Green Synthesis of Nanomaterials
Abstract
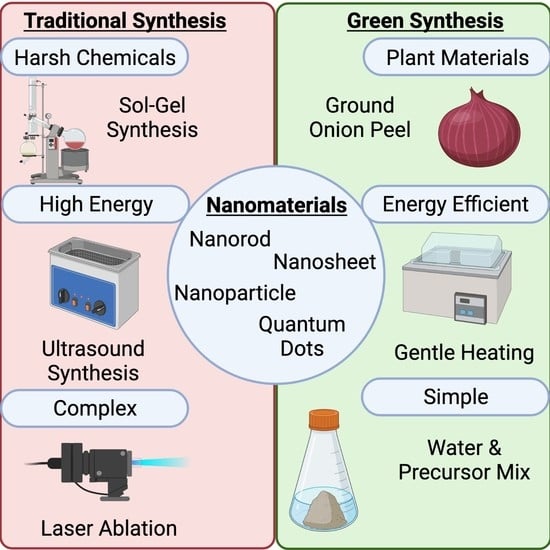
:1. Introduction
2. Traditional Synthesis Methods
2.1. Sol-Gel Synthesis
2.2. Chemical Vapor Deposition
2.3. Hydrothermal Synthesis
2.4. Ultrasound Synthesis
2.5. Laser Ablation
2.6. Flame Spray Pyrolysis
3. Green Synthesis
3.1. Background
- Prevention—Steps must be taken to prevent the production of waste.
- Atom Economy—As much as possible, the materials used in the synthesis should be incorporated into the final product.
- Less Hazardous Chemical Synthesis—Synthesis methods that require materials with minimal or no toxicity to the environment or individual should be prioritized.
- Designing Safer Chemicals—Chemicals should designed to achieve function with limited or no toxicity.
- Safer Solvents—The use of solvents and auxiliary chemicals should not be used when possible.
- Design for Energy Efficiency—Energy usage should be limited for synthesis.
- Use of Renewable Feedstocks—a feedstock should be renewable and depletion should be avoided whenever possible.
- Reduce Derivatives—Derivatives such as blocking agents and protecting/deprotecting groups should be avoided whenever possible as they cause additional waste.
- Catalysis—Catalysis agents are preferable to stoichiometric agents.
- Design for Degradation—Chemicals should be designed so that at the end of synthesis, they will break down into non-toxic derivatives.
- Real-time Analysis for Pollution Prevention—Synthesis should be monitored in real-time for toxic chemical production.
- Inherently Safer Chemistry for Accident Prevention—Agents used in product synthesis should be selected to limit the possibility of hazardous accidents.
- S—Select direct analytical technique
- I—Integrate analytical processes and operations
- G—Generate as little waste as possible and treat it properly
- N—Never waste energy
- I—Implement automation and miniaturization of methods
- F—Favor agents obtained from renewable source
- I—Increase safety of operator
- C—Carry out in-situ measurements
- A—Avoid derivatization
- N—Note the sample number and size should be minimal
- C—Choose multi-analyte or multi-parameter methods
- E—Eliminate or replace toxic reagents
3.2. 0D Nanomaterials
3.2.1. Bacteria
3.2.2. Yeast (Live and Extract)
3.2.3. Fungi
3.2.4. Algal Species
3.2.5. Plant and Plant Extract
3.3. 1D Nanomaterials
3.4. Higher Ordered Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yap, Y.H.; Azmi, A.A.; Mohd, N.K.; Yong, F.S.J.; Kan, S.-Y.; Thirmizir, M.Z.A.; Chia, P.W. Green Synthesis of Silver Nanoparticle Using Water Extract of Onion Peel and Application in the Acetylation Reaction. Arab. J. Sci. Eng. 2020, 45, 4797–4807. [Google Scholar] [CrossRef]
- Benefits and Applications of Nanotechnology. Available online: https://www.nano.gov/you/nanotechnology-benefits (accessed on 17 August 2021).
- Devatha, C.P.; Thalla, A.K. Chapter 7—Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 169–184. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, S.; Anzabi, Y.; Jafarizadeh-Malmiri, H. Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae—Fabrication and characterization. Arch. Microbiol. 2020, 202, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial Biosynthesis of Cadmium Sulfide Nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.J.; Zhang, Z.M.; Guo, Y.; Yang, G.E. Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloids Surf. B Biointerfaces 2009, 70, 142–146. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Grohens, Y.; Kokol, V.; Kalarikkal, N.; Thomas, S. Chapter 1—Synthesis and Processing of Emerging Two-Dimensional Nanomaterials. In Nanomaterials Synthesis; Beeran Pottathara, Y., Thomas, S., Kalarikkal, N., Grohens, Y., Kokol, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Wegner, K.; Schimmöller, B.; Thiebaut, B.; Fernandez, C.; Rao, T.N. Pilot Plants for Industrial Nanoparticle Production by Flame Spray Pyrolysis. KONA Powder Part. J. 2011, 29, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Ion, J.C. Laser Processing of Engineering Materials: Principal Procedure and Industrial Application; Elsevier: Oxford, UK, 2006; p. 441. [Google Scholar]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Su, D.; Ahn, H.-J.; Wang, G. Hydrothermal synthesis of α-MnO2 and β-MnO2 nanorods as high capacity cathode materials for sodium ion batteries. J. Mater. Chem. A 2013, 1, 4845–4850. [Google Scholar] [CrossRef] [Green Version]
- Hosono, E.; Saito, T.; Hoshino, J.; Okubo, M.; Saito, Y.; Nishio-Hamane, D.; Kudo, T.; Zhou, H. High power Na-ion rechargeable battery with single-crystalline Na0.44MnO2 nanowire electrode. J. Power Sources 2012, 217, 43–46. [Google Scholar] [CrossRef]
- Song, H. One-step Convenient Hydrothermal Synthesis of MoS2/RGO as a High-performance Anode for Sodium-ion Batteries. Int. J. Electrochem. Sci. 2018, 13, 4720–4730. [Google Scholar] [CrossRef]
- Lin, B.; Zhu, X.; Fang, L.; Liu, X.; Li, S.; Zhai, T.; Xue, L.; Guo, Q.; Xu, J.; Xia, H. Birnessite Nanosheet Arrays with High K Content as a High-Capacity and Ultrastable Cathode for K-Ion Batteries. Adv. Mater. 2019, 31, 1900060. [Google Scholar] [CrossRef]
- Walter, J.G.; Petersen, S.; Stahl, F.; Scheper, T.; Barcikowski, S. Laser ablation-based one-step generation and bio-functionalization of gold nanoparticles conjugated with aptamers. J. Nanobiotechnol. 2010, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Salmaso, S.; Caliceti, P.; Amendola, V.; Meneghetti, M.; Magnusson, J.P.; Pasparakis, G.; Alexander, C. Cell up-take control of gold nanoparticles functionalized with a thermoresponsive polymer. J. Mater. Chem. 2009, 19, 1608–1615. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Aboulouard, A.; Gultekin, B.; Can, M.; Erol, M.; Jouaiti, A.; Elhadadi, B.; Zafer, C.; Demic, S. Dye sensitized solar cells based on titanium dioxide nanoparticles synthesized by flame spray pyrolysis and hydrothermal sol-gel methods: A comparative study on photovoltaic performances. J. Mater. Res. Technol. 2020, 9, 1569–1577. [Google Scholar] [CrossRef]
- Pawinrat, P.; Mekasuwandumrong, O.; Panpranot, J. Synthesis of Au–ZnO and Pt–ZnO nanocomposites by one-step flame spray pyrolysis and its application for photocatalytic degradation of dyes. Catal. Commun. 2009, 10, 1380–1385. [Google Scholar] [CrossRef]
