The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action
Abstract
:1. Introduction
2. Synthesis of AgNPs
2.1. Physical Synthesis of AgNPs
2.2. Chemical Synthesis of AgNPs
2.3. Biological Synthesis of AgNPs
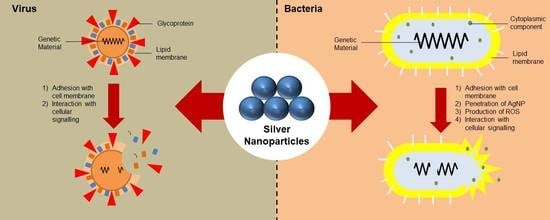
3. Mechanism of Action
3.1. Antibacterial Properties of AgNPs
3.2. Antiviral Properties of AgNPs
4. Application of AgNPs
5. Safety of AgNPs
6. Limitations of AgNPs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Zheludkevich, M.L.; Gusakov, A.G.; Voropaev, A.G.; Vecher, A.A.; Kozyrski, E.N.; Raspopov, S.A. Oxidation of silver by atomic oxygen. Oxid. Met. 2004, 61, 39–48. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Alheety, N.F.; Majeed, A.H.; Alheety, M.A. Silver nanoparticles anchored 5-methoxy benzimidazol thiomethanol (MBITM): Modulate, characterization and comparative studies on MBITM and Ag-MBITM antibacterial activities. J. Phys. Conf. Ser. 2019, 1294. [Google Scholar] [CrossRef] [Green Version]
- Pourzahedi, L.; Eckelman, M.J. Comparative life cycle assessment of silver nanoparticle synthesis routes. Environ. Sci. Nano 2015, 2, 361–369. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized bacterial cellulose as antibacterial membrane for wound-healing applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Tao, P.P.; Liu, H.; Al-Hada, M.; Amati, M.; Sezen, H.; Gregoratti, L.; Tang, Y.; House, S.D.; Tao, F.F. X-ray photoelectron spectroscopy studies of nanoparticles dispersed in static liquid. Langmuir 2018, 34, 9606–9616. [Google Scholar] [CrossRef]
- Baudot, C.; Tan, C.M.; Kong, J.C. FTIR spectroscopy as a tool for nano-material characterization. Infrared Phys. Technol. 2010, 53, 434–438. [Google Scholar] [CrossRef]
- Gorham, J.M.; MacCuspie, R.I.; Klein, K.L.; Fairbrother, D.H.; Holbrook, D. UV-induced photochemical transformations of citrate-capped silver nanoparticle suspensions. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.K.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Quan, Y.; Yu, Y.L.; Wang, J.H. Graphene quantum dot/silver nanoparticle hybrids with oxidase activities for antibacterial application. ACS Biomater. Sci. Eng. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C. Transport of silver nanoparticles capped with different stabilizers in water saturated porous media. J. Mater. Environ. Sci. 2014, 5, 231–236. [Google Scholar]
- Ribeiro, M.S.; De Melo, L.S.A.; Farooq, S.; Baptista, A.; Kato, I.T.; Núñez, S.C.; De Araujo, R.E. Photodynamic inactivation assisted by localized surface plasmon resonance of silver nanoparticles: In vitro evaluation on Escherichia coli and Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2018, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussein, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles-Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Hunt, P.R.; Keltner, Z.; Gao, X.; Oldenburg, S.J.; Bushana, P.; Olejnik, N.; Sprando, R.L. Bioactivity of nanosilver in Caenorhabditis elegans: Effects of size, coat, and shape. Toxicol. Rep. 2014, 1, 923–944. [Google Scholar] [CrossRef] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Prakash, V.; Pawar, J.; Patel, A.K.; Henry, R.; Patwardhan, A. Analysis of Nucleation and Growth Parameter of Silver Nanoparticles for Sensors. In Proceedings of the 2017 1st International Conference on Electronics, Materials Engineering and Nano-Technology (IEMENTech), Kolkata, India, 28–29 April 2017. [Google Scholar] [CrossRef]
- Slepička, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of gold and silver nanoparticles preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Soria-Aguilar, M.D.J.; Zamarripa, G.G.; Sánchez-Castillo, M.A.; García-Cerda, L.A.; Carrillo-Pedroza, F.R. Synthesis and characterization of ag nanoparticles supported in blast furnace dust microspheres. Rev. Latinoam. de Metal. y Mater. 2018, 38, 2–8. [Google Scholar]
