Scholar] [CrossRef]






















| Doping Element | Ec | Eg | Ev | Application | Further Explanation | Ref | |
|---|---|---|---|---|---|---|---|
| Phosphorus | −1.11 eV | 2.55 eV | 1.44 eV | Catalytic aromatic alcohols | The presence of P enhances the aldehyde selectivity. | [73] | |
| −0.33 eV | 2.58 eV | 2.25 eV | Photocatalytic hydrogen evolution | The g-C3N4 tube doped with P improves light absorption. The quantum efficiency of the P-doped g-C3N4 tube is 4.7 and 22.4 times higher than that of the g-C3N4 tube and bulk g-C3N4. | [74] | ||
| −1.34 eV | 2.79 eV | 1.44 eV | Photocatalytic CO2 conversion | The P-modified g-C3N4 demonstrates the highest photocatalytic efficiency. | [75] | ||
| −1.17 eV | 2.69 eV | 1.52 eV | Photocatalytic hydrogen evolution | The P-doped structure has a high efficiency in accordance with the recombination, migration, and separation of electron-hole pairs. | [76] | ||
| Sulfur | −1.04 eV | 2.92 eV | 1.88 eV | Photocatalytic nitrogen fixation | Sulfur enhances the adsorption and activation of N2 molecules of g-C3N4 porous nanosheets and uses for photocatalytic nitrogen fixation. | [77] | |
| −1.23 eV | 2.80 eV | 1.57 eV | Photocatalytic hydrogen evolution | The H2 generation rate of N-doped MoS2 and S-doped g-C3N4 is about 23 and 38 times higher than that of pure SCN and NMS with 28.8 μmol/g/h and 17.4 μmol/g/h, respectively. | [78] | ||
| −1.3 eV | 2.67 eV | 1.34 eV | Photocatalytic hydrogen evolution | The BiPO4/S-C3N4 improves photocatalytic activity by facilitating carrier transportation. | [79] | ||
| −1.3 eV | 2.69 eV | 1.39 eV | Photocatalytic bisphenol degradation | Ag–S-C3N4 enhanced the photocatalytic activity since Ag has a great electron storage ability. | [80] | ||
| −1.32 eV | 2.66 eV | 1.34 eV | Photocatalytic hydrogen evolution | Sulfur promotes the photocatalytic ability of hydrogen evolution about four times higher than the bulk g-C3N4. | [81] | ||
| Oxygen | −0.88 eV | 2.61 eV | 1.73 eV | Photocatalytic CO2 reduction | The porous O-doped graphitic carbon nitride reveals enhanced photocatalytic activity. | [82] | |
| −0.76 eV | 2.57 eV | 1.84 eV | Photocatalytic hydrogen evolution | This result of the band edge value is related to the 1.1% oxygen content mass percentage. | [83] | ||
| −0.37 eV | 2.53 eV | 2.15 eV | This result of the band edge value is related to the 2.3% oxygen content mass percentage. | ||||
| −1.08 eV | 2.93 eV | 1.85 eV | Photocatalytic hydrogen evolution and 2,4-dinitrophenol | The oxygen dopant with Pt exhibits excellent photocatalytic hydrogen evolution in overall water splitting with 29.6 μmol/(g·h), and O-g-C3N4 NR reached up to approximately 100% removal efficiency of 2,4-dinitrophenol within 75 min. | [84] | ||
| 1.51 eV | 2.70 eV | −1.19 eV | photocatalytic water splitting | This band edge value is related to CN-x = 0 (x refers to the quantity of citric acid (gr)). | [85] | ||
| 1.50 eV | 2.62 eV | −1.16 eV | This band edge value is related to CN-0.2. | ||||
| 1.46 eV | 2.52 eV | −1.06 eV | This band edge value is related to CN-0.4. | ||||
| 1.46 eV | 2.49 eV | −1.03 eV | This band edge value is related to CN-0.6. | ||||
| Carbon | −1.13 eV | 2.54 eV | 1.41 eV | Thermal oxidation etching process | C-doped g-C3N4 improves the catalytic activity by extending the visible light absorption. | [86] | |