- Joshi, D.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental risk-based ranking of solvents using the combination of a multimedia model and multi-criteria decision analysis. Green Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Akinyemi, P.A.; Adegbenro, C.A.; Ojo, T.O.; Elugbaju, O. Neurobehavioral Effects of Organic Solvents Exposure Among Wood Furniture Makers in Ile-Ife, Osun State, Southwestern Nigeria. J. Health Pollut. 2019, 9, 190604. [Google Scholar] [CrossRef]
- Mueller, R.; Jossen, R.; Pratsinis, S.E.; Watson, M.; Akhtar, M.K. Zirconia Nanoparticles Made in Spray Flames at High Production Rates. J. Am. Ceram. Soc. 2004, 87, 197–202. [Google Scholar] [CrossRef]
- Strobel, R.; Baiker, A.; Pratsinis, S.E. Aerosol flame synthesis of catalysts. Adv. Powder Technol. 2006, 17, 457–480. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Choi, K.-M.; Kim, T.-H.; Kim, K.-S.; Kim, S.-G. Case Study. J. Occup. Environ. Hyg. 2013, 10, D1–D5. [Google Scholar] [CrossRef]
- Caramazana, P.; Dunne, P.; Gimeno-Fabra, M.; McKechnie, J.; Lester, E. A review of the environmental impact of nanomaterial synthesis using continuous flow hydrothermal synthesis. Curr. Opin. Green Sustain. Chem. 2018, 12, 57–62. [Google Scholar] [CrossRef]
- Pourzahedi, L.; Eckelman, M.J. Comparative life cycle assessment of silver nanoparticle synthesis routes. Environ. Sci. Nano 2015, 2, 361–369. [Google Scholar] [CrossRef]
- Sivaraj, A.; Kumar, V.; Sunder, R.; Parthasarathy, K.; Kasivelu, G. Commercial Yeast Extracts Mediated Green Synthesis of Silver Chloride Nanoparticles and their Anti-mycobacterial Activity. J. Clust. Sci. 2020, 31, 287–291. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef]
- Ganesh Babu, M.M.; Gunasekaran, P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf. B Biointerfaces 2009, 74, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004, 14, 3303–3305. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the Surface Properties of Superparamagnetic Iron Oxide Nanoparticles through A Sol−Gel Approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum nanoparticles-doped sol–gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef]
- Sattar, U.; Ikram, M.; Junaid, M.; Aqeel, M.; Imran, M.; Ali, S. Annealing effect on synthesized ZnS/TiO2 nanocomposite for treatment of industrial wastewater. Mater. Res. Express 2019, 6, 115050. [Google Scholar] [CrossRef]
- Zapata, L.E.; Portela, G.; Suárez, O.M.; Carrasquillo, O. Rheological performance and compressive strength of superplasticized cementitious mixtures with micro/nano-SiO2 additions. Constr. Build. Mater. 2013, 41, 708–716. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, H.-E.; Jeong, S.-H. One-pot synthesis of silane-modified hyaluronic acid hydrogels for effective antibacterial drug delivery via sol–gel stabilization. Colloids Surf. B Biointerfaces 2019, 174, 308–315. [Google Scholar] [CrossRef]
- Doufène, K.; Lapinte, V.; Gaveau, P.; Félix, G.; Cacciaguerra, T.; Chopineau, J.; Robin, J.-J.; Devoisselle, J.-M.; Aubert-Pouëssel, A. Tunable vegetable oil/silica hybrid microparticles for poorly water-soluble drug delivery. Int. J. Pharm. 2019, 567, 118478. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- MacCraith, B.D.; McDonagh, C.M.; O’Keeffe, G.; McEvoy, A.K.; Butler, T.; Sheridan, F.R. Sol-gel coatings for optical chemical sensors and biosensors. Sens. Actuators B Chem. 1995, 29, 51–57. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C.; Gupta, V.; Tomar, M. Synthesis of CdS nanoparticle by sol-gel method as low temperature NO2 sensor. Mater. Chem. Phys. 2020, 239, 121975. [Google Scholar] [CrossRef]
- Abramova, A.V.; Abramov, V.O.; Bayazitov, V.M.; Voitov, Y.; Straumal, E.A.; Lermontov, S.A.; Cherdyntseva, T.A.; Braeutigam, P.; Weiße, M.; Günther, K. A sol-gel method for applying nanosized antibacterial particles to the surface of textile materials in an ultrasonic field. Ultrason. Sonochem. 2020, 60, 104788. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef]
- Martin, P.M. (Ed.) Chapter 1—Deposition Technologies: An Overview. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 1–31. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Pollard, A.J.; Nair, R.R.; Sabki, S.N.; Staddon, C.R.; Perdigao, L.M.A.; Hsu, C.H.; Garfitt, J.M.; Gangopadhyay, S.; Gleeson, H.F.; Geim, A.K.; et al. Formation of Monolayer Graphene by Annealing Sacrificial Nickel Thin Films. J. Phys. Chem. C 2009, 113, 16565–16567. [Google Scholar] [CrossRef]
- Park, S.; Lim, S.; Choi, H. Chemical Vapor Deposition of Iron and Iron Oxide Thin Films from Fe(II) Dihydride Complexes. Chem. Mater. 2006, 18, 5150–5152. [Google Scholar] [CrossRef]
- Wong, S.L.; Liu, H.; Chi, D. Recent progress in chemical vapor deposition growth of two-dimensional transition metal dichalcogenides. Prog. Cryst. Growth Charact. Mater. 2016, 62, 9–28. [Google Scholar] [CrossRef]
- Mojica, M.; Alonso, J.A.; Méndez, F. Synthesis of fullerenes. J. Phys. Org. Chem. 2013, 26, 526–539. [Google Scholar] [CrossRef]
- Cassell, A.M.; Raymakers, J.A.; Kong, J.; Dai, H. Large Scale CVD Synthesis of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 6484–6492. [Google Scholar] [CrossRef]
- Umeno, M.; Adhikary, S. Diamond-like carbon thin films by microwave surface-wave plasma CVD aimed for the application of photovoltaic solar cells. Diam. Relat. Mater. 2005, 14, 1973–1979. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Cai, X.; Ma, B.; Chen, Z.; Priydarshi, M.; Chen, K.; Gao, T.; Song, X.; Ji, Q.; et al. Direct low temperature synthesis of graphene on arbitrary glasses by plasma-enhanced CVD for versatile, cost-effective electrodes. Nano Res. 2015, 8, 3496–3504. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Jiang, L.; Xu, Z.; Huang, L.; Xue, Y.; Geng, D.; Wu, B.; Hu, W.; Yu, G.; et al. Near-Equilibrium Chemical Vapor Deposition of High-Quality Single-Crystal Graphene Directly on Various Dielectric Substrates. Adv. Mater. 2014, 26, 1348–1353. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Chen, Z.; Yuan, L.; Chen, Y.; Ning, J.; Liu, S.; Ma, D.; Song, X.; Priydarshi, M.K.; Bachmatiuk, A.; et al. Direct Chemical-Vapor-Deposition-Fabricated, Large-Scale Graphene Glass with High Carrier Mobility and Uniformity for Touch Panel Applications. ACS Nano 2016, 10, 11136–11144. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Priydarshi, M.K.; Chen, Z.; Bachmatiuk, A.; Zou, Z.; Chen, Z.; Song, X.; Gao, Y.; Rümmeli, M.H.; et al. Direct Chemical Vapor Deposition-Derived Graphene Glasses Targeting Wide Ranged Applications. Nano Lett. 2015, 15, 5846–5854. [Google Scholar] [CrossRef]
