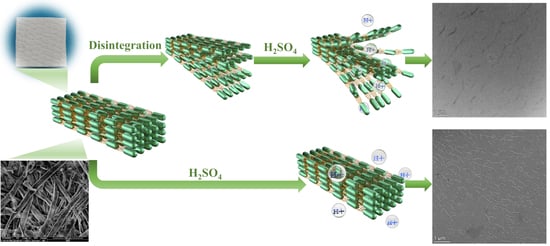
Fabrication and Properties of Tree-Branched Cellulose Nanofibers (CNFs) via Acid Hydrolysis Assisted with Pre-Disintegration Treatment
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Methods
2.2.1. Disintegration of Wood Pulps
2.2.2. Alkalization of Wood Pulps
2.2.3. Extraction of Nanocellulose
2.2.4. Preparation of Nanocellulose Films
2.3. Characterization Techniques
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Mechanical Analysis
3. Results and Discussion
3.1. Morphology of Pulps
3.2. Morphology of Nanocellulose
3.3. X-ray Diffraction (XRD) Analysis
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Thermal Stability Analysis
3.6. Mechanical Properties of Nanocellulose Films
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borrega, M.; Larsson, P.T.; Ahvenainen, P.; Ceccherini, S.; Maloney, T.; Rautkari, L.; Sixta, H. Birch wood pre-hydrolysis vs pulp post-hydrolysis for the production of xylan-based compounds and cellulose for viscose application. Carbohydr. Polym. 2018, 190, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, J.P.; Mde, F.R.; de Mde, S.F.; Nascimento, L.D.; do Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zheng, S.; Liu, Y.; Lin, J. Isolation of hierarchical cellulose building blocks from natural flax fibers as a separation membrane barrier. Int. J. Biol. Macromol. 2020, 155, 666–673. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and characterization of bacterial cellulose by Acetobacter xylinum using liquid tapioca waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Nehls, I.; Wagenknecht, W.; Philipp, B.; Stscherbina, D. Characterization of cellulose and cellulose derivatives in solution by high resolution 13C-NMR spectroscopy. Prog. Polym. Sci. 1994, 19, 29–78. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 1–35. [Google Scholar] [CrossRef]
- Harini, K.; Sukumar, M. Development of cellulose-based migratory and nonmigratory active packaging films. Carbohydr. Polym. 2019, 204, 202–213. [Google Scholar] [CrossRef]
- Mo, L.; Pang, H.; Lu, Y.; Li, Z.; Kang, H.; Wang, M.; Zhang, S.; Li, J. Wood-inspired nanocellulose aerogel adsorbents with excellent selective pollutants capture, superfast adsorption, and easy regeneration. J. Hazard. Mater. 2021, 415, 125612. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Ma, Y.; Sui, G. Characterization and properties of transparent cellulose nanowhiskers-based graphene nanoplatelets/multi-walled carbon nanotubes films. Compos. Part A Appl. Sci. Manuf. 2016, 86, 77–86. [Google Scholar] [CrossRef]
- Ajkidkarn, P.; Manuspiya, H. Novel bacterial cellulose nanocrystals/polyether block amide microporous membranes as separators for lithium-ion batteries. Int. J. Biol. Macromol. 2020, 164, 3580–3588. [Google Scholar] [CrossRef]
- Cheng, H.; Lijie, L.; Wang, B.; Feng, X.; Mao, Z.; Vancso, G.J.; Sui, X. Multifaceted applications of cellulosic porous materials in environment, energy, and health. Prog. Polym. Sci. 2020, 106, 101253. [Google Scholar] [CrossRef]
- Hassan, M.M.; Tucker, N.; le Guen, M.J. Thermal, mechanical and viscoelastic properties of citric acid-crosslinked starch/cellulose composite foams. Carbohydr. Polym. 2020, 230, 115675. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from various biomass wastes: Its preparation and potential usages towards the high value-added products. Environ. Sci. Ecotechnol. 2021, 5, 100077. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Luo, J.; Low, M.Y.; Shi, Z.; Tang, J.; Zhang, Z.; Peng, B.; Tam, K.C. Pickering emulsions stabilized by hydrophobically modified nanocellulose containing various structural characteristics. Cellulose 2019, 26, 7753–7767. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Liu, D.; Dong, Y.; Bhattacharyya, D.; Sui, G. Novel sandwiched structures in starch/cellulose nanowhiskers (CNWs) composite films. Compos. Commun. 2017, 4, 5–9. [Google Scholar] [CrossRef]
- Maciel, M.; Benini, K.; Voorwald, H.J.C.; Cioffi, M.O.H. Obtainment and characterization of nanocellulose from an unwoven industrial textile cotton waste: Effect of acid hydrolysis conditions. Int. J. Biol. Macromol. 2019, 126, 496–506. [Google Scholar] [CrossRef]