| Boron | −0.8 eV | 2.8 eV | 2 eV | Photocatalytic Oxygen evolution, Cr(VI) reduction | This structure is used for Cr(VI) reduction and O2 generation simultaneously. | [87] | |
| Nitrogen | −0.5 eV | 2.4 eV | 1.9 eV | Photocatalytic tetracycline degradation | This band edge value is related to the nitrogen-doped g-C3N4 used for Photocatalytic tetracycline degradation. | [88] | |
| −0.6 eV | 2.5 eV | 1.9 | This band edge value is related to the nitrogen-doped g-C3N4 nanosheets. | ||||
| −0.33 eV | 1.82 eV | 1.49 eV | Photocatalytic phenol degradation | N-doped g-C3N4 possesses a narrow bandgap since the N atom introduced an inter-bandgap, resulting in the redshift in the absorption UV-vis peak. | [89] | ||
| Metal | Na | −1.16 eV | 2.77 eV | 1.36 eV | 17α-ethynylestradiol mineralization | The photostable Na doped g-C3N4 content causes the photoabsorption enhancement. | [70] |
| K | −1.08 eV | 2.72 eV | 1.64 eV | Photocatalytic CO2 reduction | K content causes defects leading to improving catalytic activity by reducing the electron-hole recombination. | [90] | |
| Ti | −1.02 eV | 2.5 eV | 1.48 eV | Photocatalytic enhancement | Ti-doped g-C3N4 caused narrower bandgap and reduced carrier recombination resulting in higher absorption. | [91] | |
| Mn | −0.59 eV | 2.56 eV | 1.97 eV | Photocatalytic methylene blue degradation | The Mn-doped g-C3N4 nanoribbon reveals a great potential photocatalytic agent for water splitting coupling with MB degradation. | [92] | |
| Ag | - | 2.60 eV | - | Photocatalytic oxidation of methylene blue | The higher Ag content leads to the lower bandgap of the structure, and a lower recombination rate is observed in the Ag-doped g-C3N4. | [93] | |
| Fe | −1.10 eV | 2.50 eV | 1.40 eV | Environmental pollution control | Fe3+ with nitrogen in heptazine forms a σ-π bond and can accelerate the electron-hole separation. | [94] | |
| Co | −0.36 eV | 2.62 eV | 2.26 eV | Photo-electrochemical water oxidation | Co-doped g-C3N4 reduces the electron-hole recombination rate and demonstrates promising photocurrent and electrical conductivity. | [95] | |
| Co-doped | P, O | −0.80 eV | 2.30 eV | 1.50 eV | Photocatalytic fluoroquinolone antibiotics degradation | The degradation rate of enrofloxacin was 6.2 times higher for phosphorus and oxygen co-doped graphitic carbon nitride (POCN) than g-C3N4. | [96] |
| P, S | - | 2.6 eV | - | Photocatalytic hydrogen evolution | The high photocatalytic activity can be observed because of the synergic impact of P and S co-doping. | [97] | |
| B, F | - | 2.72 eV | - | Photocatalytic hydrogen evolution | B, F co-doped g-C3N4 improves the charge generation and the separation efficiency. | [98] | |
| Na, O | - | 2.72 eV | - | Photocatalytic hydrogen evolution | Na, O co-doped g-C3N4 reveals that photocatalytic H2 production activity was seven-fold improved by enhancing absorption of UV-vis spectra. | [99] | |
| Photocatalyst | Type of Heterojunction | Source of Light | Highest Photocatalytic Rate | Ref |
|---|---|---|---|---|
| TiO2-g-C3N4 | Type II | Asahi Spectra Hal-320 (300 mW cm−2) with a 420 nm cut off filter (λ > 420 nm) | 3.6 μmol·h−1 | [115] |
| TiO2-g-C3N4 | Type II | Xenon lamp with a 320 nm cut off filter (λ > 320 nm) | 76.25 μmol·h−1 | [414] |
| C-doped TiO2-g-C3N4 | Type II | 300 W Xe lamp (PLS-SXE300) with a 420 nm cutoff filter (λ > 420 nm) | 35.6 μmol·g−1·h−1 | [128] |
| TiO2-g-C3N4 decorated by Co-Pi | Type II | 300 W Xe lamp coupled with a monochromator | - | |