- Ma, Y.; Jang, H.; Kim, S.J.; Pang, C.; Chae, H. Copper-Assisted Direct Growth of Vertical Graphene Nanosheets on Glass Substrates by Low-Temperature Plasma-Enhanced Chemical Vapour Deposition Process. Nanoscale Res. Lett. 2015, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Tiginyanu, I.M.; Chow, L.; Ono, L.K.; Roldan Cuenya, B.; et al. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Plata, D.L.; Hart, A.J.; Reddy, C.M.; Gschwend, P.M. Early Evaluation of Potential Environmental Impacts of Carbon Nanotube Synthesis by Chemical Vapor Deposition. Environ. Sci. Technol. 2009, 43, 8367–8373. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.L.; Dahlben, L.J.; Isaacs, J.A. Environmental Assessment of Single-Walled Carbon Nanotube Processes. J. Ind. Ecol. 2008, 12, 376–393. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. 3—Metal oxide photocatalysts. In Metal Oxide-Based Photocatalysis; Zaleska-Medynska, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–209. [Google Scholar] [CrossRef]
- Schäf, O.; Ghobarkar, H.; Knauth, P. Hydrothermal Synthesis of Nanomaterials. In Nanostructured Materials: Selected Synthesis Methods Properties and Applications; Knauth, P., Schoonman, J., Eds.; Springer: Boston, MA, USA, 2002; pp. 23–41. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R. 2—Synthesis, design, and morphology of metal oxide nanostructures. In Metal Oxide Nanostructures; Nunes, D., Pimentel, A., Santos, L., Barquinha, P., Pereira, L., Fortunato, E., Martins, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–57. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.-t.; Chiang, C.-J.; Ray, D. Hydrothermal synthesis of chalcopyrite using an environmental friendly chelating agent. Mater. Lett. 2013, 98, 270–272. [Google Scholar] [CrossRef]
- Mehraz, S.; Kongsong, P.; Taleb, A.; Dokhane, N.; Sikong, L. Large scale and facile synthesis of Sn doped TiO2 aggregates using hydrothermal synthesis. Sol. Energy Mater. Sol. Cells 2019, 189, 254–262. [Google Scholar] [CrossRef]
- Patil, A.; Bhanage, B. Sonochemistry: A Greener Protocol for Nanoparticles Synthesis. In Handbook of Nanoparticles; Springer: Cham, Switzerland, 2016; pp. 143–166. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Kharissova, O.; Kharisov, B.; Valdés, J.; Ortiz Mendez, U. Ultrasound in Nanochemistry: Recent Advances. Synth. React. Inorg. Metal-Org. Nano-Met. Chem. 2011, 41, 429–448. [Google Scholar] [CrossRef]
- Young, F.R. Cavitation; World Scientific: Singapore, 1999; p. 418. [Google Scholar]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Oxley, J.D.; Mdleleni, M.M.; Suslick, K.S. Hydrodehalogenation with sonochemically prepared Mo2C and W2C. Catal. Today 2004, 88, 139–151. [Google Scholar] [CrossRef]
- Cui, C.; Quan, X.; Yu, H.; Han, Y. Electrocatalytic hydrodehalogenation of pentachlorophenol at palladized multiwalled carbon nanotubes electrode. Appl. Catal. B Environ. 2008, 80, 122–128. [Google Scholar] [CrossRef]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Cao, M.; Liu, T.; Gao, S.; Sun, G.; Wu, X.; Hu, C.; Wang, Z.L. Single-Crystal Dendritic Micro-Pines of Magnetic α-Fe2O3: Large-Scale Synthesis, Formation Mechanism, and Properties. Angew. Chem. Int. Ed. 2005, 44, 4197–4201. [Google Scholar] [CrossRef]
- Han, Y.; Radziuk, D.; Shchukin, D.; Moehwald, H. Sonochemical Synthesis of Magnetic Protein Container for Targeted Delivery. Macromol. Rapid Commun. 2008, 29, 1203–1207. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry and the environment—Providing a “green” link between chemistry, physics and engineering. Ultrason. Sonochem. 2007, 14, 476–483. [Google Scholar] [CrossRef]
- Hujjatul Islam, M.; Paul, M.T.Y.; Burheim, O.S.; Pollet, B.G. Recent developments in the sonoelectrochemical synthesis of nanomaterials. Ultrason. Sonochem. 2019, 59, 104711. [Google Scholar] [CrossRef]
- Besner, S.; Meunier, M. Laser Synthesis of Nanomaterials. In Laser Precision Microfabrication; Sugioka, K., Meunier, M., Piqué, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 163–187. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, S.; Singh, S.C.; Lal, B.; Singh, N.B. Applications of Liquid Assisted Pulsed Laser Ablation Synthesized TiO2 Nanoparticles on Germination, Growth and Biochemical Parameters of Brassica oleracea var. Capitata. Sci. Adv. Mater. 2012, 4, 522–531. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Laser ablation-based synthesis of functionalized colloidal nanomaterials in biocompatible solutions. J. Photochem. Photobiol. A Chem. 2006, 182, 330–334. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Singh, A.; Swihart, M.T.; Zavestovskaya, I.N.; Prasad, P.N. Laser-Processed Nanosilicon: A Multifunctional Nanomaterial for Energy and Healthcare. ACS Nano 2019, 13, 9841–9867. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free Silver Nanoparticles Synthesized by Laser Ablation in Organic Solvents and Their Easy Functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Laser Ablation Synthesis of Gold Nanoparticles in Organic Solvents. J. Phys. Chem. B 2006, 110, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic Nanoparticles of Iron Carbide, Iron Oxide, Iron@Iron Oxide, and Metal Iron Synthesized by Laser Ablation in Organic Solvents. J. Phys. Chem. C 2011, 115, 5140–5146. [Google Scholar] [CrossRef]
- Kushnir, D.; Sandén, B.A. Energy Requirements of Carbon Nanoparticle Production. J. Ind. Ecol. 2008, 12, 360–375. [Google Scholar] [CrossRef]
- Weidenhof, B.; Reiser, M.; Stöwe, K.; Maier, W.F.; Kim, M.; Azurdia, J.; Gulari, E.; Seker, E.; Barks, A.; Laine, R.M. High-Throughput Screening of Nanoparticle Catalysts Made by Flame Spray Pyrolysis as Hydrocarbon/NO Oxidation Catalysts. J. Am. Chem. Soc. 2009, 131, 9207–9219. [Google Scholar] [CrossRef]
- Pisduangdaw, S.; Panpranot, J.; Methastidsook, C.; Chaisuk, C.; Faungnawakij, K.; Praserthdam, P.; Mekasuwandumrong, O. Characteristics and catalytic properties of Pt–Sn/Al2O3 nanoparticles synthesized by one-step flame spray pyrolysis in the dehydrogenation of propane. Appl. Catal. A Gen. 2009, 370, 1–6. [Google Scholar] [CrossRef]
- Laine, R.M.; Hinklin, T.; Williams, G.; Rand, S.C. Low-Cost Nanopowders for Phosphor and Laser Applications by Flame Spray Pyrolysis. J. Metastable Nanocryst. Mater. 2000, 8, 500–510. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Jones, A.D.; Turn, S.Q.; Williams, R.B. Emission Factors for Polycyclic Aromatic Hydrocarbons from Biomass Burning. Environ. Sci. Technol. 1996, 30, 2462–2469. [Google Scholar] [CrossRef]