- Wu, J.; Xu, Y.; Xu, P.; Pan, Z.; Chen, S.; Shen, Q.; Zhan, L.; Zhang, Y.; Ni, W. Surface-enhanced Raman scattering from AgNP-graphene-AgNP sandwiched nanostructures. Nanoscale 2015, 7, 17529–17537. [Google Scholar] [CrossRef] [PubMed]
- Gandhewar, V.R.; Bansod, S.V.; Borade, A.B. Induction furnace—A review. Int. J. Eng. Technol. 2011, 3, 277–284. [Google Scholar]
- Zeng, H.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Zhang, D.; Choi, W.; Jakobi, J.; Kalus, M.R.; Barcikowski, S.; Cho, S.H.; Sugioka, K. Spontaneous shape alteration and size separation of surfactant-free silver particles synthesized by laser ablation in acetone during long-period storage. Nanomaterials 2018, 8, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noroozi, M.; Radiman, S.; Zakaria, A.; Soltaninejad, S. Fabrication, characterization, and thermal property evaluation of silver nanofluids. Nanoscale Res. Lett. 2014, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Gasaymeh, S.S.; Radiman, S.; Heng, L.Y.; Saion, E.; Mohamed Saeed, G.H. Synthesis and characterization of silver/Polyvinilpirrolidone (AG/PVP) nanoparticles using gamma irradiation techniques. Am. J. Appl. Sci. 2010, 7, 879–888. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Shenava, A. Synthesis of silver nanoparticles by chemical reduction method and their antifungal activity. Int. Res. J. Pharm. 2013, 4, 111–113. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng. 2009, 2, 105–111. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanoparticle Res. 2017, 19. [Google Scholar] [CrossRef]
- Song, K.C.; Lee, S.M.; Park, T.S.; Lee, B.S. Preparation of colloidal silver nanoparticles by chemical reduction method. Korean J. Chem. Eng. 2009, 26, 153–155. [Google Scholar] [CrossRef]
- Yu, D.G. Formation of colloidal silver nanoparticles stabilized by Na+-poly(γ-glutamic acid)-silver nitrate complex via chemical reduction process. Colloids Surf. B Biointerfaces 2007, 59, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A.O. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef]
- Sileikaite, A.; Prosycevas, I.; Puiso, J.; Juraitis, A.; Guobiene, A. Analysis of silver nanoparticles produced by chemical reduction of silver salt solution. Mater. Sci. 2006, 12, 287–291. [Google Scholar]
- Kheybari, S.; Samadi, N.; Hosseini, S.V.; Fazeli, A.; Fazeli, M.R. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. DARU 2010, 18, 168–172. [Google Scholar] [PubMed]
- Katas, H.; Moden, N.Z.; Lim, C.S.; Celesistinus, T.; Chan, J.Y.; Ganasan, P.; Abdalla, S.S.I. Biosynthesis and potential applications of silver and gold nanoparticles and their chitosan-based nanocomposites in nanomedicine. J. Nanotechnol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology 2018, 16. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Fang, J.; Zhong, C.; Mu, R. The study of deposited silver particulate films by simple method for efficient SERS. Chem. Phys. Lett. 2005, 401, 271–275. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Priya, K.; Nancy, F.T.; Noorlidah, A.; Ahmed, A.B.A. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind. Crops Prod. 2013, 41, 235–240. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Rao, Y.S.; Gaddam, S.A.; Prasad, T.N.V.K.V.; Reddy, A.V.; Gopal, S.V.R.S. Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus. Linn. G. Donn and its anti microbial activity. Colloids Surf. B Biointerfaces 2013, 105, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Vijay Kumar, P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameem, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- De Araujo, A.R.; Ramos-Jesus, J.; De Oliveira, T.M.; De Carvalho, A.M.A.; Nunes, P.H.M.; Daboit, T.C.; Carvalho, A.P.; Barroso, M.F.; De Almeida, M.P.; Plácido, A.; et al. Identification of Eschweilenol C in derivative of Terminalia fagifolia Mart. and green synthesis of bioactive and biocompatible silver nanoparticles. Ind. Crops Prod. 2019, 137, 52–65. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Aqarbeh, M.M.; Abdulaziz, F.M. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int. 2020, 6, 42–48. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 2020. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Popli, D.; Anil, V.; Subramanyam, A.B.; Namratha, M.N.; Ranjitha, V.R.; Rao, S.N.; Rai, R.V.; Govindappa, M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, V.; Nagasinduja, V.; Shahitha, S. Fungus-mediated synthesis and characterization of silver nanoparticles and its antibacterial activity against clinically isolated pathogens. Int. J. Curr. Res. Life Sci. 2018, 07, 1507–1512. [Google Scholar]
- Pugazhenthiran, N.; Anandan, S.; Kathiravan, G.; Udaya Prakash, N.K.; Crawford, S.; Ashokkumar, M. Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanoparticle Res. 2009, 11, 1811–1815. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.Y.; Chen, C.C.; Jean, J.S.; Chen, H.R.; Tseng, M.J.; Fan, C.W.; Wang, J.C. Biological synthesis of gold and silver nanoparticles mediated by the bacteria Bacillus subtilis. J. Nanosci. Nanotechnol. 2010, 10, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Oligodynamic effect of silver nanoparticles: A review. Bionanoscience 2018, 8, 951–962. [Google Scholar] [CrossRef]
- Peng, S.; Chen, Y.; Jin, X.; Lu, W.; Gou, M.; Wei, X.; Xie, J. Polyimide with half encapsulated silver nanoparticles grafted ceramic composite membrane: Enhanced silver stability and lasting anti‒biofouling performance. J. Memb. Sci. 2020, 611, 118340. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, H.; Zhang, H.; Zhao, P.; Zhang, Y.; Tang, Y. Polydopamine/silver hybrid coatings on soda-lime glass spheres with controllable release ability for inhibiting biofilm formation. Sci. China Mater. 2020, 63, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coat. Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.; Yu, C.P.; Esteban Fernández, G.; Hu, Z. Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res. 2010, 44, 6095–6103. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.S.I.; Katas, H.; Chan, J.Y.; Ganasan, P.; Azmi, F.; Mh Busra, M.F. Antimicrobial activity of multifaceted lactoferrin or graphene oxide functionalized silver nanocomposites biosynthesized using mushroom waste and chitosan. RSC Adv. 2020, 10, 4969–4983. [Google Scholar] [CrossRef] [Green Version]
- Wakshlak, R.B.K.; Pedahzur, R.; Avnir, D. Antibacterial activity of silver-killed bacteria: The “zombies” effect. Sci. Rep. 2015, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Katas, H.; Raja, M.A.G.; Lam, K.L. Development of chitosan nanoparticles as a stable drug delivery system for protein/siRNA. Int. J. Biomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Yi, T.; Huang, Z.; Liu, B.; Wang, J.; Yi, X.; Liu, J. Etching silver nanoparticles using DNA. Mater. Horiz. 2019, 6, 155–159. [Google Scholar] [CrossRef]