| TiO2 nanodots/g-C3N4 | S-scheme | 300 W Xe lamp (LANPU) with a 300 nm cutoff filter (λ > 300 nm) | The H2 and O2 evolution rate is 1318.3 and 638.7 μmol g−1, respectively, (roughly as same as the stoichiometric ratio of evolved H2 to O2) | [415] |
| ZnO-g-C3N4 | Type II | PLS-SXE-300C lamp with an UV light intensity of 34 mW/cm2 and visible-light intensity of 158 mW/cm2 | - | [416] |
| N-doped ZnO-g-C3N4 | Z-scheme | PLS-SXE-300C UV lamp with a 420 nm cut off filter (λ > 420 nm) | 152.7 μmol·h−1 | [417] |
| g-C3N4-WO3 | - | 300-W Xe lamp (PLS-SXE300) with a 420 nm cutoff filter (λ > 420 nm) | 963 μmol·g−1·h−1 | [141] |
| O-g-C3N4/WO3 | Z-scheme | 300 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | 15,142 μmol·g−1 | [265] |
| S-Cu2O/g-C3N4 | Z-scheme | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 24.83 μmol·h−1 | [418] |
| BiO2/g-C3N4 | Type II Z-scheme | 500 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 8,542 μmol·g−1 | [419] |
| g-C3N4/BiYO3 | Type II | - | 37.6 μmol·g−1·h−1 | [420] |
| g-C3N4/LaxCo3-xO4 | - | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 63.12 μmol·h−1 | [421] |
| Fe2O3/g-C3N4 | Z-scheme | 350 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 398.0 μmol·g−1·h−1 | [210] |
| Mn3O4/g-C3N4 | p-n heterostructure | 300 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) (PLS-SXE300D/300DUV, Beijing Perfectlight) | The H2 and O2 evolution rate is 3300 and 654 μmol g−1·h−1, respectively. | [422] |
| g-C3N4/Nitrogen-Doped Carbon Dots/WO3 | - | 300 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) (CEL-HXF 300) | 3.27 mmol g–1 h–1 | [423] |
| Mn3O4/g-C3N4 | - | 300 W Xenon lamp source (PLS-SXE300D/300DUV) | 2700 mmol·g−1·h−1 | [422] |
| NiO/g-C3N4 | Type II | Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 1.41 mmol·h−1 | [424] |
| In2O3/g-C3N4 | Type II | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 0.99 mmol·h−1 | [425] |
| MoO3-x-g-C3N4 | Z-scheme | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 22.8 mmol·h−1 | [426] |
| ZnO/Au/g-C3N4 | Z-scheme | 150 W Xenon arc lamp with a 420 nm cutoff filter (λ > 420 nm) | 3.69 μmol h−1 cm−2 | [397] |
| d-Ti3C2/TiO2/g-C3N4 | - | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | 1.62 mmol·h−1g−1 | [396] |
| TiO2/g-C3N4 | Type II | 450 W high-pressure mercury lamp | 22.4 mol·h−1 | [402] |
| TiO2-WO3-g-C3N4 | - | - | 286.6 mmol·h−1 | [427] |
| TiO2/Ti3C2/g-C3N4 | - | 300 W Xe lamp | 2592 mmol·g−1 | [428] |
| Photocatalyst | Type of Heterojunction | Source of Light | Highest Photocatalytic Rate | Ref |
|---|---|---|---|---|
| NiO-g-C3N4 | Type II | 300 W Xenon-arc lamp | 4.17 μmol·g−1·h−1 | [436] |
| g-C3N4 foam-Cu2O | Z-scheme | 350–780 nm lamp | 8.182 μmol·g−1·h−1 (CO revolution) | [437] |
| NiMoO4-g-C3N4 | Z-scheme | - | 7238 μmol·g−1·h−1 | [438] |
| CeO2-g-C3N4 | Type II | 300 W of Xenon-arc lamp | 0.590 μmol·h−1 (CO evolution) | [439] |
| ZnO/Au/g-C3N4 | Z-scheme | 300 W UV-Vis lamp | 689.7 μmol/m2 (CO evolution) | [440] |
| ZnO/g-C3N4 | Z-scheme | 300 W xenon light source with a 420 nm cutoff filter (λ > 420 nm) | ~72.24 μmol·g−1 | [435] |
| TiO2/g-C3N4 | Type II | 8 W UV lamp | The highest CH4 and CO yields of 72.2 and 56.2 μmol g−1 | [431] |
| Nb doped TiO2/g-C3N4 | Z-scheme | 1000 W Xe lamp | The CH4, CO, O2, HCOOH generation rate in the presence of 50Nb-TiO2/50 g-C3N4 is 562, 420, 1702, 698 μmol h−1 g−1, respectively. | [432] |
