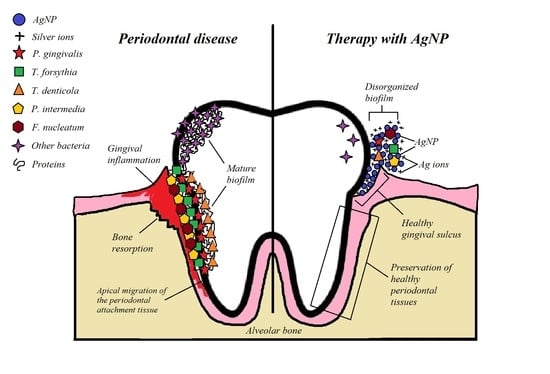
Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of AgNP
2.3. Patient Recruitment
2.4. Sampling of Oral Biofilms
2.5. Initial Bacterial Growth and Standard Microbial Suspension
2.6. Antimicrobial Test
2.7. Identification of Bacteria by Polymerase Chain Reaction (PCR)
2.8. Statistical Analysis
3. Results
3.1. Characterization of AgNP
3.2. Distribution of Patients
3.3. Bacterial Growth of Biofilms
3.4. Antimicrobial Activity of AgNP
3.5. Distribution of Periodontal Bacteria by PCR Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef]
- Gasner, N.; Schure, R. Periodontal Disease—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554590/ (accessed on 19 May 2023).
- Bárcena García, M.; Cobo Plana, J.M.; Arcos González, P.I. Prevalence and Severity of Periodontal Disease among Spanish Military Personnel. BMJ Mil. Health 2022, 168, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The Role of the Microbiota in Periodontal Disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Levin, L. Inequities in Periodontal Disease Prevalence, Prevention, and Management. Quintessence Int. 2022, 53, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S2), S286–S291. [Google Scholar] [CrossRef] [Green Version]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The Subgingival Microbiome in Health and Periodontitis and Its Relationship with Community Biomass and Inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D. Microbial Ecology of Dental Plaque and Its Significance in Health and Disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.C.; Moore, L.V.H. The Bacteria of Periodontal Diseases. Periodontol. 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Hong, B.-Y.; Furtado Araujo, M.V.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P.I. Microbiome Profiles in Periodontitis in Relation to Host and Disease Characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S.S.; Haffajee, A.D. Periodontal Microbial Ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Chackartchi, T.; Hamzani, Y.; Shapira, L.; Polak, D. Effect of Subgingival Mechanical Debridement and Local Delivery of Chlorhexidine Gluconate Chip or Minocycline Hydrochloride Microspheres in Patients Enrolled in Supportive Periodontal Therapy: A Retrospective Analysis. Oral Health Prev. Dent. 2019, 17, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Poklepovic, T.; Worthington, H.V.; Johnson, T.M.; Sambunjak, D.; Imai, P.; Clarkson, J.E.; Tugwell, P. Interdental Brushing for the Prevention and Control of Periodontal Diseases and Dental Caries in Adults. Cochrane Database Syst. Rev. 2013, 2013, CD009857. [Google Scholar] [CrossRef] [PubMed]
- Sälzer, S.; Graetz, C.; Dörfer, C.E.; Slot, D.E.; Van der Weijden, F.A. Contemporary Practices for Mechanical Oral Hygiene to Prevent Periodontal Disease. Periodontol. 2000 2020, 84, 35–44. [Google Scholar] [CrossRef]
- Worthington, H.V.; MacDonald, L.; Poklepovic Pericic, T.; Sambunjak, D.; Johnson, T.M.; Imai, P.; Clarkson, J.E. Home Use of Interdental Cleaning Devices, in Addition to Toothbrushing, for Preventing and Controlling Periodontal Diseases and Dental Caries. Cochrane Database Syst. Rev. 2019, 4, CD012018. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.S.; Miglani, R.; Srinivasan, M.R.; Indira, R. Antifungal Efficacy of 5.25% Sodium Hypochlorite, 2% Chlorhexidine Gluconate, and 17% EDTA With and Without an Antifungal Agent. J. Endod. 