One-Pot Synthesis of Rubber Seed Shell-Derived N-Doped Ultramicroporous Carbons for Efficient CO2 Adsorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Pretreatment
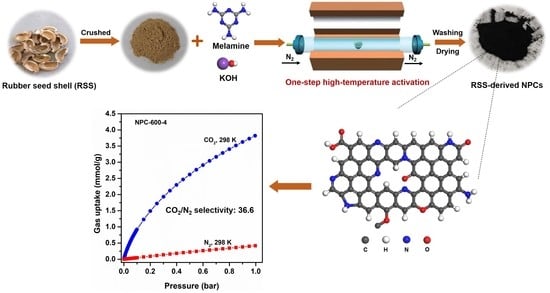
2.2. Preparation of RSS-Derived NPCs
2.3. Instrumentation
2.4. Gas Adsorption Tests
3. Results and Discussion
3.1. Chemical Structures and Morphology
3.2. Textural Properties
3.3. CO2 Adsorption and Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jevrejeva, S.; Jackson, L.P.; Riva, R.E.; Grinsted, A.; Moore, J.C. Coastal sea level rise with warming above 2 degrees C. Proc. Natl. Acad. Sci. USA 2016, 113, 13342–13347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, M.A.; Knutson, T.R.; Tuleya, R.E.; Sirutis, J.J.; Vecchi, G.A.; Garner, S.T.; Held, I.M. Modeled Impact of Anthropogenic Warming on the Frequency of Intense Atlantic Hurricanes. Science 2010, 327, 454–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, B.N.; Venugopal, V.; Sengupta, D.; Madhusoodanan, M.S.; Xavier, P.K. Increasing Trend of Extreme Rain Events Over India in a Warming Environment. Science 2006, 314, 1442–1445. [Google Scholar] [CrossRef] [Green Version]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- Rochelle, G. Conventional amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Bae, T.-H.; Hudson, M.R.; Mason, J.A.; Queen, W.L.; Dutton, J.J.; Sumida, K.; Micklash, K.J.; Kaye, S.S.; Brown, C.M.; Long, J.R. Evaluation of cation-exchanged zeolite adsorbents for post-combustion carbon dioxide capture. Energy Environ. Sci. 2012, 6, 128–138. [Google Scholar] [CrossRef]
- Tumurbaatar, O.; Lazarova, H.; Popova, M.; Mitova, V.; Shestakova, P.; Koseva, N. CO2 Adsorption on the N- and P-Modified Mesoporous Silicas. Nanomaterials 2022, 12, 1224. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frame-works for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2016, 5, 1334–1347. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Zhang, Z.; Cano, Z.P.; Luo, D.; Dou, H.; Yu, A.; Chen, Z. Rational design of tailored porous carbon-based materials for CO2 capture. J. Mater. Chem. A 2019, 7, 20985–21003. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Luo, J.; Liu, B.; Shi, R.; Guo, Y.; Feng, Q.; Liu, Z.; Li, L.; Norinaga, K. The effects of nitrogen functional groups and narrow micropore sizes on CO2 adsorption onto N-doped biomass-based porous carbon under different pressure. Microporous Mesoporous Mater. 2021, 327, 111404. [Google Scholar] [CrossRef]
- Saha, D.; Kienbaum, M.J. Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater. 2019, 287, 29–55. [Google Scholar] [CrossRef]
- Ma, C.; Lu, T.; Shao, J.; Huang, J.; Hu, X.; Wang, L. Biomass derived nitrogen and sulfur co-doped porous carbons for efficient CO2 adsorption. Sep. Purif. Technol. 2021, 281, 119899. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, C.; Chen, F.; Xu, G.; Pang, R.; Qian, X.; Shao, J.; Hu, X. Water caltrop shell-derived nitrogen-doped porous carbons with high CO2 adsorption capacity. Biomass Bioenergy 2021, 145, 105969. [Google Scholar] [CrossRef]