- Aislabie, J.; Saul, D.J.; Foght, J.M. Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles 2006, 10, 171–179. [Google Scholar] [CrossRef]
- Dibble, J.T.; Bartha, R. Effect of environmental parameters on the biodegradation of oil sludge. Appl. Environ. Microbiol. 1979, 37, 729. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Obbard, J.P. Effect of Nutrient Amendments on Indigenous Hydrocarbon Biodegradation in Oil-Contaminated Beach Sediments. J. Environ. Qual. 2003, 32, 1234–1243. [Google Scholar] [CrossRef] [Green Version]
- Anastas, P.; Warner, J. Green Chemistry; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the significance mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Tăbăran, A.-F.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver Nanoparticles for the Therapy of Tuberculosis. Int. J. Nanomed. 2020, 15, 2231–2258. [Google Scholar] [CrossRef] [Green Version]
- Moulton, M.C.; Braydich-Stolle, L.K.; Nadagouda, M.N.; Kunzelman, S.; Hussain, S.M.; Varma, R.S. Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale 2010, 2, 763–770. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V. Chapter 2—Sustainable Delivery Systems Through Green Nanotechnology. In Nano- and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Lu, Y.; Ozcan, S. Green nanomaterials: On track for a sustainable future. Nano Today 2015, 10, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z. Green Chemistry: Recent Advances in Developing Catalytic Processes in Environmentally-Benign Solvent Systems. In Frontiers of Chemistry; University of Pittsburgh: Pittsburgh, PA, USA, 2018. [Google Scholar]
- Stephenson, P.; Licence, P.; Ross, S.K.; Poliakoff, M. Continuous catalytic asymmetric hydrogenation in supercritical CO2. Green Chem. 2004, 6, 521–523. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Lane, M.K.M.; Zimmerman, J.B. Controlling metal oxide nanoparticle size and shape with supercritical fluid synthesis. Green Chem. 2019, 21, 3769–3781. [Google Scholar] [CrossRef]
- Shen, H.; Duan, C.; Guo, J.; Zhao, N.; Xu, J. Facile in situ synthesis of silver nanoparticles on boron nitride nanosheets with enhanced catalytic performance. J. Mater. Chem. A 2015, 3, 16663–16669. [Google Scholar] [CrossRef]
- Markus, J.; Mathiyalagan, R.; Kim, Y.-J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzym. Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef]
- Mathivanan, K.; Selva, R.; Chandirika, J.U.; Govindarajan, R.K.; Srinivasan, R.; Annadurai, G.; Duc, P.A. Biologically synthesized silver nanoparticles against pathogenic bacteria: Synthesis, calcination and characterization. Biocatal. Agric. Biotechnol. 2019, 22, 101373. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Mao, J.; Kwak, I.S.; Yun, Y.S. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010, 162, 989–996. [Google Scholar] [CrossRef]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.V.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular Synthesis of Crystalline Silver Nanoparticles and Molecular Evidence of Silver Resistance from Morganella sp.: Towards Understanding Biochemical Synthesis Mechanism. ChemBioChem 2008, 9, 1415–1422. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M.A. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, S.; Hu, W.; Shi, S.; Shen, W.; Zhang, X.; Wang, H. In situ synthesis of CdS nanoparticles on bacterial cellulose nanofibers. Carbohydr. Polym. 2009, 76, 509–512. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K.; Kulkarni, A.R. Lactobacillus assisted synthesis of titanium nanoparticles. Nanoscale Res. Lett. 2007, 2, 248. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.-J.; Zhang, Z.-M.; Gong, J. Biological Synthesis of Semiconductor Zinc Sulfide Nanoparticles by Immobilized Rhodobacter sphaeroides. Biotechnol. Lett. 2006, 28, 1135–1139. [Google Scholar] [CrossRef]
- Mann, S. Structure, Morphology, and Crystal Growth of Bacterial Magnetite. In Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism; Kirschvink, J.L., Jones, D.S., MacFadden, B.J., Eds.; Springer: Boston, MA, USA, 1985; pp. 311–332. [Google Scholar] [CrossRef] [Green Version]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Purdon, A.M.; Chu, V.; Westervelt, R.M. Controlled Assembly of Magnetic Nanoparticles from Magnetotactic Bacteria Using Microelectromagnets Arrays. Nano Lett. 2004, 4, 995–998. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.-W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S.K. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520. [Google Scholar] [CrossRef]
- Labrenz, M.; Druschel, G.K.; Thomsen-Ebert, T.; Gilbert, B.; Welch, S.A.; Kemner, K.M.; Logan, G.A.; Summons, R.E.; Stasio, G.D.; Bond, P.L.; et al. Formation of Sphalerite (ZnS) Deposits in Natural Biofilms of Sulfate-Reducing Bacteria. Science 2000, 290, 1744. [Google Scholar] [CrossRef] [Green Version]
- Gow, N.A.R.; Yadav, B. Microbe Profile: Candida albicans: A shape-changing, opportunistic pathogenic fungus of humans. Microbiology 2017, 163, 1145–1147. [Google Scholar] [CrossRef]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2002, 14, 95–100. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K. A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 2009, 43, 303–306. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Monodisperse Gold Nanoparticles by a Novel Extremophilic Actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Agnihotri, M.; Joshi, S.; Kumar, A.R.; Zinjarde, S.; Kulkarni, S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 2009, 63, 1231–1234. [Google Scholar] [CrossRef]
- Prasad, K.; Jha, A.K. Biosynthesis of CdS nanoparticles: An improved green and rapid procedure. J. Colloid Interface Sci. 2010, 342, 68–72. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Green synthesis of AgNPs using Cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.; Awad, M.; Abo-Dahab, N.; Elkady, M. Extracellular synthesis of silver nanoparticles by marine derived fungus Aspergillus terreus MALEX. Nanomed. Nanotechnol. Biol. Med. 2014, 11, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 2008, 11, 2079. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.; Kumar, R.; Sastry, M. Extra-/Intracellular Biosynthesis of Gold Nanoparticles by an Alkalotolerant Fungus, Trichothecium sp. J. Biomed. Nanotechnol. 2005, 1, 47–53. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef]