| ZnO/g-C3N4 | Z-scheme | 350 W Xe lamp | The CH3OH production rate was 1.32 μmol h−1 g−1 | [429] |
| ZnO/g-C3N4 | Type II | 300 W xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | H2, CH4, and CO production rates of 22.7 μmol·g-Cat−1·h−1, 30.5 μmol·g-Cat−1·h−1, and 16.8 μmol·g-Cat−1·h−1 | [433] |
| ZnO/g-C3N4 | Type II | 350 W Xe arc lamp | 45.6 mol·g-Cat−1·h−1 | [441] |
| TiO2/g-C3N4 | Type II | 450 W Xe lamp | 22.5 μmol·g−1 and 70 μmol·g−1 for CO and CH4 yield, respectively | [442] |
| g-C3N4/3D ordered microporous (3DOM)-WO3 | Z-scheme | visible light (λ ≥ 420 nm) | 48.7 μmol g−1 h−1 | [443] |
| NiTO3/g-C3N4 | Z-scheme | 300 W xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | The highest yield of CH3OH production is 13.74 μmol∙g−1∙h−1 | [444] |
| Photocatalyst | Type of Heterojunction | Source of Light | Application | Highest Photocatalytic Rate | Stability | Ref |
|---|---|---|---|---|---|---|
| TiO2-g-C3N4 | Z-scheme | 300 W Xenon arc lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of Rhodamine B and tetracycline hydrochloride | The RhB removal rates for 5 layer TiO2, 3, 5, 7 layers g-C3N4 (0.5)/TiO2 were 5.1%, 17.9%, 31.2%, and 22.6%, respectively | - | [503] |
| P/O co-doped g-C3N4/anatase TiO2 | Z-scheme | 350 W Xenon-arc lamp as a light source with a 420 nm cutoff filter (λ > 420 nm) | Degradation of enrofloxacin | ~98.5% | 1 h | [504] |
| TiO2@ g-C3N4 | Z-scheme | 100-W xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of RhB | 95.68% | [505] | |
| MoS2-g-C3N4@TiO2 | - | 350 W Xenon lamp | Degrdation of Methylene Blue | 97.55 | 1 h | [506] |
| g-C3N4 and polyaniline-co-modified TiO2 | - | xenon lamp containing an optical filter | Degradation of tetrabromobisphenol A | 92.42% | 16 h | [507] |
| N-TiO2/O-doped N vacancy g-C3N4 | Type II Z-scheme | Lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of tetracycline hydrochloride and Cr(VI) | TC-HCl and Cr(VI) removal efficiency is 79.9% and 89.5%, respectively | - | [508] |
| TiO2@g-C3N4 | Type II | 300 W xenon lamp | Degradation of tetracycline antibiotic | TiO2@g-C3N4 photocatalyst shows the The highest tetracycline degradation rate is 2.2 mg/min, which is 2 times higher than that of TiO2 and 2.3 times higher than that of bulk g-C3N4. | - | [509] |
| TiO2/g-C3N4/persulfate (PS) | Type II | 300 W xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of micropollutant (phenol, bisphenol A and carbamazepine | 99.3%. | [510] | |
| TiO2 nanowire/g-C3N4 nanosheet/graphene (G) heterostructures | - | 300 W xenon lamp | Degradation of nitrobenzene | 97% | 4 h | [119] |
| Ti3+ and O doped TiO2/g-C3N4 | Type II | 30 W cold visible light-emitting diode | Degradation of Rhodamine B | The photodegradation reaction rate constant based on this heterojunction is 0.0356 min−1, which is 3.87 and 4.56 times higher than those of pristine Ti3+-TiO2 and g-C3N4, respectively. | - | [125] |
| TiO2/g-C3N4 | Type II | - | Degrdation of ciprofloxacin (CIP) | 68.1% | 3 h | [511] |
| ZnO-g-C3N4 | Z scheme | 500 W Xe lamp, with a 420 nm cutoff filter (λ > 420 nm) | Degradation of Methylene Blue (MB) | 75% | 3 h | [512] |
| ZnO-g-C3N4 | Z scheme | 300 W xenon lamp | Degradation of cephalexin oxidation | 98.9% | 1 h | [513] |