2010, 36, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of Chlorhexidine Rinses after Periodontal or Implant Surgery: A Systematic Review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Haydari, M.; Bardakci, A.G.; Koldsland, O.C.; Aass, A.M.; Sandvik, L.; Preus, H.R. Comparing the Effect of 0.06% -, 0.12% and 0.2% Chlorhexidine on Plaque, Bleeding and Side Effects in an Experimental Gingivitis Model: A Parallel Group, Double Masked Randomized Clinical Trial. BMC Oral Health 2017, 17, 118. [Google Scholar] [CrossRef] [Green Version]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current Uses of Chlorhexidine for Management of Oral Disease: A Narrative Review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.S.; Faveri, M. Systemic Antibiotics in the Treatment of Periodontitis. Periodontol. 2000 2015, 67, 131–186. [Google Scholar] [CrossRef]
- Walker, C.; Karpinia, K. Rationale for Use of Antibiotics in Periodontics. J. Periodontol. 2002, 73, 1188–1196. [Google Scholar] [CrossRef]
- Blair, F.M.; Chapple, I.L.C. Prescribing for Periodontal Disease. Prim. Dent. J. 2014, 3, 38–43. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic Resistance in Human Chronic Periodontitis Microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.H.J.; Ahmed, M.G.; Chinnam, S.; Paul, K.; Ashrafuzzaman, M.; Chavali, M.; Gahtori, R.; Pandit, S.; Kesari, K.K.; Gupta, P.K. Current Trends in Bio-Waste Mediated Metal/Metal Oxide Nanoparticles for Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 71, 103305. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.-H. Metal and Metal Oxide Nanoparticles in Caries Prevention: A Review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Maboza, E.; Meyer, M.; Geerts, G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Sun, Q.; Li, J. Preparation, Characterization, and Antibacterial Properties of Silver Nanoparticles Embedded into Cellulose Aerogels. Polym. Compos. 2016, 37, 1137–1142. [Google Scholar] [CrossRef]
- de Almeida, J.; Cechella, B.; Bernardi, A.; de Lima Pimenta, A.; Felippe, W. Effectiveness of Nanoparticles Solutions and Conventional Endodontic Irrigants against Enterococcus Faecalis Biofilm. Indian J. Dent. Res. 2018, 29, 347. [Google Scholar] [CrossRef]
- Vanitha, G.; Rajavel, K.; Boopathy, G.; Veeravazhuthi, V.; Neelamegam, P. Physiochemical Charge Stabilization of Silver Nanoparticles and Its Antibacterial Applications. Chem. Phys. Lett. 2017, 669, 71–79. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Cruz Pena, D.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; de Jesús Pozos Guillén, A.; Tapia-Pérez, H.; Martínez Castañón, G. The Antimicrobial Sensitivity of Streptococcus Mutans to Nanoparticles of Silver, Zinc Oxide, and Gold. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.F.; Ruiz, F. Antibacterial Effect of Silver Nanoparticles against Streptococcus Mutans. Mater. Lett. 2009, 63, 2603–2606. [Google Scholar] [CrossRef]
- May, A.; Kopecki, Z.; Carney, B.; Cowin, A. Practical Extended Use of Antimicrobial Silver (PExUS). ANZ J. Surg. 2022, 92, 1199–1205. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Dahake, P.T.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Siddiqi, A.G.; Maggavi, U.R. In Vitro Evaluation of Antimicrobial Property of Silver Nanoparticles and Chlorhexidine against Five Different Oral Pathogenic Bacteria. Saudi Dent. J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.-L.; Tang, J.; Chu, C.-H. Developing Biocompatible Silver Nanoparticles Using Epigallocatechin Gallate for Dental Use. Arch. Oral Biol. 2019, 102, 106–112. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-Dependent Antibacterial Activities of Silver Nanoparticles against Oral Anaerobic Pathogenic Bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Halkai, K.; Halkai, R.; Mudda, J.; Shivanna, V.; Rathod, V. Antibiofilm Efficacy of Biosynthesized Silver Nanoparticles against Endodontic-Periodontal Pathogens: An in Vitro Study. J. Conserv. Dent. 2018, 21, 662. [Google Scholar] [CrossRef] [PubMed]