- Li, Q.; Lu, T.; Wang, L.; Pang, R.; Shao, J.; Liu, L.; Hu, X. Biomass based N-doped porous carbons as efficient CO2 adsorbents and high-performance supercapacitor electrodes. Sep. Purif. Technol. 2021, 275, 119204. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem. Eng. J. 2018, 345, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Yang, Y.; Wu, Q.; Liu, B.; Li, D.; Chen, R.; Wang, C.; Li, H.; Zeng, Z.; Li, L. Underlying mechanism of CO2 uptake onto biomass-based porous carbons: Do adsorbents capture CO2 chiefly through narrow micropores? Fuel 2020, 282, 118727. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Rashidi, A.; Jafarinejad, S. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J. Environ. Chem. Eng. 2018, 6, 6653–6663. [Google Scholar] [CrossRef]
- Yang, P.; Rao, L.; Zhu, W.; Wang, L.; Ma, R.; Chen, F.; Lin, G.; Hu, X. Porous Carbons Derived from Sustainable Biomass via a Facile One-Step Synthesis Strategy as Efficient CO2 Adsorbents. Ind. Eng. Chem. Res. 2020, 59, 6194–6201. [Google Scholar] [CrossRef]
- Yang, Q.; Teng, D.; Qu, J.; Li, P.; Cao, Y. Solvent-Free Synthesis of N-Doped Porous Carbons from Chitosan for an Efficient CO2 Capture. Ind. Eng. Chem. Res. 2021, 60, 13023–13030. [Google Scholar] [CrossRef]
- Patel, H.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2016, 10, 1303–1317. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Oluodo, L.A.; Huda, N.; Komilus, C.F. Potential utilization of rubber seed meal as feed and food. Int. J. Eng. Technol. 2018, 7, 64–71. [Google Scholar]
- Yan, K.Z.; Zaini, M.A.A.; Arsad, A.; Nasri, N.S. Rubber seed shell based activated carbon by physical activation for phenol removal. Chem. Eng. Trans. 2019, 72, 151–156. [Google Scholar]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and Modelling Studies of Activated Carbon Produced from Rubber-Seed Shell Using KOH for CO2 Adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef] [Green Version]
- Borhan, A.; Abdullah, N.A.; Rashidi, N.A.; Taha, M.F. Removal of Cu2+ and Zn2+ from Single Metal Aqueous Solution Using Rubber-Seed Shell Based Activated Carbon. Procedia Eng. 2016, 148, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Pagketanang, T.; Artnaseaw, A.; Wongwicha, P.; Thabuot, M. Microporous Activated Carbon from KOH-Activation of Rubber Seed-Shells for Application in Capacitor Electrode. Energy Procedia 2015, 79, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Jiang, J.C. Preparation and characterization of activated carbon from rubber-seed shell by physical activation with steam. Biomass Bioenergy 2010, 34, 539–544. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Li, X.; Jia, C.Q.; Jiang, W. Preparation of high-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for toluene. RSC Adv. 2018, 8, 30171–30179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafin, J.; Cruz, O.F. Promising activated carbons derived from common oak leaves and their application in CO2 storage. J. Environ. Chem. Eng. 2022, 10, 107642. [Google Scholar] [CrossRef]