| djembe-like ZnO- g-C3N4 | - | 150 W Xenon light sources | Degradation of MB and RhB | MB and RhB degradation efficiency are ~95% and ~97%, respectively. | 50 min | [514] |
| WO3-g-C3N4 | 300 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of tetracycline | 90.54% | 1 h | [515] | |
| WO3-g-C3N4 | Z-scheme | 450 W xenon lamps | Degradation of AO7 | 100% | 75 min | [516] |
| WO3-g-C3N4 | Z-scheme | 35-W Xe lamp with a radiation intensity of 1380 μW∙cm2 | Degradation of orange G | 98% | 1 h | [517] |
| Ag-WO3/g-C3N4 | Z-scheme | 500 W Xe lamp (Beijing Bofei Technology. Co. Ltd., Beijing, China) | degradation of oxytetracycline hydrochloride | 97.74 | 1 h | [273] |
| WO3@g-C3N4@MWCNT | Z-scheme | 5 mW cm−2 Xe lamp (SANEI electronics-JAPAN) | Degradation of tetracycline | 79.54% | 2 h | [518] |
| g-C3N4/WO3 | Z-scheme | - | Degradation of nitenpyram | The photocatalyst rate constant is 0.036 min−1 which is about 1.7 and 25 times higher than that of pure g-C3N4 and WO3, respectively. | - | [519] |
| Bi2O3- g-C3N4 | Z-scheme | 350 W xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of Rhodamine B | 98% | 80 min | [520] |
| Bi2O3- g-C3N4 | Z-scheme | 250 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of tetracycline | 80.2% | 50 min | [521] |
| g-C3N4-CeO2 | Type II | 300W Xe arc lamp | Degradation of antibiotic doxycycline hydrochloride | 84% | 1 h | [522] |
| Shuttle-like g-C3N4-CeO2 | - | 300 W Xe arc lamp | Degradation of norfloxacin | 88.6% | 1 h | [523] |
| Kaolin/CeO2/g-C3N4 | - | 500 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Removal of ciprofloxacin | 90% | 150 min | [524] |
| Fe3O4/CeO2/g-C3N4 | - | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of tetracycline hydrochloride | 96.63% | 3 h | [525] |
| Au/g-C3N4 nanosheets/CeO2 | Z-scheme | 500 W Xe lamp with a 400 nm cutoff filter (λ > 400 nm) | Reduction of hexavalent chromium Oxidation of oxytetracycline hydrochloride | 88.2% 95.1% | 150 min | [411] |
| Co3O4-g-C3N4 | - | 250 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of Methyl Orange | 100% | 3 h | [526] |
| NiO-g-C3N4 | Type II | 500 W Xe-lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of Methylene Blue | 6.3 wt. % NiO loading shows a 2.3 times higher MB degradation rate than that of the pristine g-C3N4. | 80 min | [527] |
| V2O5-g-C3N4 | Z-scheme | 500 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of Congo Red and Cr (VI) reduction | -. | 90 min | [528] |
| MoO3-g-C3N4 | Z-scheme | 150 W-Xe lamp having 1.5 AM filter which allows wavelength larger than 400 nm for the visible light-based catalytic reaction | Degradation of Rhodamine B | 93% | 3 h | [529] |
| MoO3-g-C3N4 | Z-scheme | 500W Xenon lamp | Degradation of Rhodamine B | 100% | 10–15 min | [530] |
| MoO3-g-C3N4 | Z-scheme | visible light (λ > 420 nm) | Degradtion of tetracycline | 85.9% | 100 min | [531] |
| BiMoO6-g-C3N4 | Z-scheme | 300 W Xe lamp | Degradation of ciprofloxacin | ~100 | 30 min | [532] |
| g-C3N4/SnO2 | S-scheme | A 300 W Osram, 230 V with a 420 nm cut-off filter as used as the visible light source. | Degradation of NO | 44.17% | 30 min | [533] |
| g-C3N4-NiO | Z-scheme | 30 W LED-light source | Degradation of Methyl Orange (MO) | 96.8% | 2 h | [461] |
| ZnO/g-C3N4 | Type II | 150 W Xe lamp | Degradation of MB and RhB | The MB and RhB degradation was ~95% and ~97%, respectively. | 50 min | [514] |
| g-C3N4/CuOx | - | 350W Xe lamp | Degradation of Methyl Orange (MO) | ~62.5%. | 70 min | [534] |
