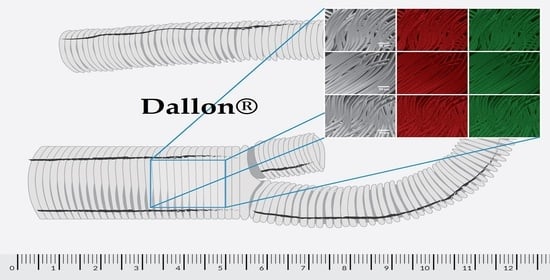
The Influence of a Knitted Hydrophilic Prosthesis of Blood Vessels on the Activation of Coagulation System—In Vitro Study
Abstract
:1. Introduction
- alcoholic solution of a surface-active substance, e.g., Tween 80 and drying or,
- methanol solution and exposing them to the action of ultrasounds [10].
2. Materials and Methods
2.1. Materials
2.2. Physic-Chemical Characteristic
2.3. Differential Scanning Calorimetry
- -
- atmosphere–nitrogen, 30 cm3/min;
- -
- vessel–standard aluminium lid;
- -
- warming velocity—20 °C/min;
- -
- cooling velocity—20 °C/min;
- -
- range of temperature: 32–197 °C;
- -
- weight of sample: 6–10 mg.
- ΔHm—melting enthalpy;
- ΔHc—crystallization enthalpy;
- ΔHref—refers to 100% crystalline polymer which in the case of poly-(ethyleneterephalate) equals to 140 ± 20 J/g.
2.4. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy
2.5. Coagulation System Studies
3. Results
3.1. Physic-Chemical Characteristic
3.2. Differential Scanning Calorimetry
3.3. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy
3.4. Coagulation System Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorobisz, A.T.; Rybak, Z.; Skóra, J.; Pupka, A.; Patrzałek, D.; Stȩpiński, P.; Korta, K.; Barć, P. Iatrogenic injuries of the carotid arteries. VASA J. Vasc. Dis. 2005, 34, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Ginalska, G.; Osinska, M.; Uryniak, A.; Urbanik-Sypniewska, T.; Belcarz, A.; Rzeski, W.; Wolski, A. Antibacterial activity of gentamicin-bonded gelatin-sealed polyethylene terephthalate vascular prostheses. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Galdbart, J.O.; Branger, C.; Andreassian, B.; Lambert-Zechovsky, N.; Kitzis, M. Elution of six antibiotics bonded to polyethylene vascular grafts sealed with three proteins. J. Surg. Res. 1996, 66, 174–178. [Google Scholar] [CrossRef]
- Nagae, T.; Wilson, S.E.; Tsuchida, H.; Furukawa, K.; Peng, S.K. Composite porosity of expanded polytetrafluoroethylene vascular prosthesis. Vascular 1995, 3, 479–484. [Google Scholar] [CrossRef]
- Basmadjian, D.; Sefton, M.V.; Baldwin, S.A. Coagulation on biomaterials in flowing blood: Some theoretical considerations. Biomaterials 1997, 18, 1511–1522. [Google Scholar] [CrossRef]
- Nygren, H.; Broberg, M. Specific activation of platelets by surface-adsorbed plasma proteins. J. Biomater. Sci. Polym. Ed. 1998, 9, 817–831. [Google Scholar] [CrossRef]
- Pessinaba, S.; Kane, A.; Ndiaye, M.B.; Mbaye, A.; Bodian, M.; Dia, M.M.; Sarr, S.A.; Diao, M.; Sarr, M.; Kane, A.; et al. Vascular complications of infective endocarditis. Med. Mal. Infect. 2012, 42, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccafoschi, F.; Ramella, M.; Fusaro, L.; Catoira, M.C.; Casella, F. Biological Grafts: Surgical Use and Vascular Tissue gineering Options for Peripheral Vascular Implants. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128012383. [Google Scholar]
- Struszczyk, M.H.; Bednarek, P.; Raczyski, K. Synthetic vascular prostheses. Polim. Med. 2002, 32, 13–22. [Google Scholar] [PubMed]
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. Part A 2015, 103, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Paluch, D.; Szymonowicz, M.; Pielka, S.; Buczyńska, H. Influence of materials with various wettability of surface on hematological parameters of blood. Eng. Biomater. 2001, 4, 22–24. [Google Scholar]
- Milewski, A.; Staniszewska-Kuś, J.; Rutowski, R.; Solski, L.; Pielka, S. Tissue reaction following the implantation of a DALLON H vascular prosthesis in the thoracic aorta defect. Experimental tests. Polim. Med. 2002, 32, 23–40. [Google Scholar] [PubMed]
- Duhamel, R.C.; Eldridge, S.; Kelley, B.; McCrea, B. Method for Making Vascular Grafts. U.S. Patent 5,584,875, 17 December 1996. [Google Scholar]
- Lesiakowska, K.; Dyczka, A.; Raczyński, K.; Gąsiorowski, T. Method of Modifying Vascular Prostheses. Polish Patent 325090, 30 September 2003. [Google Scholar]
- ISO10993-2:2006: Biological Evaluation of Medical Devices—Part 2: Animal Welfare Requirements; ISO: Geneva, Switzerland, 2006.
