Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water
Abstract
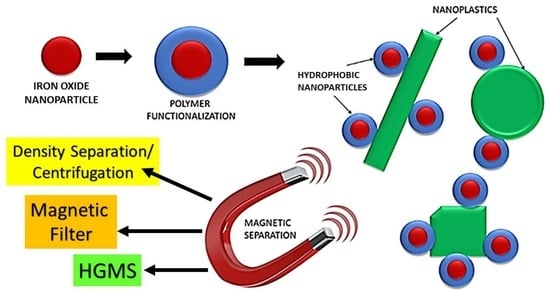
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of IONPs
2.3. Capping Procedure
2.4. Characterization of IONPs
2.5. Determination of Hydrophobicity via Contact Angle Measurements
2.6. Interaction and Magnetization of Plastic Nurdles and Fibers
2.7. Nanoparticle Removal from Water
3. Results
3.1. TEM Characterization of IONPs
3.2. X-ray Diffraction
3.3. Zeta Potential
3.4. Dynamic Light Scattering
3.5. Magnetic Characterization
3.6. Fourier Transform Infrared Spectroscopy
3.7. UV-Vis Spectrophotometry
3.8. Contact Angle Measurement Results
3.9. Interactions with Plastic Particles and Fibers
3.10. Binding to PS NPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Mofijur, M.; Ahmed, S.; Rahman, S.A.; Siddiki, S.Y.A.; Islam, A.S.; Shahabuddin, M.; Ong, H.C.; Mahlia, T.I.; Djavanroodi, F.; Show, P.L. Source, distribution and emerging threat of micro-and nanoplastics to marine organism and human health: Socio-economic impact and management strategies. Environ. Res. 2021, 195, 110857. [Google Scholar] [CrossRef]
- Estahbanati, M.K.; Kiendrebeogo, M.; Mostafazadeh, A.K.; Drogui, P.; Tyagi, R.D. Treatment processes for microplastics and nanoplastics in waters: State-of-the-art review. Mar. Pollut. Bull. 2021, 168, 112374. [Google Scholar] [CrossRef]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Löder, M.G.; Gerdts, G. Methodology used for the detection and identification of microplastics—A critical appraisal. In Marine Anthropogenic Litter; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 201–227. [Google Scholar]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Danish, M. Removal of Zn (II) and Cd (II) ions from aqueous solutions using treated sawdust of sissoo wood as an adsorbent. Holz Als Roh-Und Werkst. 2007, 65, 429–436. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xin, Q.; Wu, X.; Chen, Z.; Yan, Y.; Liu, S.; Wang, M.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Shen, S.-L.; Wang, S.-Q.; Huang, J.; Su, P.; Wang, Q.-R.; Zhao, B.-X. A dual function magnetic nanomaterial modified with lysine for removal of organic dyes from water solution. Chem. Eng. J. 2014, 239, 250–256. [Google Scholar] [CrossRef]
- Vindedahl, A.M.; Strehlau, J.H.; Arnold, W.A.; Penn, R.L. Organic matter and iron oxide nanoparticles: Aggregation, interactions, and reactivity. Environ. Sci. Nano 2016, 3, 494–505. [Google Scholar] [CrossRef]
- Zhang, W.X.; Elliott, D.W. Applications of iron nanoparticles for groundwater remediation. Remediat. J. J. Environ. Cleanup Costs Technol. Tech. 2006, 16, 7–21. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Krajangpan, S.; Chisholm, B.J.; Khan, E.; Bermudez, J.J.E. Entrapment of iron nanoparticles in calcium alginate beads for groundwater remediation applications. J. Hazard. Mater. 2009, 166, 1339–1343. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Armijo, L.M.; Brandt, Y.I.; Mathew, D.; Yadav, S.; Maestas, S.; Rivera, A.C.; Cook, N.C.; Withers, N.J.; Smolyakov, G.A.; Adolphi, N.L. Iron oxide nanocrystals for magnetic hyperthermia applications. Nanomaterials 2012, 2, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Armijo, L.M.; Wawrzyniec, S.J.; Kopciuch, M.; Brandt, Y.I.; Rivera, A.C.; Withers, N.J.; Cook, N.C.; Huber, D.L.; Monson, T.C.; Smyth, H.D. Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms. J. Nanobiotechnol. 2020, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Sötebier, C.; Michel, A.; Fresnais, J. Polydimethylsiloxane (PDMS) coating onto magnetic nanoparticles induced by attractive electrostatic interaction. Appl. Sci. 2012, 2, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Prucker, O.; Rühe, J. Synthesis of poly (styrene) monolayers attached to high surface area silica gels through self-assembled monolayers of azo initiators. Macromolecules 1998, 31, 592–601. [Google Scholar] [CrossRef]