- Sadoon, A.A.; Khadka, P.; Freeland, J.; Gundampati, R.K.; Manso, R.H.; Ruiz, M.; Krishnamurthi, V.R.; Thallapuranam, S.K.; Chen, J.; Wang, Y. Silver ions caused faster diffusive dynamics of histone-like nucleoid-structuring proteins in live bacteria. Appl. Environ. Microbiol. 2020, 86, 1–16. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Mi, Y.; Si, Y. Silver nanoparticles induced cell apoptosis, membrane damage of azotobacter vinelandii and nitrosomonas europaea via generation of reactive oxygen species. Bull. Environ. Contam. Toxicol. 2019, 103, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Chatterjee, S.; Saha, A.; Devi, P.S.; Suresh Kumar, G. Unraveling the interaction of silver nanoparticles with mammalian and bacterial DNA. J. Phys. Chem. B 2016, 120, 5313–5324. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold-silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Kora, A.J.; Arunachalam, J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J. Microbiol. Biotechnol. 2011, 27, 1209–1216. [Google Scholar] [CrossRef]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lü, C. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C 2020, 110, 110735. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnology 2012, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Ouda, S.M. Some nanoparticles effects on Proteus sp. and Klebsiella sp. isolated from water. Am. J. Infect. Dis. Microbiol. 2014, 2, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, O.S.; Shittu, E.O.; Akpor, O.B.; Rotimi, D.; Batiha, G.E.S. Silver nanoparticles restrict microbial growth by promoting oxidative stress and DNA damage. EXCLI J. 2020, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G.P. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnology 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Huy, T.Q.; Hien Thanh, N.T.; Thuy, N.T.; Van Chung, P.; Hung, P.N.; Le, A.T.; Hong Hanh, N.T. Cytotoxicity and antiviral activity of electrochemical – synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and immunomodulatory activity of silver nanoparticles in experimental rsv infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.K.; Che, C.-M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Fu, K.; Mu, C.; Zhen, X.; Wang, G. L166P mutant DJ-1 promotes cell death by dissociating Bax from mitochondrial Bcl-XL. Mol. Neurodegener. 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Sarkar, D.S. Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. J. Curr. Med. Res. Opin. 2020, 3, 449–450. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Zhang, W.; He, T.; Shu, M.; Deng, J.; Wang, J.; Li, W.; Bai, J.; Lin, Q.; Luo, F.; et al. Evaluation of the genotoxic and oxidative damage potential of silver nanoparticles in human NCM460 and HCT116 cells. Int. J. Mol. Sci. 2020, 21, 1618. [Google Scholar] [CrossRef] [Green Version]
- El-Shishtawy, R.M.; Asiri, A.M.; Abdelwahed, N.A.M.; Al-Otaibi, M.M. In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Gupta, A.; Low, W.L.; Radecka, I.; Britland, S.T.; Mohd Amin, M.C.I.; Martin, C. Characterisation and in vitro antimicrobial activity of biosynthetic silver-loaded bacterial cellulose hydrogels. J. Microencapsul. 2016, 33, 725–734. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Quaroni, L.; Chumanov, G. Preparation of Polymer-coated functionalized silver nanoparticles. J. Am. Chem. Soc. 1999, 121, 10642–10643. [Google Scholar] [CrossRef]
- Hajtuch, J.; Hante, N.; Tomczyk, E.; Wojcik, M.; Radomski, M.W.; Santos-Martinez, M.J.; Inkielewicz-Stepniak, I. Effects of functionalized silver nanoparticles on aggregation of human blood platelets. Int. J. Nanomed. 2019, 14, 7399–7417. [Google Scholar] [CrossRef] [Green Version]
- Dowling, D.P.; Betts, A.J.; Pope, C.; McConnell, M.L.; Eloy, R.; Arnaud, M.N. Anti-bacterial silver coatings exhibiting enhanced activity through the addition of platinum. Surf. Coat. Technol. 2003, 163–164, 637–640. [Google Scholar] [CrossRef]