| WO3/g-C3N4 | Z-scheme | 300 W Xe arc lamp | Degradation of sulfamethoxazole | 91.7% | 4 h | [453] |
| g-C3N4 Nanosheets/ZnO | - | 500 W Xe lamp | Photocatalytic reduction of aqueous chromium(VI) | 70% | 4 h | [535] |
| ZnO/g-C3N4 | - | 500 W xenon lamp | Photodegradation of Direct Blue 199 (DB) | 99% | 100 min | [536] |
| ZnO/g-C3N4 | - | A 150 W xenon lamp | Degradation of tetracycline hydrochloride | 97% | 30 min | [452] |
| MoS2/Al2O3/g-C3N4 | - | 150 W tungsten halogen lamp with a 420 nm cutoff filter (λ > 420 nm) | Degradation of crystal violet (CV) | 97.3%. | 90 min | [537] |
| g-C3N4/ZnO | Z-scheme | 300 W Xenon lamp cutoff filter with a 420 nm cutoff filter (λ > 420 nm | Degradation of 4-chlorophenol | ~ 95% | 1 h | [538] |
| Gd2O3 NPs@g-C3N4 | - | 300 W Xe lamp with a 420 nm cutoff filter (λ > 420 nm) (PLS-SXE300, Beijing Perfectlight Technology Co., Ltd., Beijing, China) | Degradation of Methyl Orange (MO) Methyl Blue (MB) Rhodamine B | 72.4% 95.5% 100% | 2 h | [539] |
| MoO3/g-C3N4/peroxydisulfate (PDS) | Z-scheme | 350 W Xenon lamp with a 420 nm cutoff filter (λ > 420 nm) | degradation of ofloxacin (OFLX) | 94.4% | 2 h | [540] |
| ZnO/g-C3N4 | Z-scheme | 300 W Xe lamp | Degradation of cephalexin | 98.9% | 1 h | [513] |
| Ce2O/Bi2O3/g-C3N4 | Z-scheme | 75 W halogen lamp | Degradation of Malachite green and Rose Bengal | - | 1 h | [541] |
| CuO/ZnO/g-C3N4 | Z-scheme | 400 W hallow lamp | Degradation of Methylene Blue and ammonia-nitrogen | The MB and ammonia-nitrogen degradation efficiency are ~98% in 45 min and 91% in 6 h. | 45 min, and 6 h | [542] |
| Fe3O4/ZnO/g-C3N4 | Type II | 23 W white LED | Degradation of pantoprazole | 97.09%. | 90 min | [543] |
| Fe3O4/TiO2/g-C3N4 | - | 500 W Xenon Lamp with a 420 nm cutoff filter (λ > 420 nm | Degradation of Rhodamine B (RhB) and Methylene Orange (MO) | The photocatalyst shows the RhB, and MO degradation efficiency is ~96.4% in 80 min and 90% in120 min. | 8 min and 120 min | [544] |
| g-C3N4/ZnO/TiO2 | - | 1000 W xenon lamp irradiation system equipped with a 410 nm cutoff filter under room temperature. | Degradation of p-toluenesulfonicacid (p-TSA) | 100% | 60 min | [545] |
| ZnO/Ag2O/g-C3N4 | - | high pressure xenon short arc lamp with the light intensity of 100 mW cm−2 | Degradation of ciprofloxacin | 97.4% | 48 min | [196] |
| WO3/TiO2@g-C3N4 | - | 500 W metal halide lamp | Removal of acetylsalicylate (aspirin) and methyl-theobromine (caffeine) | 98% | 90 min | [546] |
| TiO2@g-C3N4/Co3O4 | - | 300 W xenon lamp | Degradation of tetracycline (TC) and Methylene Orange (MO) | The TC (10 mg/L) and MO (25 mg/L) degradation efficiency are 91.6% and 97.8%, respectively. | 1 h | [547] |
| g-C3N4/Bi2O3/TiO2 | - | Xe-lamp light source with a 420 nm cutoff filter (λ > 420 nm | Degradation of Methylene Blue (MB) | The MB removal efficiency is 77.5%. | 3 h | [548] |
| SnO2/ZnO@g-C3N4 | - | 300 W xenon lamp | Degradation of Rhodamine B dye and H2 production | 99% | 1 h | [494] |
| g-C3N4/NiO/ZnO/Fe3O4 | - | - | removal of esomeprazole | 95.05 ± 1.72% | 70 min | [513] |
| Photocatalyst | Application | Explanation | Ref |
|---|---|---|---|
| MoO3-g-C3N4 | Detection of Furazolidone | The high electroactive surface area (0.3788 cm2), as well as enhanced heterogeneous electron transfer rate (K°eff = 4.91 × 10−2 cm·s−1 can detect Furazolidone with low limit of detection (LOD) (1.4 nM) with a working range of 0.01–228 μM. | [528] |