- Kante, K.; Nieto-Delgado, C.; Rangel-Mendez, J.R.; Bandosz, T.J. Spent coffee-based activated carbon: Specific surface features and their importance for H2S separation process. J. Hazard. Mater. 2012, 201–202, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, K.; Zhang, K.; He, J.; Zhao, Y.; Yu, D. Combinatorial Vibration-Mode Assignment for the FTIR Spectrum of Crystalline Melamine: A Strategic Approach toward Theoretical IR Vibrational Calculations of Triazine-Based Compounds. J. Phys. Chem. A 2016, 120, 7427–7433. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Komatsu, T.; Nakamura, T. Polycondensation/pyrolysis of tris-s-triazine derivatives leading to graphite-like carbon nitrides. J. Mater. Chem. 2000, 11, 474–478. [Google Scholar] [CrossRef]
- Li, D.; Chen, W.; Wu, J.; Jia, C.Q.; Jiang, X. The preparation of waste biomass-derived N-doped carbons and their application in acid gas removal: Focus on N functional groups. J. Mater. Chem. A 2020, 8, 24977–24995. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Full Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Huang, J. Porphyrin-Based Triazine Polymers and Their Derived Porous Carbons for Efficient CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 3205–3212. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, H.; Wei, Z.; Xie, L.; Wang, Y. An Efficient Way To Introduce Hierarchical Structure into Biomass-Based Hydrothermal Carbonaceous Materials. ACS Sustain. Chem. Eng. 2014, 2, 2435–2441. [Google Scholar] [CrossRef]
- Mi, Y.; Hu, W.; Dan, Y.; Liu, Y. Synthesis of carbon micro-spheres by a glucose hydrothermal method. Mater. Lett. 2008, 62, 1194–1196. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas Solid Systems with Special Reference to the Determination of Surface-Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Li, Z.; Xing, B.; Ding, Y.; Li, Y.; Wang, S. A high-performance biochar produced from bamboo pyrolysis with in-situ nitrogen doping and activation for adsorption of phenol and methylene blue. Chin. J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Chu, S.; Wang, C.; Feng, J.; Wang, Y.; Zou, Z. Melem: A metal-free unit for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 13519–13526. [Google Scholar] [CrossRef]
- Borhan, A.; Yusuf, S. Activation of Rubber-Seed Shell Waste by Malic Acid as Potential CO2 Removal: Isotherm and Kinetics Studies. Materials 2020, 13, 4970. [Google Scholar] [CrossRef]
- Rong, M.; Yang, L.; Wang, L.; Xing, H.; Yu, J.; Qu, H.; Liu, H. Fabrication of Microporous Aminal-Linked Polymers with Tunable Porosity toward Highly Efficient Adsorption of CO2, H2, Organic Vapor, and Volatile Iodine. Ind. Eng. Chem. Res. 2019, 58, 17369–17379. [Google Scholar] [CrossRef]