- Sedira, S.; Ayachi, A.A.; Lakehal, S.; Fateh, M.; Achour, S. Silver nanoparticles in combination with acetic acid and zinc oxide quantum dots for antibacterial activities improvement—A comparative study. Appl. Surf. Sci. 2014, 311, 659–665. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Laurent-Maquin, D.; Pina, S.; Rebelo, A.H.S.; Faure, J.; Ferreira, J.M.F. An in vitro biological and anti-bacterial study on a sol-gel derived silver-incorporated bioglass system. Dent. Mater. 2008, 24, 1343–1351. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Baudrit, J.R.V. Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Front. Chem. 2017, 5, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, B.H.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.B.; Lee, J.H.; Lee, S.H.; Lee, A.Y.; Choi, J.S.; Ahn, Y.S. A case of argyria following colloidal silver ingestion. Ann. Dermatol. 2009, 21, 308–310. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Y.; Zhang, H. Long-term effects of sliver nanoparticles on the abundance and activity of soil microbiome. J. Environ. Sci. (China) 2018, 69, 3–4. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat. Phys. Chem. 2020, 168, 108616. [Google Scholar] [CrossRef]




| Analytical Technique | Functions | References |
|---|---|---|
| X-ray diffraction | Measure the degree of crystallinity at the atomic scale. Used to analyze the structure of nanoparticles, particle sizes, for compounds identification, and to determine structure imperfections in the structures. The analysis depends on the formation of diffraction patterns | [6] |
| X-ray photoelectron spectroscopy | Determine the electronic states by atoms which include the oxidation state, and electron transfer in the nanoparticles. Estimate the empirical formulae by surface chemical analysis. Characterize the nanoparticles’ surface in the liquid forms. | [7] |
| Fourier transform infrared spectroscopy | Characterize various chemical bonding in nanomaterials. | [8] |
| UV–vis spectroscopy | Evaluate the stability and characteristics of AgNPs. Absorption of AgNPs depends on the dielectric medium, particle size, and the chemical environment. Size depends on surface plasmon for metal nanoparticles ranging from 2 to 100 nm. | [9] |
| Transmission electron microscopy | Measure of particle size, morphology, and size distribution. Provide better spatial resolution compared to SEM. | [10] |
| Scanning electron microscopy | Evaluate the morphology of AgNPs. Histogram obtains from images. Manually measure and count the particles or using specific software. | [11] |
| Dynamic light scattering | Measure nanoparticles size. Evaluate their stability over time in suspension at different pH and temperature conditions. | [12] |
| Localized surface plasmon resonance | Determine spatial oscillation of non-excited or excited (near-visible light) electron. Evaluate the molecular interaction on the surface of a nanoparticle. Depends on several factors: particle’s size and shape, electronic properties, dielectric media, and temperature | [13] |
| Reaction | Results | References |
|---|---|---|
| Turkevich method: Reduction of silver nitrate with sodium citrate | The particles size of 14 nm with a mean diameter of 10 nm | [29] |
| Reduction of silver nitrate with a mixture of hydrazine hydrate and sodium citrate as reductants; sodium dodecyl sulfate as a stabilizer | Colloidal solution with the particles size range of 8 to 50 nm with a mean diameter of 24 nm | [30] |
| Reduction of silver nitrate with a mixture of two different reducing agents which are tannic acid and sodium nitrate | Combination of reducing agents able to produce monodisperse spherical silver nanoparticles in 5 to 140 nm | [31] |