| Co3O4-g-C3N4 | Detection of environmental phenolic hormones | This composite showed a wide detection range and a low limit of detection LOD (10−9 mol L−1) | [565] |
| g-C3N4-Fe3O4 | Determination of Tramadol in Human Biological Fluids | LOD of this composite is ~0.1 μΜ | [566] |
| V2O5-g-C3N4 | Detection of folic acid | The sensitivity, the LOD, and noise-to-signal ratio of the sensor is 19.02 μA mM−1 cm−2, and 0.00174 μM, and 3 (S/N = 3), respectively | [567] |
| g-C3N4-NiO | Detection of quercetin | The dynamic range and LOD of g-C3N4-NiO for sensing quercetin is 10 nM to 250 and 0.002 μM, respectively | [568] |
| Cu2O-g-C3N4 | Humidity sensor | The response time and recovery time were 180–200 s and 5–10 s, respectively. | [569] |
| NiO-Co3O4-g-C3N4 | Detection of tetrabromobisphenol-A | This structure showed a LOD of ~0.1 mmol L−1 | [570] |
| ZnO @ g-C3N4 | Detection of CCRF-CEM cells | The LOD of this compsite is ~20 cell/mL | [571] |
| ZnO flower-rod/g-C3N4-gold nanoparticle | Detection of carcinoembryonic antigen (CEA) | The PEC aptasensor for CEA determination is from 0.01 to 2.5 ng·mL−1 with detection of 1.9 pg·mL−1. | [572] |
| g-C3N4/ZnO | Ethanol sensing | Compared to the ZnO, the g-C3N4/ZnO-8% composites revealed an excellent response ~60 orders of magnitude at room temperature. | [559] |
| ZnO/g-C3N4 | NO2 sensing | The response, recovery time, and LOD of ZnO/g-C3N4-10 wt % are 142, 190 s, and 38 ppb, respectively. | [561] |
| SnO2/g-C3N4 | Ethanol gas Sensing | The composite with 7 wt % g-C3N4 content exhibited a promising gas sensing property to ethanol, which has better response and selectivity than that of the pure SnO2 based sensor. | [321] |
| g-C3N4/ZnO | CH4 Sensing | The higher active sites can be obtained in this structure due to the larger specific surface area leading to the great response toward 1000 ppm CH4. | [560] |
| g-C3N4-Mn3O4 | H2S sensor | The LOD (S/N = 3, LOD) is 0.13 μg mL−1 | [573] |
| α-Fe2O3/g-C3N4 | H2S sensor | The linear detection range and detection limit of the H2S gas sensor were 0.88–7.01 μg mL−1(r = 0.998) and 0.5 μg mL−1(S/N = 3), respectively. | [562] |
| O vacancy WO2.9/g-C3N4 | 4-nitrophenol Sensing | Compared to other research works, this heterojunction showed the linear range of 0.4–100 μmol/L and a lower detection limit of 0.133 μmol/L. | [574] |
| b-Bi2O3/g-C3N4 | Detection of chlorpyrifos | The linear detection range and detection limit of this sensor were 0.1–80 ng mL−1 a03 μg mL−1, respectively. | [575] |
| Fe3O4/Bi2O3/g-C3N4 | Determination of Cd2+ and Pb2+ | The minimum quantity for Cd2+ and Pb2+ that can be detected is 3 × 10−9 and 1 × 10−9 mol/L, respectively. | [576] |
| WO3/g-C3N4 | Detection of phosmet | The limit of detection (LOD) and limit of quantification (LOQ) of this device was calculated 3.6 nM and 11.2 nM, respectively. | [577] |
| WO3/g-C3N4/MnO2 | Detection of oxytetracycline cathodic | This sensor exhibited a wide detection range from 1 pM to 150 nM and a low detection limit of 0.1 pM. | [578] |
| CuO-g-C3N4 | Aflatoxin B1 sensing | The limit of detection of 6.8 pg mL−1 for AFB1. | [579] |
| Photocatalyst | Source of Light | Application | Highest Photocatalytic Rate | Ref |
|---|---|---|---|---|