- ISO 10993-12:2012: Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2012.
- ISO 10993-1:2009: Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing; ISO: Geneva, Switzerland, 2009.
- ISO 10993-4:2009 Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood; ISO: Geneva, Switzerland, 2009.
- Paluch, D.; Szymonowicz, M.; Pielka, S.; Rutowski, R. In vitro studies of the influence polyester materials with a different degree of surface wettability have on blood haematological parameters and coagulation and fibrinolysis system parameters. Polim. Med. 2002, 32, 41–64. [Google Scholar] [PubMed]
- Szelest-Lewandowska, A.; Masiulanis, B.; Szymonowicz, M.; Pielka, S.; Paluch, D. Modified polycarbonate urethane: Synthesis, properties and biological investigation in vitro. J. Biomed. Mater. Res. Part A 2007, 82, 509–520. [Google Scholar] [CrossRef]
- Szymonowicz, M.; Łowkis, B. In vitro testing method of polymers candidate destined for contact with blood. Polym. Med. 1990, 20, 43–45. [Google Scholar]
- Abosdera, M.M.; Almasry, A.E.; Abdel-Moneim, E.S. Coagulation defects in thalassemic patients. Pediatr. Neonatol. 2017, 58, 421–424. [Google Scholar] [CrossRef] [Green Version]
- ACL 9000/10000 System Operator’s Manual Text Part No 190834-29; Rev.02; Instrumentation Laboratory: Bedford, MA, USA, 2003.
- Benington, I.C.; Biagioni, P.A.; Briggs, J.; Sheridan, S.; Lamey, P.-J. Thermal changes observed at implant sites during internal and external irrigation. Clin. Oral Implant. Res. 2002, 13, 293–297. [Google Scholar] [CrossRef]
- Struszczyk, M. Vascular Prostheses—Harmonized Standards. In Proceedings of Vth International Scientific Conference; Med-Tex: Warszawa, Poland, 2005; p. 32. ISBN 83-911012-3-1,32. [Google Scholar]
- Paluch, D.; Szymonowicz, M.; Rutowski, R.; Milewski, A.; Pielka, S.; Solski, L.; Raczyński, K. Intraoperative studies and studies on selected parameters of coagulation and fibrinolysis following implantation of DALLON H prostheses with greater surface wettability. Polim. Med. 2002, 32, 65–79. [Google Scholar]
- Kmiecik, B.; Skotny, A.; Batycka, M.; Wawrzaszek, R.; Rybak, Z. Influence of oxidative stress on tissue regeneration. Polim. Med. 2013, 43, 191–197. [Google Scholar]
- Grunkemeier, J.M.; Tsai, W.B.; McFarland, C.D.; Horbett, T.A. The effect adsorbed fibrinogen, fibronectin, von Willebrand factor and vitronectin on procoagulant state of adherent platelets. Biomaterials 2000, 21, 2243–2252. [Google Scholar] [CrossRef]
- Goodman, S.L.; Goodman, S.L. Sheep, pig and human platelet-material interactions with model cardiovascular biomaterials. J. Biomed. Mater. Res. 1999, 45, 240–250. [Google Scholar] [CrossRef]