- Jun, D.; Guomin, Z.; Mingzhu, P.; Leilei, Z.; Dagang, L.; Rui, Z. Crystallization and mechanical properties of reinforced PHBV composites using melt compounding: Effect of CNCs and CNFs. Carbohydr. Polym. 2017, 168, 255–262. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind. Eng. Chem. Res. 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Wang, Q.; Wolcott, M. Mechanical activation and characterization of micronized cellulose particles from pulp fiber. Ind. Crops. Prod. 2019, 141, 111750. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Zhang, Y.; Kong, L. Homogeneous isolation of nanocellulose from eucalyptus pulp by high pressure homogenization. Ind. Crops. Prod. 2017, 104, 237–241. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Liu, D.Y.; Yuan, X.W.; Bhattacharyya, D.; Easteal, A.J. Characterisation of solution cast cellulose nanofibre—Reinforced poly(lactic acid). Express Polym. Lett. 2010, 4, 26–31. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, Y.; Lv, Y.; Wang, J.; Jia, C.; Liu, J.; Zhang, X.; Sun, J.; Shao, Z. Carboxymethyl Cellulose Nanofibrils with a Treelike Matrix: Preparation and Behavior of Pickering Emulsions Stabilization. ACS Sustain. Chem. Eng. 2019, 7, 12887–12896. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Sebe, G.; Ham-Pichavant, F.; Ibarboure, E.; Koffi, A.L.; Tingaut, P. Supramolecular structure characterization of cellulose II nanowhiskers produced by acid hydrolysis of cellulose I substrates. Biomacromolecules 2012, 13, 570–578. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Du, H.; Wang, X.; Liu, W.; Duan, Y.; Zhang, X.; Sun, L.; Zhang, X.; Si, C. Highly efficient preparation of functional and thermostable cellulose nanocrystals via H2SO4intensified acetic acid hydrolysis. Carbohydr. Polym. 2020, 239, 116233. [Google Scholar] [CrossRef]
- Tang, X.; Liu, G.; Zhang, H.; Gao, X.; Li, M.; Zhang, S. Facile preparation of all-cellulose composites from softwood, hardwood, and agricultural straw cellulose by a simple route of partial dissolution. Carbohydr. Polym. 2021, 256, 117591. [Google Scholar] [CrossRef]
- Larsson, A.T.P.T.; Iversen, T. On the accessibility and structure of xylan in birch kraft pulp. Cellulose 2001, 8, 209–215. [Google Scholar] [CrossRef]
- Rauhala, T.; King, A.W.T.; Zuckerstätter, G.; Suuronen, S.; Sixta, H. Effect of autohydrolysis on the lignin structure and the kinetics of delignification of birch wood. Mech. Pulping 2011, 26, 386–391. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Zhang, Q.; French, A.D.; Wu, Q. Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: Morphology, crystallinity and percentage estimation. Carbohydr. Polym. 2015, 133, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; McClements, D.J.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Preparation and characterization of okara nanocellulose fabricated using sonication or high-pressure homogenization treatments. Carbohydr. Polym. 2021, 255, 117364. [Google Scholar] [CrossRef]
- Li, B.; Xu, W.; Kronlund, D.; Eriksson, J.-E.; Maattanen, A.; Willfor, S.; Xu, C. Comparable characterization of nanocellulose extracted from bleached softwood and hardwood pulps. Pap. Biomater. 2018, 3, 35–44. [Google Scholar]
- Lahtinen, P.; Liukkonen, S.; Pere, J.; Sneck, A.; Kangas, H. A comparative study of fibrillated fibers from different mechanical and chemical pulps. Bioresources 2014, 9, 2115–2127. [Google Scholar] [CrossRef]
- Spiliopoulos, P.; Spirk, S.; Paakkonen, T.; Viljanen, M.; Svedstrom, K.; Pitkanen, L.; Awais, M.; Kontturi, E. Visualizing degradation of cellulose nanofibers by acid hydrolysis. Biomacromolecules 2021, 22, 1399–1405. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II nanocrystals produced by sulfuric acid hydrolysis of Tetra pak cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef]
- Yavorov, N.; Valchev, I.; Radeva, G.; Todorova, D. Kinetic investigation of dilute acid hydrolysis of hardwood pulp for microcrystalline cellulose production. Carbohydr. Res. 2020, 488, 107910. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Jabli, M.; Tka, N.; Ramzi, K.; Saleh, T.A. Physicochemical characteristics and dyeing properties of lignin-cellulosic fibers derived from Nerium oleander. J. Mol. Liq. 2018, 249, 1138–1144. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, T.Q.; Jang, H.F.; Grant, E. Modification of xylan in alkaline treated bleached hardwood kraft pulps as classified by attenuated total-internal-reflection (ATR) FTIR spectroscopy. Carbohydr. Polym. 2015, 127, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Tka, N.; Jabli, M.; Saleh, T.A.; Salman, G.A. Amines modified fibers obtained from natural Populus tremula and their rapid biosorption of Acid Blue 25. J. Mol. Liq. 2018, 250, 423–432. [Google Scholar] [CrossRef]