| g-C3N4/TiO2/Ag | Xe lamp with a 420 nm cutoff filter (λ > 420 nm) | Bactericidal efficiency against E. coli | the optimal bacterial inhibition of g-C3N4/TiO2/Ag was 84% | [102] |
| g-C3N4/Cu2O | 300 W xenon lamp with 400 nm cutoff filter (λ > 400 nm) | Inactivation efficiencies of E. coli as well as Fusarium graminearum | g-C3N4 with 45%Cu2O composition revealed the inactivation efficiencies of ~7 log E. coli | [584] |
| TiO2/g-C3N4/SnO2 | 300 W xenon lamp with 420 nm cutoff filter (λ > 420 nm) | Bactericidal efficiency against E. coli | TiO2/g-C3N4/SnO2 structure showed a good E. coli disinfection efficiency under visible and UV irradiation, with ~−6.7 log E. coli and −8.2 log E. coli, respectively. | [314] |
| α-Fe2O3/CeO2 decorated g-C3N4 | 500 W Xe light with the cut-off filter of ~420 nm (λ > 420 nm) | Antibacterial activities against S. aureus (G+) and E. coli (G−) bacteria | α-Fe2O3/CeO2 decorated g-C3N4 exhibited perfect antibacterial to E. coli and S.aureus activity with the maximum zone of inhibition (ZOI) of 11 ± 0.5, 12 ± 0. | [585] |
| g-C3N4-m-Bi2O4 | 500 W halogen lamp with UV-cut off filter (λ > 420 nm) | Bactericidal efficiency E. coli and S.aureus bacteria | The ZOI value for E.coli and S. aureus bacterial strains was ~11 ± 0.5 mm and 12-13±0.5. | [586] |
| Ag/ZnO/g-C3N4 | 300 W xenon arc lamp | Bactericidal efficiency against E. coli | The E. coli disinfection efficiency of Ag/ZnO/g-C3N4 structure is ~7.4 log E. coli. | [190] |
| Ag/AgO-g-C3N4 | 100 W tungsten lamp | Bactericidal efficiency against E. coli | The 1, 2, 3, and 4 mg of catalyst showed low quantitative E. coli growth inhibition, which was ~17% and 65%, 97%, 99%, respectively. | [587] |
| Cu2O-g-C3N4 | 36 W fluorescent lamp with the cut-off filter of ~400 nm (λ > 400 nm) | Bactericidal efficiency against B. subtilis, E. coli, S. aureus and P. aeruginosa | The maximum ZOI for Cu2O-g-C3N4 to B. subtilis E.coli, S. aureus and P. aeruginosa is 22 ± 1.67, 15 ± 1.08, 11 ± 1.22, 6 ± 0.09, respectively. | [587] |
| g-C3N4/TiO2 Kaolinite | Xenon lamp with a 400 nm cut-off filter | Disinfection ability towards S. aureus | The disinfection efficiency of g-C3N4/TiO2/kaolinite is ~4.3 log cfu/mL in 5 h. | [588] |
| TiO2/g-C3N4 | 500 W xenon arc lamp with a 400 nm cut-off filter | Anti-fouling ability of E. coli | TiO2/g-C3N4 showed an excellent E. coli removal with a permeate flux of 2 times higher than that of filtration alone. | [589] |
| TiO2/g-C3N4 | - | Bactericidal efficiency against E. coli | The bacterial survival rate for the TiO2 nanotube/g-C3N4 nanofilms is ~16%. | [590] |
| g-C3N4/Ag-TiO2 | Xenon lamp | Bactericidal efficiency against both Gram-negative 18 Escherichia-coli and Gram-positive Staphylococcus-aureus | The presence of Ag in g-C3N4/Ag-TiO2 structure could enhance water disinfection under visible light. | [591] |
| g-C3N4/MoS2-Bi2O3 | 300 W xenon arc lamp | Bactericidal efficiency against E. coli | g-C3N4/Bi2O4 with the ratio of 1:0.5, could entirely inactivate 6-log10 cfu/mL E. coli. | [586] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. https://doi.org/10.3390/nano12020294
Alaghmandfard A, Ghandi K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials. 2022; 12(2):294. https://doi.org/10.3390/nano12020294
Chicago/Turabian StyleAlaghmandfard, Amirhossein, and Khashayar Ghandi. 2022. "A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing" Nanomaterials 12, no. 2: 294. https://doi.org/10.3390/nano12020294