- Devi, L.S.; Bareh, D.A.; Joshi, S.R. Studies on Biosynthesis of Antimicrobial Silver Nanoparticles Using Endophytic Fungi Isolated from the Ethno-medicinal Plant Gloriosa superba L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 1091–1099. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.K.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef]
- Ingle, A.; Gade, A.; Pierrat, S.; Sönnichsen, C.; Rai, M. Mycosynthesis of Silver Nanoparticles Using the Fungus Fusarium acuminatum and its Activity Against Some Human Pathogenic Bacteria. Curr. Nanosci. 2008, 141–144. [Google Scholar] [CrossRef]
- Mohammadian, A. Fusarium oxysporum Mediates Photogeneration of Silver Nanoparticles. Sci. Iran. 2007, 14, 323–326. [Google Scholar]
- Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Fungus mediated synthesis of silver nanoparticles: A novel biological approach. Indian J. Phys. 2004, 78, 101–105. [Google Scholar]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Shaligram, N.S.; Bule, M.; Bhambure, R.; Singhal, R.S.; Singh, S.K.; Szakacs, G.; Pandey, A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009, 44, 939–943. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei, E.; Naghibi, F. Green Synthesis of Silver Nanoparticles Induced by theFungus Penicillium citrinum. Trop. J. Pharm. Res. 2013, 12, 7–11. [Google Scholar] [CrossRef]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.A.; Dhivya, B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar] [CrossRef]
- Datta, D.; Desai, D. Green synthesis of silver antimicrobials for its potential application in control of nosocomial infections. Asian J. Pharm. Clin. Res. 2015, 8, 219–223. [Google Scholar]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tsuruda, Y.; Furukawa, T.; Negishi, L.; Imura, Y.; Sakuda, S.; Yoshimura, E.; Suzuki, M. Synthesis of CdSe Quantum Dots Using Fusarium oxysporum. Materials 2016, 9, 855. [Google Scholar] [CrossRef]
- Riddin, T.L.; Gericke, M.; Whiteley, C.G. Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 2006, 17, 3482–3489. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, W.W.; Mahmood, N.A.; Hyde, E.G. Natural Toxins from Cyanobacteria (Blue-Green Algae). In Marine Toxins; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 87–106. [Google Scholar]
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater. Lett. 2012, 79, 116–118. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Microalga Scenedesmus sp.: A potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. 2014, 24, 522–533. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Pal, R. Synthesis and Characterization of Nanosilver—A blue green approach. Indian J. Appl. Res. 2011, 4, 54–56. [Google Scholar] [CrossRef]
- Sinha, S.N.; Paul, D.; Halder, N.; Sengupta, D.; Patra, S.K. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci. 2015, 5, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Ponnuchamy, K.; Selvi, S.; Prabha, L.; Premkumar, K.; Ganeshkumar, R.; Munisamy, G. Synthesis of silver nanoparticles from Sargassum tenerrimum and screening phytochemicals for its anti-bacterial activity. Nano Biomed. Eng. 2012, 4, 12–16. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Nuraje, N.; Lei, Y.; Belcher, A. Virus-templated visible spectrum active perovskite photocatalyst. Catal. Commun. 2014, 44, 68–72. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Dinesh, D.; Panneerselvam, C.; Subramaniam, J.; Kumar, P.M.; Suresh, U.; Roni, M.; et al. Sargassum muticum-synthesized silver nanoparticles: An effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 2015, 114, 4305–4317. [Google Scholar] [CrossRef] [PubMed]
- Salari, Z.; Danafar, F.; Dabaghi, S.; Ataei, S.A. Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. J. Saudi Chem. Soc. 2016, 20, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Murugan, K.; Samidoss, C.M.; Panneerselvam, C.; Higuchi, A.; Roni, M.; Suresh, U.; Chandramohan, B.; Subramaniam, J.; Madhiyazhagan, P.; Dinesh, D.; et al. Seaweed-synthesized silver nanoparticles: An eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol. Res. 2015, 114, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef] [Green Version]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Isaac, G.; Renitta, E. Brown algae mediated synthesis, characterization of gold nano particles using padina pavonica and their antibacterial activity against human pathogens. Int. J. PharmTech Res. 2015, 8, 31–40. [Google Scholar]
- Yang, F.; Li, Y.; Liu, T.; Xu, K.; Zhang, L.; Xu, C.; Gao, J. Plasma synthesis of Pd nanoparticles decorated-carbon nanotubes and its application in Suzuki reaction. Chem. Eng. J. 2013, 226, 52–58. [Google Scholar] [CrossRef]
- Arockiya Aarthi Rajathi, F.; Parthiban, C.; Ganesh Kumar, V.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef]
- Singh, R.; Misra, V.; Mudiam, M.K.R.; Chauhan, L.K.S.; Singh, R.P. Degradation of γ-HCH spiked soil using stabilized Pd/Fe0 bimetallic nanoparticles: Pathways, kinetics and effect of reaction conditions. J. Hazard. Mater. 2012, 237–238, 355–364. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.V.; Nachane, R.P.; Balasubramanya, R.H. Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf. B Biointerfaces 2006, 53, 55–59. [Google Scholar] [CrossRef]
- Momeni, S.; Nabipour, I. A Simple Green Synthesis of Palladium Nanoparticles with Sargassum Alga and Their Electrocatalytic Activities Towards Hydrogen Peroxide. Appl. Biochem. Biotechnol. 2015, 176, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, K.; Chelliah, R.; Shanmugam, S.; Varukattu, N.B.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. J. Photochem. Photobiol. B 2018, 185, 126–135. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Vidrevich, M.B.; Brainina, K.Z. The Effect of the Antioxidant Activity of Plant Extracts on the Properties of Gold Nanoparticles. Nanomaterials 2019, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Fardsadegh, B.; Jafarizadeh, H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process. Synth. 2019, 8, 399–407. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Sasidharan, D.; Namitha, T.R.; Johnson, S.P.; Jose, V.; Mathew, P. Synthesis of silver and copper oxide nanoparticles using Myristica fragrans fruit extract: Antimicrobial and catalytic applications. Sustain. Chem. Pharm. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Giordano, C.; Musetti, R. In vivo synthesis of nanomaterials in plants: Location of silver nanoparticles and plant metabolism. Nanoscale Res. Lett. 2014, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-González, M.T.; Díaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chávez Morales, R.M.; Regalado Soto, D.I.; Treviño González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef] [Green Version]