- Sehgal, A.; Lalatonne, Y.; Berret, J.-F.; Morvan, M. Precipitation− redispersion of cerium oxide nanoparticles with poly (acrylic acid): Toward stable dispersions. Langmuir 2005, 21, 9359–9364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douadi-Masrouki, S.; Frka-Petesic, B.; Sandre, O.; Cousin, F.; Dupuis, V.; Perzynski, R.; Cabuil, V. Neutron reflectivity on polymer multilayers doped with magnetic nanoparticles. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd.: Bäch, Switzerland, 2009; pp. 194–197. [Google Scholar]
- Casula, M.F.; Jun, Y.-W.; Zaziski, D.J.; Chan, E.M.; Corrias, A.; Alivisatos, A.P. The concept of delayed nucleation in nanocrystal growth demonstrated for the case of iron oxide nanodisks. J. Am. Chem. Soc. 2006, 128, 1675–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketteler, G.; Weiss, W.; Ranke, W.; Schlögl, R. Bulk and surface phases of iron oxides in an oxygen and water atmosphere at low pressure. Phys. Chem. Chem. Phys. 2001, 3, 1114–1122. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Huang, X.; Retrum, J.; Schmucker, A.; Pink, M.; Stein, B.D.; Dragnea, B. Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem. Mater. 2007, 19, 3624–3632. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic characterization of iron oxide nanoparticles for biomedical applications. In Biomedical Nanotechnology; Humana Press: New York, NY, USA, 2017; pp. 47–71. [Google Scholar]
- Gaboury, S.R.; Urban, M.W. Quantitative analysis of the Si-H groups formed on poly (dimethylsiloxane) surfaces: An ATR FTi. r. approach. Polymer 1992, 33, 5085–5089. [Google Scholar] [CrossRef]
- Silva, F.A.; Chagas-Silva, F.A.; Florenzano, F.H.; Pissetti, F.L. Poly (dimethylsiloxane) and poly [vinyltrimethoxysilane-co-2-(dimethylamino) ethyl methacrylate] based cross-linked organic-inorganic hybrid adsorbent for copper (II) removal from aqueous solutions. J. Braz. Chem. Soc. 2016, 27, 2181–2191. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields, C.W., IV; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.; Varshney, S.K. Simple approach for the scale-up production of block copolymer of polydimethylsiloxane with (meth) acrylic ester monomers. Des. Monomers Polym. 2002, 5, 79–95. [Google Scholar] [CrossRef]
- Ashkani, M.; Bouhendi, H.; Kabiri, K.; Rostami, M. Synthesis of poly (2-acrylamido-2-methyl propane sulfonic acid) with high water absorbency and absorption under load (AUL) as concrete grade superabsorbent and its performance. Constr. Build. Mater. 2019, 206, 540–551. [Google Scholar] [CrossRef]
- Hwang, S.; Umar, A.; Dar, G.; Kim, S.; Badran, R. Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sens. Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- He, B.; Lee, J.; Patankar, N.A. Contact angle hysteresis on rough hydrophobic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2004, 248, 101–104. [Google Scholar] [CrossRef]
- Nakashima, S.; Sturgeon, R.; Willie, S.; Berman, S. Determination of trace metals in seawater by graphite furnace atomic absorption spectrometry with preconcentration on silica-immobilized 8-hydroxyquinoline in a flow-system. Fresenius’ Z. Für Anal. Chem. 1988, 330, 592–595. [Google Scholar] [CrossRef]
- Yiying, J.; Huan, L.; Mahar, R.B.; Zhiyu, W.; Yongfeng, N. Combined alkaline and ultrasonic pretreatment of sludge before aerobic digestion. J. Environ. Sci. 2009, 21, 279–284. [Google Scholar]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Lindeque, P.K.; Smerdon, G.R. Temporal transcription of two Antennapedia class homeobox genes in the marine copepod Calanus helgolandicus. Mar. Biotechnol. 2003, 5, 604–615. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [PubMed] [Green Version]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, L.M.A.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials 2022, 12, 2348. https://doi.org/10.3390/nano12142348
Martin LMA, Sheng J, Zimba PV, Zhu L, Fadare OO, Haley C, Wang M, Phillips TD, Conkle J, Xu W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials. 2022; 12(14):2348. https://doi.org/10.3390/nano12142348
Chicago/Turabian StyleMartin, Leisha M. A., Jian Sheng, Paul V. Zimba, Lin Zhu, Oluniyi O. Fadare, Carol Haley, Meichen Wang, Timothy D. Phillips, Jeremy Conkle, and Wei Xu. 2022. "Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water" Nanomaterials 12, no. 14: 2348. https://doi.org/10.3390/nano12142348