| Reduction of silver nitrate by sodium borohydride in presences of sodium dodecyl sulfate as a stabilizer Ag+ + BH4 − + 3H2O → Ago + B(OH)3 + 3.5 H2 | Formation of colloidal silver nanoparticles with particles diameter in a range from 30 to 40 nm | [32] |
| Reduction of silver nitrate with dextrose as reducing agent in presence of Na+-carrying poly[γ-glutamic acid] (PGA) 2Ag+ + 2OH− → Ag2O + H2O Ag2O + 4NH3 + H2O → 2[Ag(NH3)2]+ + 2OH | Formation of silver nanoparticles with an average size of 37.3 ± 5.5 nm for 0.5 wt% PGA-AgNP and 17.3 ± 3.4 nm for 2 wt% of PGA-AgNP | [33] |
| Reduction of silver nitrate with aniline in the presence of cetyltrimethylammonium bromide (CTAB) Ph-NH2 + Ag+ → Ph-NH2-Ag+ Ph-NH2-Ag+ → Anilino radical + Ago + H2O | Formation of spherical nanoparticles in size range from 10 to 30 nm and wide size distribution | [34] |
| Reduction of silver nitrate with trisodium citrate 4Ag+ + C6H5O7Na3 + 2H2O → 4Ago + C6H5O7H3 + 3Na+ + H+ + O2↑ | Formation of silver nanoparticle with particle size range from 5 to 100 nm | [35] |
| Reduction of silver nitrate with two different reducing agents which are ethylene glycol (EG) and glucose in the presence of poly[N-vinylpyrolidone] (PVP) as a stabilizer 2AgNO3 + R-CHO + 2 NaOH → 2Ag + R-COOH + 2NaNO3 + H2O | Spherical silver nanoparticles with particle size range from 10 to 250 nm | [36] |
| Substances | Results | Reference |
|---|---|---|
| (a) Plants | ||
| Artemisia nilagirica extract | The size diameters of the nanoparticles are in the range of 70 to 90 nm and the size distribution in the range of 2 to 4 keV. There was no impurity found. | [42] |
| Leaves extract of Catharanthus roseus. Linn. G. Donn | The size of nanoparticles is 27 ± 2 nm with zeta-potential of −63.1 mV which indicate good dispersity and stability. | [43] |
| Boerhaavia diffusa plant extract | The average particles size is 25 nm with cubic morphology of silver nanoparticles. | [44] |
| Ethanolic extract of Terminalia fagifolia Mart. | Formation of spherical or polygonal silver nanoparticles with a size range of 66 to 81 nm with high polydispersity. | [45] |
| Rosemary leaf aqueous extract | The silver nanoparticles are in a spherical shape with a diameter size of 14 nm with high purity. | [46] |
| Butea monosperma (BM) leaf extract | Formation of triangular and spherical nanoparticles with a size range of 20 to 80 nm. | [15] |
| Curcumin:hydroxypropyl-β-cyclodextrin (CUR:HPβCD) | Formation of spherical silver nanoparticles with an average size of 42.71 ± 17.97 nm and homogeneous dispersion of nanoparticles. | [47] |
| (b) Fungus | ||
| Biosorption by Aspergillus flavus | Production of monodisperse silver nanoparticles with an average particle size of 8.92 ± 1.61 nm and size distribution about 1000 nanoparticles. | [48] |
| Cladosporium cladosporioides | Formation of high-density silver nanoparticles with average size of 24 nm with uniform dispersion. | [49] |
| Guignardia sp. | The size of silver nanoparticles in the range of 5 nm and 20 nm with fairly monodisperse nature. | [50] |
| (c) Bacteria | ||
| Bacillus sp. | Development of silver nanoparticles with a size range of 5 to 15 nm observed in the periplasmic space of bacterial cells. | [51] |
| Bacillus subtilis | The average size of silver nanoparticles produces is 6.1 ± 1.6 nm. | [52] |
| Escherichia coli | The process yielded an average size particle of 50 nm with a uniform distribution at 50 nm. | [53] |
| Bacteria | Type of Bacteria | Silver Nanoparticles Size | Mechanism of Action | References |
|---|---|---|---|---|
| Pseudomonas aeruginosa | Gram-negative | Average particles size of 45 nm | Interaction with ROS and attachment of AgNPs at microbial cell wall | [77] |