- Ghanbar, F.; Mirzaie, A.; Ashrafi, F.; Noorbazargan, H.; Dalirsaber Jalali, M.; Salehi, S.; Sadat Shandiz, S.A. Antioxidant, antibacterial and anticancer properties of phyto-synthesised Artemisia quttensis Podlech extract mediated AgNPs. IET Nanobiotechnol. 2017, 11, 485–492. [Google Scholar] [CrossRef]
- Almalah, H.; Alzahrani, H.; Abdelkader, H. Green Synthesis of Silver Nanoparticles using Cinnamomum Zylinicum and their Synergistic Effect against Multi-Drug Resistance Bacteria. J. Nanotechnol. Res. 2019, 1, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Kelkawi, A.H.A.; Abbasi Kajani, A.; Bordbar, A.K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017, 11, 370–376. [Google Scholar] [CrossRef]
- Butola, B.S.; Gupta, A.; Roy, A. Multifunctional finishing of cellulosic fabric via facile, rapid in-situ green synthesis of AgNPs using pomegranate peel extract biomolecules. Sustain. Chem. Pharm. 2019, 12, 100135. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, J.A.; Khan, E.; Perwez, M.; Gupta, M.; Kumar, S.; Raza, K.; Sardar, M. Exposure of biosynthesized nanoscale ZnO to Brassica juncea crop plant: Morphological, biochemical and molecular aspects. Sci. Rep. 2020, 10, 8531. [Google Scholar] [CrossRef]
- Lin, L.; Wang, W.; Huang, J.; Li, Q.; Sun, D.; Yang, X.; Wang, H.; He, N.; Wang, Y. Nature factory of silver nanowires: Plant-mediated synthesis using broth of Cassia fistula leaf. Chem. Eng. J. 2010, 162, 852–858. [Google Scholar] [CrossRef]
- Horta-Piñeres, S.; Britto Hurtado, R.; Avila-Padilla, D.; Cortez-Valadez, M.; Flores-López, N.S.; Flores-Acosta, M. Silver nanoparticle-decorated silver nanowires: A nanocomposite via green synthesis. Appl. Phys. A 2019, 126, 15. [Google Scholar] [CrossRef]
- Gnanaprakasam, P.; Selvaraju, T. Green synthesis of self assembled silver nanowire decorated reduced graphene oxide for efficient nitroarene reduction. RSC Adv. 2014, 4, 24518–24525. [Google Scholar] [CrossRef]
- Venkataramanan, N.S.; Matsui, K.; Kawanami, H.; Ikushima, Y. Green synthesis of titania nanowire composites on natural cellulose fibers. Green Chem. 2007, 9, 18–19. [Google Scholar] [CrossRef]
- Yang, W.; Yang, C.; Sun, M.; Yang, F.; Ma, Y.; Zhang, Z.; Yang, X. Green synthesis of nanowire-like Pt nanostructures and their catalytic properties. Talanta 2009, 78, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Flores-González, M.; Talavera-Rojas, M.; Soriano-Vargas, E.; Rodríguez-González, V. Practical mediated-assembly synthesis of silver nanowires using commercial Camellia sinensis extracts and their antibacterial properties. New J. Chem. 2018, 42, 2133–2139. [Google Scholar] [CrossRef]
- Jeevika, A.; Ravi Shankaran, D. Seed-free synthesis of 1D silver nanowires ink using clove oil (Syzygium aromaticum) at room temperature. J. Colloid Interface Sci. 2015, 458, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varma, R.S. Green Synthesis of Ag and Pd Nanospheres, Nanowires, and Nanorods Using Vitamin B2: Catalytic Polymerisation of Aniline and Pyrrole. J. Nanomater. 2008, 2008, 782358. [Google Scholar] [CrossRef] [Green Version]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; García-Balboa, C.; Ballester, A. Biosynthesis of gold nanowires using sugar beet pulp. Process Biochem. 2011, 46, 1076–1082. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Kharat, A.N. One pot green synthesis of gold nanowires using pomegranate juice. Mater. Lett. 2014, 134, 64–66. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Guo, Z.; Gu, N. Biological Synthesis of Gold Nanowires Using Extract of Rhodopseudomonas capsulata. Biotechnol. Prog. 2008, 24, 476–480. [Google Scholar] [CrossRef]
- Parial, D.; Patra, H.K.; Dasgupta, A.K.R.; Pal, R. Screening of different algae for green synthesis of gold nanoparticles. Eur. J. Phycol. 2012, 47, 22–29. [Google Scholar] [CrossRef]
- Das, S.K.; Das, A.R.; Guha, A.K. Microbial Synthesis of Multishaped Gold Nanostructures. Small 2010, 6, 1012–1021. [Google Scholar] [CrossRef]
- Intrchom, W.; Thakkar, M.; Hamilton, R.F.; Holian, A.; Mitra, S. Effect of Carbon Nanotube-Metal Hybrid Particle Exposure to Freshwater Algae Chlamydomonas reinhardtii. Sci. Rep. 2018, 8, 15301. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Azim, A.A.; Mouez, F.A.; Rehim, S.S.A. Challenges on synthesis of carbon nanotubes from environmentally friendly green oil using pyrolysis technique. J. Anal. Appl. Pyrolysis 2017, 126, 218–229. [Google Scholar] [CrossRef]
- Hakim, Y.; Yulizar, Y.; Nurcahyo, A.; Surya, M. Green Synthesis of Carbon Nanotubes from Coconut Shell Waste for the Adsorption of Pb(II) Ions. Acta Chim. Asiana 2018, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, N.; Pavelyev, V.; Islam, S.S. Synthesis of carbon nanotubes using green plant extract as catalyst: Unconventional concept and its realization. Appl. Nanosci. 2017, 7, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.J.B.; Lucas, J.M.F.; Silva, F.M.; Girão, A.V.; Oliveira, F.J.; Marques, P.A.A.P.; Freire, C.S.R. Bio-based synthesis of oxidation resistant copper nanowires using an aqueous plant extract. J. Clean. Prod. 2019, 221, 122–131. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Biosynthesis and structural characterization of selenium nanoparticles mediated by Zooglea ramigera. Powder Technol. 2013, 244, 26–29. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B Biointerfaces 2010, 80, 94–102. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, W.; Liu, G. Hierarchical electrode design of high-capacity alloy nanomaterials for lithium-ion batteries. Nano Today 2015, 10, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.; Flynn, C.E.; Hayhurst, A.; Sweeney, R.; Qi, J.; Georgiou, G.; Iverson, B.; Belcher, A.M. Viral assembly of oriented quantum dot nanowires. Proc. Natl. Acad. Sci. USA 2003, 100, 6946. [Google Scholar] [CrossRef] [Green Version]





| NPs | Species | Active Molecules | Morphology/Size (nm) | References |
|---|---|---|---|---|
| Ag | Bacillus cereus | Tyrosine and tryptophan | Spherical/4–5 | [35] |
| Bacillus licheniformis | NADPH-dependent Nitrate Reductase | Irregular/50 | [66] | |