- Rong, M.; Yang, L.; Wang, L.; Yu, J.; Qu, H.; Liu, H. Fabrication of ultramicroporous triphenylamine-based polyaminal networks for low-pressure carbon dioxide capture. J. Colloid Interface Sci. 2019, 548, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Krungleviciute, V.; Heroux, L.; Migone, A.D.; Kingston, C.T.; Simard, B. Isosteric Heat of Argon Adsorbed on Single-Walled Carbon Nanotubes Prepared by Laser Ablation. J. Phys. Chem. B 2005, 109, 9317–9320. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Everett, D.H.; Marshall, C.T.; Paniego, A.R.; Powl, J.C.; Rodriguez-Reinoso, F. Thermodynamics of the high temperature adsorption of some permanent gases by porous carbons. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1974, 70, 2154–2169. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, G.; Wu, C.; Liu, J.; Li, G.; Zhao, Y.; Wang, Y.; Xu, Y. A cost-effective synthesis of heteroatom-doped porous carbon by sulfur-containing waste liquid treatment: As a promising adsorbent for CO2 capture. J. Environ. Chem. Eng. 2021, 9, 105165. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2020, 103, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, A.; Chen, J.; Bao, Y. A green route to CO2 adsorption on biomass chitosan derived nitrogen-doped mi-cropore-dominated carbon nanosheets by different activators. J. Environ. Chem. Eng. 2022, 10, 107021. [Google Scholar] [CrossRef]
- Bai, R.; Yang, M.; Hu, G.; Xu, L.; Hu, X.; Li, Z.; Wang, S.; Dai, W.; Fan, M. A new nanoporous nitrogen-doped highly-efficient carbonaceous CO2 sorbent synthesized with inexpensive urea and petroleum coke. Carbon 2015, 81, 465–473. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption. Ind. Crop. Prod. 2018, 128, 290–297. [Google Scholar] [CrossRef]
- Shao, L.; Liu, M.; Huang, J.; Liu, Y.N. CO2 capture by nitrogen-doped porous carbons derived from nitrogen-containing hy-per-cross-linked polymers. J. Colloid Interface Sci. 2018, 513, 304–313. [Google Scholar] [CrossRef]
- Luo, L.; Chen, T.; Li, Z.; Zhang, Z.; Zhao, W.; Fan, M. Heteroatom self-doped activated biocarbons from fir bark and their excellent performance for carbon dioxide adsorption. J. CO2 Util. 2018, 25, 89–98. [Google Scholar] [CrossRef]
- Shao, L.; Sang, Y.; Huang, J. Imidazole-based hyper-cross-linked polymers derived porous carbons for CO2 capture. Microporous Mesoporous Mater. 2018, 275, 131–138. [Google Scholar] [CrossRef]
- Yue, L.; Xia, Q.; Wang, L.; Wang, L.; DaCosta, H.; Yang, J.; Hu, X. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell. J. Colloid Interface Sci. 2018, 511, 259–267. [Google Scholar] [CrossRef] [PubMed]













| Samples | EA (wt%) | XPS b (wt%) | |||||
|---|---|---|---|---|---|---|---|
| C | N | H | O a | C | N | O | |
| RSS | 51.36 | 0.26 | 6.29 | 57.91 | -- | -- | -- |
| NPC-700-1 | 77.43 | 5.21 | 2.82 | 14.54 | 78.59 | 6.32 | 9.02 |
| NPC-700-2 | 76.97 | 6.65 | 2.76 | 13.62 | 78.37 | 6.56 | 8.95 |
| NPC-700-3 | 76.08 | 7.06 | 2.84 | 14.02 | 76.44 | 6.73 | 10.33 |
| NPC-700-4 | 75.71 | 7.36 | 1.87 | 15.06 | 75.07 | 8.54 | 9.59 |
| NPC-700-5 | 74.04 | 7.52 | 2.67 | 15.77 | 70.03 | 11.93 | 10.33 |
| NPC-500-4 | 68.72 | 5.33 | 3.60 | 22.35 | 66.76 | 5.42 | 19.99 |
| NPC-600-4 | 72.84 | 6.60 | 4.39 | 16.17 | 70.23 | 7.28 | 14.97 |
| NPC-800-4 | 84.27 | 1.58 | 1.10 | 13.05 | 84.22 | 1.59 | 9.49 |