| Escherichia coli AB1157 | Gram-negative | Average mean diameter 8.3 ± 1.9 nm | Damage the cellular DNA by influencing the base excision repair system | [78] |
| Staphylococcus aureus ATCC25923 | Gram-positive | Average size of 3.91 nm, 2.29 nm, and 1.59 nm | Destruction of microbial cell membrane and rise of ROS concentration | [79] |
| Escherichia coli ATCC25922 | Gram-negative | |||
| Escherichia coli DH5α | Gram-negative | Average size of 30 nm | Accumulation of AgNPs in the cell wall and cell membrane of bacterial cell | [80] |
| Bacillus Calmette-Guérin | Acid-fast Gram-positive | |||
| Multidrug resistant Escherichia coli (MC-2) | Gram-negative | Average size of 18 ± 3 nm | Disruption of cell membrane through formation of ROS | [81] |
| Multidrug resistant Staphylococcus aureus (MMC-20) | Gram-positive | |||
| Proteus sp. | Gram-negative | Average size of 38 nm | Cell wall ruptured and inhibit DNA replication thus inhibit the bacterial growth | [82] |
| Klebsiella sp. | Gram-negative | |||
| Staphylococcus aureus | Gram-positive | Size of nanoparticles should be lower than 100 nm. The articles do not mention the size of AgNPs | Oxidative stress which cause alteration in kynurenine protein. Activation of kynurenine pathways thus inhibit the bacterial growth | [83] |
| Escherichia coli | Gram-negative | |||
| Pseudomonas aeruginosa | Gram-negative | |||
| Bacillus subtilis | Gram-positive | |||
| Klebsiella pneumoniae | Gram-negative |
| Virus | Family | Silver Nanoparticles Composition | Mechanism of Action | References |
|---|---|---|---|---|
| Herpes simplex virus type 2 (HSV-2) | Herpesviridae | Tannic acid-modified silver nanoparticles (13 nm) | Interact with viral glycoproteins thus interfere with cell attachment | [84] |
| Bacteriophage MS2 | Leviviridae | Magnetic hybrid colloid silver nanoparticles (15 nm) | Damage proteins of the viral coat | [85] |
| Murine novovirus | Caliciviridae | |||
| Herpes simplex virus type 1 and type 2 (HSV-1 & HSV-2) | Herpesviridae | Mycosynthsized silver nanoparticles (4–31 nm) | Block interaction of virus and cells | [86] |
| Human parainfluenza virus type 3 (hPIV3) | Paramyxoviridae | |||
| Human immunodeficiency virus (HIV) | Retroviridae | PVP-coated silver nanoparticles (30–50 nm) | Inhibit the interaction between gp120 and cell membrane receptors | [87] |
| H1N1 influenza A | Orthomyxoviridae | Chitosan-coated silver nanoparticles (3.5, 6.5, and 12.9 nm) | Inhibit the viral contact with host cells and interaction of silver nanoparticles with viral glycoproteins | [88] |
| Poliovirus | Pure silver nanoparticles (7.1 nm) | Bind with the viral particles thus prevent binding with host receptor and inhibition of viral proteins | [89] | |
| Respiratory syncytial virus (RSV) | Paramyxoviridae | PVP-coated silver nanoparticles (10 nm) | Interfere with virus attachment by binding with gp120 glycoprotein | [90] |
| Hepatitis B virus (HBV) | Hepadnaviridae | Silver nanoparticles (10 and 50 nm) | Reduce the formation of HBV DNA by binding with the HBV dsDNA and virions | [91] |
| Adenovirus type 3 (Ad3) | Adenoviridae | Silver nanoparticles (11.4 nm) | Damaging the viral particles and bind to the viral DNA | [92] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. https://doi.org/10.3390/nano10081566
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N, Fauzi MB. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials. 2020; 10(8):1566. https://doi.org/10.3390/nano10081566
Chicago/Turabian StyleSalleh, Atiqah, Ruth Naomi, Nike Dewi Utami, Abdul Wahab Mohammad, Ebrahim Mahmoudi, Norlaila Mustafa, and Mh Busra Fauzi. 2020. "The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action" Nanomaterials 10, no. 8: 1566. https://doi.org/10.3390/nano10081566