| Bacillus sp. KMS2-2 | Phospholipids and proteins | Spherical/18–153 | [117] | |
| Corynebacterium sp. SH09 | Aldose and ketose | ND/10–15 | [118] | |
| Corynebacterium glutamicum | Organic molecules of cell wall | Irregular/5–50 | [119] | |
| Escherichia coli | Protein amino acids on cell wall | Irregular/50 | [4] | |
| Morganella sp. | Protein amino acids on cell wall | Spherical/20 ± 5 | [120] | |
| Au | Bacillus subtilis 168 | Aldose and ketose | Octahedral/5–25 | [118] |
| Escherichia coli | Protein amino acids on cell wall | Triangles, hexagons/20–30 | [4] | |
| Pseudomonas aeruginosa | Excreted cell wall reduction enzymes | Irregular/15–30 | [121] | |
| Lactobacillus Kimchicus | Sugars and NADPH-dependent reductase | Spherical/5–15 | [116] | |
| Ureibacillus thermosphaericus | ND | Irregular/50–70 | [122] | |
| CdS | Escherichia coli | Glutathione | Spherical, elliptical/2–5 | [6] |
| Gluconoacetobacter xylinus | Bacterial-cellulose nanofibers | Spherical/30 | [123] | |
| Lactobacillcus | ND | Spherical/4.9 | [124] | |
| Rhodopseudomonas palustris | cysteine desulfhydrase | Cubic/8 | [7] | |
| Rhodobacter sphaeroides | cysteine desulfhydrase | Hexagonal/8 | [125] | |
| Fe3O4 | Aquaspirillum magnetotacticum | ND | Octahedral prism/40–50 | [126] |
| Magnetospirillum magnetotacticum | Amino and carboxylate acids | Cubo-octohedrons/47.1 | [127] | |
| Magnetospirillum magnetotacticum (MS-1) | ND | Cuboctahedral/~50 | [128] | |
| Shewanella oneidensis | Proteins, reductases, quinones and electron transferase | Rectangular, rhombic, hexagonal/40–50 | [129] | |
| Hg | Enterobacter sp. | ND | Spherical/2–5 | [122] |
| MnO | Bacillus sp. | cardiolipins | Orthorhombic/4.62 | [130] |
| PbS | Rhodobacter sphaeroides | cysteine desulfhydrase | Spherical/10.5 ± 0.15 | [7] |
| Ti | Lactobacillus sp. | ND | Spherical/40–60 | [124] |
| UO2 | Shewanella oneidensis | Proteins, reductases, quinones, electron transferase | (UO2-EPS)/1–5 | [129] |
| ZnS | Desulfobacteraceae | Cysteine desulfhydrase | Bio-film/2–5 | [131] |
| NPs | Species | Active Molecules | Morphology/Size (nm) | References |
|---|---|---|---|---|
| Au | Rhodococcus sp. | Enzymes in cell wall and cytoplasm | ND/5–15 | [136] |
| Thermomonospora sp. | Chloroaurate reduction and capping enzyme | Spherical/ND | [137] | |
| Yarrowia lipolytica | NADH-dependent reductases and protease | Triangles/15 | [138] | |
| Ag | Saccharomyces cerevisiae | Primary amine of proteins | Spherical/17 | [32] |
| Yeast strain MKY3 | Excreted biochemical reducing agents | Hexagonal/2–5Irregular polygonal/9–25 | [133] | |
| Cd | Schizosaccharomyces pombe | Phytochelatin synthase | Wurtzite-hexagonal/1–1.5 | [134] |
| Yeast | Oxidoreductase | Spherical/3.6 | [139] | |
| Fe3O4 | Yeast | Protein with 2 amides | Wormhole-like/<100 | [138] |
| PbS | Torulopsis sp. | Phytochelatin synthase | Spherical/2–5 | [134] |
| Sb2O3 | Saccharomyces cerevisiae | Oxidoreductase and quinone | Spherical/2–10 | [135] |
| Se | Magnusiomyces ingens | Proteins, phenols, flavonoids, amino groups | Spherical/87.82 ± 2.71 | [140] |
| Saccharomyces cerevisiae | Oxidoreductase and quinone | Spherical/75–709 | [5] |
| NPs | Species | Active Molecules | Morphology/Size (nm) | References |
|---|---|---|---|---|
| Ag | Alternaria solani | NADH-dependent reductase | Spherical/5–20 | [149] |
| Aspergillus fumigatus | Proteins and proteinic components | Spherical/5–25 | [150] | |
| Aspergillus niger | Nitrate-dependent reductase and quinone | Spherical/20 | [145] | |
| Aspergillus terreus | NADH-dependent reductase and nitrate reductase | Spherical/15–29 | [141] | |
| Cladosporium cladosporioides | ND | Spherical/10–100 | [151] | |
| Coriolus versicolor | Amine and cysteine amino residues | Spherical/25–75 | [143] | |
| Fusarium acuminatum | Nitrate-dependent reductase | Spherical/5–40 | [152] | |
| Fusarium oxysporum | Citrate | Variable/5–15 | [153] | |
| Fusarium oxysporum | Enzymes in cell membrane | ND/5–50 | [154] | |
| Fusarium oxysporum | NADH-dependent reductase | Spherical, triangular/5–15 | [155] | |
| Fusarium semitectum | NADH-dependent reductase | Spherical/10–60 | [156] | |
| Fusarium solani USM 3799 | Carboxylate, amine, cysteine amino residues | Spherical/16.23 | [144] | |
| Penicillium brevicompactum | Nitrate-dependent reductase and quinone | ND/58.35 ± 17.88 | [157] | |
| Penicillium citrinum | Amide amino acids | Spherical/109 | [158] | |
| Penicillium fellutanum | Nitrate reductase | Spherical/5–25 | [159] | |
| Penicillium funiculosum | NADH-dependent reductase | Spherical/5–10 | [160] | |
| Penicllium Notatum | Citrate | Spherical/30–40 | [160] | |
| Phenerochaete chrysosporium | NADH-dependent reductase | Spherical, oval/34–90 | [161] | |
| Phoma glomerata | Proteins | Spherical/60–80 | [34] | |
| Verticillium | Enzymes in cell membrane | Spherical/25 ± 12 | [142] | |
| Verticillium sp. | Enzymes in cell membrane | Spherical/5–50 | [140] | |
| Au | Colletotrichum sp. | Terpenoids, polypeptides, enzymes | Spherical/20–40 | [162] |
| Fusarium oxysporum | Oxidoreductase | Spherical, triangular/20–40 | [148] | |
| Trichothecium sp. | Enzymes | Triangular, hexagonal/5–200 | [146] | |
| Trichothecium sp. | Enzymes | Spheres, rods/ND | [146] | |
| Verticillium luteoalbum and isolate 6-3 | Enzymes | Spheres, rods/<10 | [147] | |
| Au, Au/Ag | Neurospora crassa | Proteins and enzymes | Spherical/32, 20–50 | [163] |
| Au, Ag, Au-g | Volvariella volvacea | Amino and carboxylate acids | Spherical, hexagonal/20–150 | [127] |
| CdSe | Fusarium oxysporum | Superoxidedisumutase | Spherical/9–15 | [164] |
| Pt | “Fusarium oxysporum f. sp. lycopersici” | Hydrogenase | Triangle, hexagons, square, rectangles/10–50 | [165] |
| Si | Fusarium oxysporum | Proteins | Quasi-spherical/5–15 | [166] |
| Ti | Fusarium oxysporum | Proteins | Spherical/6–13 | [166] |
| TiO2 | Fusarium oxysporum | Proteins | Spherical/6–13 | [166] |
| Zr | Fusarium oxysporum | Amide amino acids | Quasi-spherical/3–11 | [36] |
| ZrO2 | Fusarium oxysporum | Amide amino acids | Spherical/3–11 | [36] |
| NPs | Species | Active Molecules | Morphology/Size (nm) | References |
|---|---|---|---|---|
| Ag | Caulerpa racemosa | Peptides | Spherical, triangular/05–25 | [175] |
| Chaetomorpha linum | Carboxyl groups | Clusters/03–44 | [176] | |
| Cystophora moniliformis | Metabolites and phenol compounds | Spherical/50–100 | [170] | |
