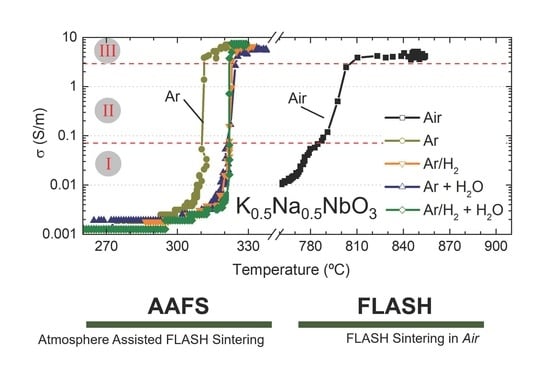
3.2. FLASH Sintering in Dry and Humidified Atmospheres
The electrical conductivity as a function of furnace temperature is represented in
Figure 3 for BM and AM powders, in (a) and (b), respectively. Equal electrical and thermal conditions but different sintering atmospheres were used to FLASH sinter such compacts—namely, dry ones (Air, Ar and Ar + H
2) and humidified (Ar + H
2O and Ar + H
2 + H
2O). The data shows rather similar FLASH processes independently of the employed atmosphere; however, that is not the case for the FLASH temperature, T
F.
A clear and prominent dependence of T
F with the operating atmosphere is revealed.
Table 3 summarizes the FLASH temperatures estimated from
Figure 3. Each experimental condition was repeated at least once, and the standard deviation from the mean value of T
F was calculated when three or more repetitions were performed. Additionally,
Table 3 gives information on the relative final density (ρ
sint) of the BM and AM sintered compacts.
A first analysis on BM compacts revealed that the use of Ar atmosphere allows a TF decrease from 870 °C (Air) to 276 °C. If a hydrogen-containing dry atmosphere is used (Ar/H2), the FLASH temperature is slightly increased to 295 °C. A similar tendency is observed when humidified reducing atmospheres are employed, with Ar + H2O and Ar/H2 + H2O giving slightly higher TF (284 and 306 °C, respectively) than the correspondent dry atmospheres.
Previously reported for ZnO [
6] and KNN [
11], the use of low oxygen content (reducing) atmospheres dramatically decreases T
F, which is related to the defect chemistry of materials, a topic discussed later on. Additionally, when hydrogenized [
6] and/or humidified [
5] atmospheres are used to FLASH sinter ZnO, the T
F is further decreased (ultimately, to room temperature [
5]).
However, in opposition to that, both hydrogenized (Ar/H2) and humidified atmospheres (Ar + H2O and Ar/H2 + H2O) used in AAFS of KNN faintly increased TF, in comparison with the correspondent non-hydrogenized and/or dry atmospheres. These observations must have a relationship with the interaction of different gases and humidity with the material, which is not known yet.
In parallel,
Table 3 and
Figure 3 reveal that AAFS of BM compacts resulted in low final densification (ρ
sint). For compacts AAFSed under Ar, Ar/H
2 and Ar/H
2 + H
2O, the final density is not increased when compared with the un-sintered compacts, meaning that sintering did not occur. Nonetheless, the densification of compacts FLASH sintered in Ar + H
2O, is already appreciable, leading to specimens with 74% of the relative density.
Even though water’s role on AAFS is not yet well understood, it was demonstrated to be associated with the achievement of high density for ZnO (98%) by a process based on increased mass transport and consequent greater densification [
5]. In the case of KNN, water shows a promising effect towards the increase in density for AAFS in an Ar + H
2O atmosphere.
When comparing BM with finer particle AM compacts,
Table 3 shows that the final densities of both are similar, regardless of the operating atmosphere during FLASH. However, that is not the case for T
F, as shown in
Figure 3 and
Table 3. For experiments in Air, the T
F of AM is decreased when compared with that of BM (785 and 870 °C, respectively). This is related to the effects of the particle-size reduction and correspondent particle-contact-density increase, as previously reported and discussed [
12].
When AAFS is employed in AM compacts, a general observation reveals that a similar trend is obtained as in BM with reducing atmospheres contributing for a strong decrease in T
F. In a parallel link, the use of hydrogenized and humidified atmospheres also resulted in slight T
F increases. Additionally,
Figure 3 and
Table 3 reveal that, while in Air, the T
F is decreased for AM powders when compared with BM ones, the use of reducing atmospheres promoted the opposite tendency, with AM compacts AAFSed in Ar and Ar/H
2 presenting a higher T
F than the BM ones.
The fact that the decrease of particle size augments the density of particle-to-particle contacts per volume unit explains the decrease of T
F in AM compacts compared with BM ones when Air FLASH is performed. However, it does not remain valid to explain the opposite tendency observed during AAFS. As presented before,
Table 2 and
Figure 2 show that in green BM compacts the pores are coarser than in AM.
Coarser porosity channels should increase the gas permeability in the green pellets and a more pronounced effect of its interaction with the powder’s surfaces. This effect must overlap the effect of the lower particle contact density in the compacts with coarser powder (BM) when compared to finer particle ones (AM), explaining its lower TF during AAFS. The increase in TF when using Ar/H2 in comparison with simple Ar remains a topic to be clarified.