- Souza, A.G.; Santos, D.F.; Ferreira, R.R.; Pinto, V.Z.; Rosa, D.S. Innovative process for obtaining modified nanocellulose from soybean straw. Int. J. Biol. Macromol. 2020, 165, 1803–1812. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Lin, K.H.; Enomae, T.; Chang, F.C. Cellulose nanocrystal isolation from hardwood pulp using various hydrolysis conditions. Molecules 2019, 24, 3724. [Google Scholar] [CrossRef] [Green Version]
- Hirn, U.; Schennach, R. Comprehensive analysis of individual pulp fiber bonds quantifies the mechanisms of fiber bonding in paper. Sci. Rep. 2015, 5, 10503. [Google Scholar] [CrossRef] [Green Version]
- Thomson, C.I.; Lowe, R.M.; Ragauskas, A.J. Imaging cellulose fibre interfaces with fluorescence microscopy and resonance energy transfer. Carbohydr. Polym. 2007, 69, 799–804. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Dixit, S.; Pal, D.B.; Mishra, P.K.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of nanocellulose on mechanical and barrier properties of PVA–banana pseudostem fiber composite films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose films with combined cellulose nanofibers and nanocrystals: Tailored thermal, optical and mechanical properties. Cellulose 2017, 25, 1103–1115. [Google Scholar] [CrossRef]
- Reising, A.B.; Moon, J.P. Youngblood. Effect of particle alignment on mechanical properties of neat cellulose nanocrystal films. J-FOR 2012, 2, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Geng, B.; Chen, Y.; Liu, H.; Li, G.; Wu, Q. Preparation and characteristics of TEMPO-oxidized cellulose nanofibrils from bamboo pulp and their oxygen-barrier application in PLA films. Front. Chem Sci Eng. 2017, 11, 554–563. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Li, P.; Zhang, K.; Lian, H.; Liimatainen, H. Enhancement of the nanofibrillation of birch cellulose pretreated with natural deep eutectic solvent. Ind. Crops. Prod. 2020, 154, 112677. [Google Scholar] [CrossRef]
- Bardet, R.; Reverdy, C.; Belgacem, N.; Leirset, I.; Syverud, K.; Bardet, M.; Bras, J. Substitution of nanoclay in high gas barrier films of cellulose nanofibrils with cellulose nanocrystals and thermal treatment. Cellulose 2015, 22, 1227–1241. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Kotcharat, P.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of bacterial cellulose and polycaprolactone (PCL) based composite for medical material. Sustain. Chem. Pharm. 2021, 20, 100404. [Google Scholar] [CrossRef]









| Sample | Initial Degradation Temperature (°C) | Maximum Decomposition Temperature Tmax(°C) | Maximum Mass Loss Rate (%/°C) |
|---|---|---|---|
| BSP | 251 | 362 | 2.51 |
| BHP | 247 | 362.1 | 1.93 |
| CBSP | 135 | 183.0 | 0.43 |
| 223.2 | 0.48 | ||
| 379.4 | 0.18 | ||
| CBHP | 135 | 185.9 | 0.62 |
| 214.9 | 0.36 | ||
| 386.6 | 0.17 | ||
| CBSP-D | 137 | 187.9 | 0.36 |
| 224.8 | 0.53 | ||
| 379.4 | 0.19 | ||
| CBHP-D | 135 | 183.5 | 0.36 |
| 225.6 | 0.46 | ||
| 384.4 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, D.; Li, J.; Yang, F.; Sui, G.; Dong, Y. Fabrication and Properties of Tree-Branched Cellulose Nanofibers (CNFs) via Acid Hydrolysis Assisted with Pre-Disintegration Treatment. Nanomaterials 2022, 12, 2089. https://doi.org/10.3390/nano12122089
Li J, Liu D, Li J, Yang F, Sui G, Dong Y. Fabrication and Properties of Tree-Branched Cellulose Nanofibers (CNFs) via Acid Hydrolysis Assisted with Pre-Disintegration Treatment. Nanomaterials. 2022; 12(12):2089. https://doi.org/10.3390/nano12122089
Chicago/Turabian StyleLi, Jun, Dongyan Liu, Junsheng Li, Fei Yang, Guoxin Sui, and Yu Dong. 2022. "Fabrication and Properties of Tree-Branched Cellulose Nanofibers (CNFs) via Acid Hydrolysis Assisted with Pre-Disintegration Treatment" Nanomaterials 12, no. 12: 2089. https://doi.org/10.3390/nano12122089