| Gelidium amansii (live) | Carboxyl groups | Spherical/27–54 | [37] | |
| Gracilaria corticata | Phenols and peptides | Spherical/18–46 | [174] | |
| Laminaria japonica (extract) | Carboxy groups of alginic acid and amide groups of proteins | Spherical to oval/31 | [37] | |
| Leptolyngbya valderianum | Protein | Spherical/02–20 | [172] | |
| Pithophora oedogonia | Sulfate moeity of polysaccharide | Cubical, hexagonal/25–44 | [173] | |
| Porphyra vietnamensis | Sulfate moeity of polysaccharide | Spherical/13 ± 03 | [177] | |
| Sargassum tenerrimum | Phenols and peptides | Spherical/20 | [174] | |
| Sargassum muticum (extract) | Sulfate or hydroxyl groups | Spherical/43–79 | [178] | |
| Sargassum wighti | ND | ND/08–27 | [105] | |
| Scenedesmus sp. | Peptides | Spherical, crystalline/15–20 | [171] | |
| Spirogyra varians (extract) | ND | Quasi–spheres/35 | [179] | |
| Ulva lactuca (extract) | Polyphenols | Spherical/– | [180] | |
| Au | Chlorella vulgaris (extract) | Phytochemicals with hydroxyl, carboxyl and amino functional groups | Spatial array of self assembled structures/02–10 | [181] |
| Padina gymnospora (live) | Hydroxyl groups of polysaccharides in algal cell wall | Spherical/53–67 | [182] | |
| Padina pavonica (live) | - | Spherical/30–100 | [183] | |
| Sargassum muticum | ND | Spherical/5.42 ± 1.18 | [1] | |
| Sargassum wightii (extract) | ND | Spherical/08–12 | [184] | |
| Stoechospermum marginatum (extract) | Hydroxyl groups of diterpenoids | Spherical, hexagonal, triangular/19–94 | [185] | |
| Tetraselmis kochinensis (live) | Enzymes on cell wall and cytoplasm | Spherical, triangular/05–35 | [169] | |
| CuO | Bifurcaria bifurcate (extract) | ND | Spherical/05–45 | [186] |
| Fe | Chlorococcum sp. MM11 (live) | Reductases and hydrosylates of polysaccharides | Spherical/20–50 | [187] |
| Pd | Sargassum bovinum (extract) | Sulfated polysaccharides | Octahedral/05–10 | [188] |
| NPs | Species | Active Molecules | Morphology/Size (nm) | Application | References |
|---|---|---|---|---|---|
| Ag | Acadia rigidula | ND | Spherical/22.46 ± 10.83 | Antimicrobial | [197] |
| Allium cepa L. | Flavonoids, quercetin, glucosides | Spherical/12.5 | Catalyst | [1] | |
| Artemisia quttensis | ND | ND/ND | Antibacterial, anti-cancer, antioxidant | [198] | |
| Artemisia Tournefortiana Rchb | Terpenoids | Spherical/22.89 ± 14.82 | Antibacterial, anti-cancer | [198] | |
| Berberis vulgaris | Terpenoids, flavonoids, carboxylic acid | Spherical/30–70 | Antimicrobial | [192] | |
| Cannabis sativa | Phenols, proteins, flavonoids | Spherical/26.52 | Antibacterial, anti-yeast | [140] | |
| Cinnamomum Zylinicum | ND | Spherical/10–78.9 | Antimicrobial | [199] | |
| Gardenia Jasminoides Ellis | Phenols, terpenoids, proteins | Spherical/20 | Antimicrobial, dye, degradation, antioxidant | [189] | |
| Mentha pulegium | Phenols, terpenoids, proteins | Anisotropic/5–50 | Antibacterial, antifungal, anti-cancer | [200] | |
| P. granatum L. | Flavonoids | Spherical/10–50 | Antimicrobial, dyeing, antioxidant | [201] | |
| Ag, Cu | Myristica fragrans | Flavonoids, quercetin, phenols | Spherical/10–50 | Antimicrobial, catalyst | [194] |
| Au | Fragaria × ananassa | Phenols, flavonoids, terpenoids | Spherical/5–31 | Medical applications | [190] |
| Ribes nigrum | Phenols, flavonoids, terpenoids | Spherical/6–44 | Medical applications | [190] | |
| Ribes uva-crispa | Phenols, flavonoids, terpenoids | Spherical/8–47 | Medical applications | [190] | |
| CuO | Adiantum lunulatum | ND | Spherical/6.5 ± 1.5 | Agriculture | [202] |
| Se | Aloe Vera | Polysaccharides, phenols, flavonoids, proteins | Spherical/50 | Antibacterial, anti-yeast | [191] |
| ZnO | Aplha Amylase | ND | Spherical/11 | Agriculture | [203] |
| NPs | Species | Active Molecules | Morphology/Size (nm) | References |
|---|---|---|---|---|
| Ag | Brassica junecea | Glucose, fructose | ND/ND | [195] |
| Festuca rubra | Ascorbic acid, citric acid, polyphenols | ND/ND | [195] | |
| Medicago sativa | ND | Spherical/1–100 | [195] |
| NPs | Species | Active Molecules | Morphology/Diameter (nm)/Length (μm) | Application | References |
|---|---|---|---|---|---|
| Ag | Abelmoschus esculentus (Extract) | Catalyst | Decorated nanowire/92/100–200 | Sensors | [206] |
| Camellia sinensis (Extract) | ND | Nanowire/50/1.3 | Antibacterial | [209] | |
| Cassia fistula (Extract) | Alkaloids, flavonoids, or polysaccharides | Nanowire/50–60/10 | Electronic and Optical | [204] | |
| Mangifera indica (Extract) | Phenols and sugars | Decorated nanowire/~70/10 | Medical (antibacterial) | [205] | |
| Syzygium Aromaticum (Extract) | Eugenol | Nanowire/39/3 | Electrical | [210] | |
| Vitamin B2 | ND | Wire, rod/20-Oct/100–200 | Biological | [211] | |
| Au | Beta vulgaris (Extract) | Sugars and proteins | Nanowire/15/0.2–0.4 | Sensors | [212] |
| Punica granatum (Extract) | ND | Irregular wires/30–90/ND | Optical | [213] | |
| Rhodopseudomonas capsulata (Bacteria, Extract) | Surface bound proteins | Nanowire/20–30/ND | Electronics | [214] | |
| Phormidium valderianum | Polypeptides | Nanowire/32/411 | Medical | [215] | |
| Rhizopus oryzae | ND | Nanowire/10/ND | Medical | [216] | |
| C | Chlamydomonas reinhardtii (Algae, Live) | ND | Nanotube/20–30/2.6 ± 0.8 | Electronics | [217] |
| Cocos nucifera (Extract) | ND | Rod, bundle/ND/ND | Medical | [218] | |
| Cocos nucifera (Extract) | ND | Tube/123/1 | Pb2 Ion adsorption | [219] | |
| J. Regia (Extract) | ND | Tube/15-Aug/3.6 | Medical | [220] | |
| Olea Europea (Extract) | ND | SWCNT/27-31/ND | Medical | [218] | |
| Cu | Eucalyptus globulus (Extract) | Oleyl groups | Nanowire/44,245/5–60 | Electronic and Optical | [221] |
| Pd | Vitamin B2 | ND | Wire, rod/20/100–200 | Biological | [211] |
| Pt | Dextran | ND | Nanowire-like/2.2/100 | Electronic | [208] |
| Se | Zooglea ramigera (Bacteria, Live) | ND | Nanorod/30–150/ND | Medical | [222] |
| Bacillus subtilis (Bacteria, Live) | ND | Nanowire/50/5 | H2O2 Sensor | [223] | |
| Ti | Cellulose | ND | Nanowire/ND/100–500 (nm) | Electronic and Optical | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. https://doi.org/10.3390/nano11082130
Huston M, DeBella M, DiBella M, Gupta A. Green Synthesis of Nanomaterials. Nanomaterials. 2021; 11(8):2130. https://doi.org/10.3390/nano11082130
Chicago/Turabian StyleHuston, Matthew, Melissa DeBella, Maria DiBella, and Anisha Gupta. 2021. "Green Synthesis of Nanomaterials" Nanomaterials 11, no. 8: 2130. https://doi.org/10.3390/nano11082130