Despite the detailed discussion on T
F dependencies,
Figure 3 also suggests that the conductive behavior of KNN powders FLASH sintered in Air or in reducing atmospheres is different for both BM and AM compacts. While a slow increase in σ with temperature occurs in Air, the transition from low conductivity (0.001 S/m) towards the FLASH event (σ ≳ 0.07 S/m)—stage I to stage II—during the AAFS processes is faster, identifiable by the minor quantity of data points in
Figure 2. In parallel,
Table 3 reveals that the final density is affected by the AAFS in non-oxidizing atmospheres. Therefore, the mechanisms that promote FLASH must be different than those operating for Air FLASH, which is why they were investigated.
Figure 4 shows the Arrhenius representation of the electrical conductivity using the estimated specimen temperature (Tcalc) obtained from the non-equilibrium adaptation of the Black Body Radiation model [
15,
16], in which dT/dt is different from zero during stage I of FLASH. In a general observation, one can state that the dependency of the conductivity on the temperature is similar to both BM and AM compacts when FLASH sintered in Air. However, that dependence is different when non-oxidizing AAFS is performed.
In detail, the maximum calculated temperature achieved during stage I of FLASH in Air and Atmosphere-Assisted is significantly different, independently of the powder (BM and AM). In the first case (Air), stage I is completed at 1000/Tcalc 0.8 K−1, or 977 °C. Instead, for the AAFS in reducing conditions, the stage I maximum temperature is roughly independent of the atmosphere and occurs at 1000/Tcalc 1.4 K−1, or 441 °C. Exceptions are made for both compacts in Ar/H2 + H2O in which the temperature at the end of stage I is roughly 350 to 390 °C.
There is more than a 100 °C difference between humidified-hydrogenized-Argon and the remaining AAFS atmospheres and about a 500 °C difference between the later and Air experiments. Moreover, the transition regime, associated with stage II, is also dissimilar, as previously stated (
Figure 3). A significantly larger number of data points, acquired constantly with a time interval of 1 s in all experiments, was recorded for Air FLASH. In contrast, a fast transition occurs for AAFS during stage II, with only a few points being recorded.
This was especially evident in the Ar/H2 + H2O atmosphere. These conditions together induce a smaller generation of heat by Joule effect, observed clearly by the lower calculated temperature during stage I for AAFS when compared with Air FLASH. One can say that thermal runaway phenomena is not ruling in AAFS of KNN, thereby, hindering the densification.
As indicated by J. Nie and co-workers [
5], in the water-assisted FLASH sintering of ZnO, densification only occurs when the estimated sample temperature is above a threshold (1100 °C, in that case). For AAFS of KNN, we postulate that the minimum estimated temperature for the transition between stage I and II must be close to 1000 °C (Air conditions) so that high densification is achieved.
In parallel, the apparent activation energy for conduction, E
a(σ), during stage I was estimated from the Arrhenius representation of conductivity over the estimated temperature and is also shown in
Figure 4. The results confirm the thermally activated processes for all the pellets and atmospheres. However, differences in the apparent E
a(σ) as a dependence of the process were found. In detail, when FLASH sintered in Air, BM and AM, the pellets presented E
a(σ) between 2.6 and 2.7 eV. In contrast, when reducing atmospheres were used to perform AAFS, E
a(σ) was between 0.9 and 1.5 eV, independently of the powder and atmosphere. The exception was again the Ar/H
2 + H
2O atmosphere, in which BM compacts presented an activation energy of 0.43 eV for conduction, while for AM ones, E
a(σ) = 2.74 eV.
Studies on the DC conductivity of the perovskite-structured La-doped BFO indicate that, if the condition 0.2 < E
a(σ) < 0.45 eV is satisfied, a conduction mechanism dominated by p-type polaron hopping occurs [
17,
18,
19]. In parallel, K
0.5Na
0.5NbO
3 has been reported to present p- or n-type behavior when sintered in Air or N
2, respectively. Moreover, high dielectric losses were found for the ceramics sintered under a reducing N
2 atmosphere, which was attributed to a higher concentration of oxygen vacancies,
, in such ceramics [
20]. In low PO
2 atmospheres, the formation of
is facilitated (Equation (2)). As Nb
5+ is a d-cation, it would accommodate the excess of electrons [
20]; however, it is possible that such excess of electrons also contributes to the conduction.
Complementarily, the activation energies for conduction in ferroelectric perovskites reported between 0.4 and 1.2 eV have been associated with charge transport by ionized oxygen defects [
21,
22]. This was confirmed previously for KNN ceramics and single crystals [
12,
23]. On the other hand, ionic-based conducting mechanisms have been reported in KNN single crystals to present activation energies higher than 1.2–1.3 eV [
23].
The data reported in
Figure 4 suggests that the conducting mechanism during the Air FLASH sintering of dry KNN powders is suitable with ionic-based conduction because of the condition E
a(σ) > 1.2 eV. This conduction process can be interpreted as intrinsic conduction.