| NPC-900-4 | 85.99 | 1.17 | 1.06 | 11.78 | 86.27 | 1.33 | 8.21 |
| NPC-1000-4 | 87.32 | 0.82 | 1.64 | 10.22 | 92.29 | 0.92 | 4.26 |
| Samples | SBETa (m2/g) | Smicrob (m2/g) | Vmicrob (cm3/g) | Vultramicroc (cm3/g) | Vtotald (cm3/g) | Vmicro/Vtotal |
|---|---|---|---|---|---|---|
| RSS | 40 | 0 | 0 | 0 | 0.049 | 0 |
| NPC-700–1 | 398 | 114 | 0.053 | 0.043 | 0.369 | 0.144 |
| NPC-700-2 | 823 | 436 | 0.187 | 0.135 | 0.575 | 0.325 |
| NPC-700-3 | 1017 | 792 | 0.323 | 0.158 | 0.555 | 0.582 |
| NPC-700-4 | 1190 | 1010 | 0.411 | 0.210 | 0.603 | 0.682 |
| NPC-700-5 | 1139 | 828 | 0.348 | 0.148 | 0.674 | 0.516 |
| NPC-500-4 | 734 | 551 | 0.226 | 0.285 | 0.411 | 0.550 |
| NPC-600-4 | 1246 | 1144 | 0.452 | 0.179 | 0.565 | 0.800 |
| NPC-800-4 | 2163 | 1209 | 0.544 | 0.061 | 1.323 | 0.411 |
| NPC-900-4 | 2152 | 844 | 0.386 | 0.052 | 1.482 | 0.260 |
| NPC-1000-4 | 1494 | 680 | 0.301 | 0.071 | 1.037 | 0.290 |
| Sample | CO2 Uptake (mmol/g) | Q0 a (kJ/mol) | IAST Selectivity b | |
|---|---|---|---|---|
| 273 K | 298 K | CO2/N2 | ||
| NPC-700-1 | 2.44 | 1.22 | 33.9 | 24.6 |
| NPC-700-2 | 3.25 | 1.60 | 34.1 | 26.4 |
| NPC-700-3 | 3.85 | 1.90 | 34.3 | 26.9 |
| NPC-700-4 | 4.45 | 2.33 | 36.4 | 31.1 |
| NPC-700-5 | 3.69 | 2.66 | 34.2 | 28.9 |
| NPC-500-4 | 3.49 | 1.67 | 36.6 | 44.6 |
| NPC-600-4 | 5.81 | 3.82 | 37.4 | 36.6 |
| NPC-800-4 | 3.85 | 1.90 | 26.1 | 10.8 |
| NPC-900-4 | 3.21 | 1.63 | 25.0 | 8.9 |
| NPC-1000-4 | 2.80 | 1.66 | 21.8 | 8.0 |
| Samples | SBET (m2 g−1) | CO2 Uptakes (mmol g−1) | IAST CO2/N2 Selectivity at 298 K | Ref. | |
|---|---|---|---|---|---|
| 273 K/1.0 bar | 298 K/1.0 bar | ||||
| NPC-600-4 | 1246 | 5.81 | 3.82 | 36.6 | This work |
| LS-600-0.3 | 1188 | 5.11 | 3.68 | 16 | [23] |
| HS-500-3 | 1600 | 6.43 | 4.30 | 17 | [17] |
| CN-600-3 | 1082 | 5.12 | 3.71 | 13 | [62] |
| UC-650-2 | 1394 | 6.27 | 4.40 | 17 | [57] |
| NDPC-10%-3 | 1153 | 5.55 | 3.34 | 20.8 (273 K) | [59] |
| NPC-2 | 1384 | 5.86 | - | 18 (273 K) | [61] |
| ACBK3 | 1377 | 7.0 | 5.20 | 32.3 | [60] |
| WSM-550-2 | 1535 | 5.86 | 4.32 | 19 | [18] |
| UC-15-2-600 | 1113 | - | 4.80 | 22 | [58] |
| COL-900 | 1382 | 4.41 | 2.88 | 47 | [34] |
| CN6-750-KOH | 1928 | 5.57 | 3.91 | 15 | [56] |
| PC-SK-2-3-800 | 1418 | 5.61 | 3.82 | 13 | [54] |
| LCM-550-2 | 1487 | 5.44 | 3.87 | 20 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Rong, M.; Cao, H.; Tan, T. One-Pot Synthesis of Rubber Seed Shell-Derived N-Doped Ultramicroporous Carbons for Efficient CO2 Adsorption. Nanomaterials 2022, 12, 1889. https://doi.org/10.3390/nano12111889
Zhang X, Rong M, Cao H, Tan T. One-Pot Synthesis of Rubber Seed Shell-Derived N-Doped Ultramicroporous Carbons for Efficient CO2 Adsorption. Nanomaterials. 2022; 12(11):1889. https://doi.org/10.3390/nano12111889
Chicago/Turabian StyleZhang, Xiaoxia, Meng Rong, Hui Cao, and Tianwei Tan. 2022. "One-Pot Synthesis of Rubber Seed Shell-Derived N-Doped Ultramicroporous Carbons for Efficient CO2 Adsorption" Nanomaterials 12, no. 11: 1889. https://doi.org/10.3390/nano12111889