- Karaszewska, A.; Buchenska, J. Polyester vascular prostheses—Antibacterial and athrombogenic biomaterials. Part I. Two-stage modification of vascular prostheses. Polimery 2012, 57, 722–727. [Google Scholar] [CrossRef]
- Karaszewska, A.; Buchenska, J. Polyester vascular prostheses—Antibacterial and athrombogenic biomaterials. Part II. Effect of two-stage modification of polyester vascular prostheses on the selected physicochemical, mechanical and microbiological properties. Polimery 2013, 58, 33–38. [Google Scholar] [CrossRef]

















| Lp. | Wavelength [cm−1] | Suggested Infrared Band Assignment |
|---|---|---|
| 1 | 3445 | O–H stretching bond |
| 2 | 3050 | Aromatic C–H stretch |
| 3 | 3000–2800 | Stretching C–H bond |
| 4 | 1720, 1630 | C=O |
| 5 | 1580, 1500 | C–C stretch (in-ring) |
| 6 | 1310 | C–H bending (in-plane) |
| 7 | 730 | C–H bending (out-of-plane) |
| 8 | 1260, 1100 | C–O bond from ester group |
| 9 | 973 | Trans configuration (crystalline band) |
| 10 | 927 | Cis configuration |
| 11 | 900 | Gauche configuration |
| ΔCp | Melting Temperature | Melting Enthalpy | Crystallization Temperature | Crystallization Enthalpy | Reference Enthalpy | Crystallinity |
|---|---|---|---|---|---|---|
| [J/g K] | Tm [C] | ΔHm | Tc [C] | ΔHc | ΔHref | XDSC |
| Dallon® | ||||||
| 0.12 | 259 | 64.9 | 207 | 43.5 | 140 | 0.15 |
| Dallon® H | ||||||
| 0.14 | 260 | 62.6 | 207 | 46.6 | 140 | 0.11 |
| Parameters | Reference Values Range |
|---|---|
| APTT | 25.0–37.0 s |
| PT | 9.0–12.6 s |
| PT INR | 0.8–1.2 |
| Fb | 2.0–4.5 g/L |
| TT | 11.0–18.0 s |
| Factor XII | 50–150% activity |
| Factor IX | 65–150% activity |
| Factor VIII | 50–150% activity |
| AT III | 75–122% activity |
| Protein C | 6–134% activity |
| Plazminogen | 75–150% activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymonowicz, M.; Dobrzynski, M.; Targonska, S.; Rusak, A.; Rybak, Z.; Struszczyk, M.H.; Majda, J.; Szymanski, D.; Wiglusz, R.J. The Influence of a Knitted Hydrophilic Prosthesis of Blood Vessels on the Activation of Coagulation System—In Vitro Study. Nanomaterials 2021, 11, 1600. https://doi.org/10.3390/nano11061600
Szymonowicz M, Dobrzynski M, Targonska S, Rusak A, Rybak Z, Struszczyk MH, Majda J, Szymanski D, Wiglusz RJ. The Influence of a Knitted Hydrophilic Prosthesis of Blood Vessels on the Activation of Coagulation System—In Vitro Study. Nanomaterials. 2021; 11(6):1600. https://doi.org/10.3390/nano11061600
Chicago/Turabian StyleSzymonowicz, Maria, Maciej Dobrzynski, Sara Targonska, Agnieszka Rusak, Zbigniew Rybak, Marcin H. Struszczyk, Jacek Majda, Damian Szymanski, and Rafal J. Wiglusz. 2021. "The Influence of a Knitted Hydrophilic Prosthesis of Blood Vessels on the Activation of Coagulation System—In Vitro Study" Nanomaterials 11, no. 6: 1600. https://doi.org/10.3390/nano11061600