On the other hand, the movement of thermally activated ionized appears to be the ruling mechanism in AAFS because the condition 0.4 < Ea(σ) < 1.2 eV is satisfied. Therefore, extrinsic conduction occurs. Once again, exception made for AM compact in Ar/H2 + H2O, where ionic conductivity seems to take place, which is an unexpected result.
The limited number of points in the Arrhenius plots of
Figure 4 and deviations from the assumptions assumed in Equation (1)—namely, the contribution of the heat dissipation by conduction/convection in the sample temperature estimation of AAFS samples increases the error associated to the determined activation energy values. In the case of Ar/H
2 + H
2O, there is a higher fluctuation of the conductivity values, probably coming from the lack of uniformity of the humidity in the atmosphere and which is believed to be the reason for the unexpected, calculated differences on the activation energies values; however, this topic requires further investigation.
It is suggested that, in AAFS, there was an increase in the concentration of
at low temperature promoted by the low partial pressure of oxygen, facilitated by the polaron hoping and excess of electrons that allow conduction at T < 330 °C; in contrast, in Air FLASH, ionic conduction is activated. The presence of oxygen does not allow a relevant contribution of oxygen vacancies for the conduction. In that case, a significant local heating by Joule effect (thermal runaway) promotes the partial melting of particles’ contacts and the following densification of KNN compacts [
9,
11]. This process does not occur in AAFS due to the significantly lower furnace temperature and higher concentration of conducting defects (extrinsic) hindering the Joule heating generation and thermal runaway process.
Figure 4 shows that similar conducting mechanisms are obtained for each powder (AM and BM), if the same type of atmosphere is considered. This observation indicates that, independently of the particle contact density and pore morphology, the conducting mechanisms during FLASH are the same for each atmosphere, and T
F is changed only by the interaction of the gas and moisture with the KNN compacts. These results contrast with the observations in ZnO [
5,
6], where AAFSF led to high densification at room temperature (98% of relative density).
The analysis of operating conducting mechanisms during AAFS may lead to the conclusion that the initial particle size and consequent pore morphology do not affect the FLASH-sintering process. However, the final density of AAFSed KNN is dependent on the atmosphere and the presence of water (
Table 3). On the one hand, when FLASH sintering both BM and AM compacts in Air, the whitish color of compacts was found to be kept on the sintered ceramics. On the other hand, that is not the case of AAFS. While a uniform dark color was identified in all AAFSed AM compacts, BM pellets appeared with dark non-uniform localized areas. As an example, the final macroscopic appearance and respective microstructures of ceramics AAFSed in Ar + H
2O of BM and AM powders are shown in
Figure 5, (a) and (b), respectively. Dark colors in KNN ceramics are typically associated with the presence of oxygen vacancies.
Hence, by direct observation of
Figure 5, it is possible to infer that AM pellets present a significantly more uniform distribution of these defects, when compared with coarser BM powders, independently of the operating atmospheres during AAFS. As revealed in (a), the localized darker areas in BM ceramics are associated with higher density as a consequence of current localization and hotspots [
11]; on the other hand, white areas are not well densified. In opposition,
Figure 5b shows a uniform ceramic with a homogeneous microstructure; nonetheless, the microstructure inset reveals a still low final density, in accordance with
Table 3.
The dissimilarities in pore size and distribution in AM and BM compacts may explain their different AAFS behavior (
Table 3 and
Figure 3) and consequent appearance and microstructure (
Figure 5). The atmosphere interaction with the powders is achieved through the pore channels available for gas adsorption. Therefore, the coarser, less uniformly distributed pore channels in BM compacts are prone to promote current localization when a FLASH-sintering atmosphere-dependent process is performed, contributing to the hotspot formation (
Figure 5) in accordance with previous work [
11]. Furthermore, the finer and more uniformly distributed pores in AM explain the limit conditions found for T
F, where the use of reducing dry and humidified atmospheres (except for Ar) gives approximately the same FLASH temperature (
Figure 3: T
F = 319 to 322 °C).
As the gas interaction with the powder is limited by the amount of that gas (and humidity) that can be adsorbed in the particles through pores, a smaller pore size induces a limited concentration of gas to interact with the particles. However, this interaction is achieved uniformly through the compact, avoiding current localization and hotspots. The validity of such affirmation is ensured because there is no pressurization of gases during AAFS. Therefore, it is suggested that, while the difference in Ea(σ) of Air and AAFS might explain the low densification obtained on the latter case, the pore-size distribution has a fundamental role to promote a uniform interaction of gases and humidity during AAFS.
Regarding the final density of AAFSed ceramics, an increase in density (from the green state) was observed for both AM and BM compacts sintered in reducing dry atmospheres; on the other hand, when humidified atmospheres were used—namely, argon—an already appreciable densification (~74 to 75%) was found. However, hotspots occurred in coarser BM powder compacts, while a uniform atmosphere interaction with AM compacts was found. Despite that the conducting mechanisms may be similar for wet/dry and simple/hydrogenized reducing atmospheres, the different TF and ρfinal of AAFSed ceramics indicate that water plays a role in increasing the densification.