- Zorraquín-Peña, I.; Cueva, C.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. Glutathione-Stabilized Silver Nanoparticles: Antibacterial Activity against Periodontal Bacteria, and Cytotoxicity and Inflammatory Response in Oral Cells. Biomedicines 2020, 8, 375. [Google Scholar] [CrossRef]
- Constantin, M.; Lupei, M.; Bucatariu, S.-M.; Pelin, I.M.; Doroftei, F.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. PVA/Chitosan Thin Films Containing Silver Nanoparticles and Ibuprofen for the Treatment of Periodontal Disease. Polymers 2022, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrillo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and Antimicrobial Properties of Silver Nanoparticles against Streptococcus Mutans on Brackets and Wires Used for Orthodontic Treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Ramírez, A.J.; Martínez-Martínez, R.E.; Ayala-Herrera, J.L.; Zaragoza-Contreras, E.A.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Silva-Benítez, E.L.; Espinosa-Cristóbal, L.F. Antimicrobial Activity of Silver Nanoparticles against Clinical Biofilms from Patients with and without Dental Caries. J. Nanomater. 2021, 2021, 5587455. [Google Scholar] [CrossRef]
- Martinez-Martinez, R.E.; Abud-Mendoza, C.; Patiño-Marin, N.; Rizo-Rodríguez, J.C.; Little, J.W.; Loyola-Rodríguez, J.P. Detection of Periodontal Bacterial DNA in Serum and Synovial Fluid in Refractory Rheumatoid Arthritis Patients. J. Clin. Periodontol. 2009, 36, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, R.E.; Moreno-Castillo, D.F.; Loyola-Rodríguez, J.P.; Sánchez-Medrano, A.G.; Miguel-Hernández, J.H.S.; Olvera-Delgado, J.H.; Domínguez-Pérez, R.A. Association between Periodontitis, Periodontopathogens and Preterm Birth: Is It Real? Arch. Gynecol. Obstet. 2016, 294, 47–54. [Google Scholar] [CrossRef]
- Tran, S.D.; Rudney, J.D. Multiplex PCR Using Conserved and Species-Specific 16S RRNA Gene Primers for Simultaneous Detection of Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis. J. Clin. Microbiol. 1996, 34, 2674–2678. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, S.; Park, S.F.; Bishop, P.A.; Lewis, M.A.O. Direct Detection of Prevotella Intermedia and P. Nigrescens in Suppurative Oral Infection by Amplification of 16S RRNA Gene. J. Med. Microbiol. 1999, 48, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Frommel, T.O. Porphyromonas Gingivalis, Actinobacillus Actinomycetemcomitans and Treponema Denticola Detection in Oral Plaque Samples Using the Polymerase Chain Reaction. J. Clin. Periodontol. 1996, 23, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ashimoto, A.; Chen, C.; Bakker, I.; Slots, J. Polymerase Chain Reaction Detection of 8 Putative Periodontal Pathogens in Subgingival Plaque of Gingivitis and Advanced Periodontitis Lesions. Oral Microbiol. Immunol. 1996, 11, 266–273. [Google Scholar] [CrossRef]
- Poulsen, K.; Ennibi, O.-K.; Haubek, D. Improved PCR for Detection of the Highly Leukotoxic JP2 Clone of Actinobacillus Actinomycetemcomitans in Subgingival Plaque Samples. J. Clin. Microbiol. 2003, 41, 4829–4832. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, Q.; Jiang, C.; Yang, D.; Liu, X.; Xu, S. One-Step Synthesis of Biocompatible Gold Nanoparticles Using Gallic Acid in the Presence of Poly-(N-Vinyl-2-Pyrrolidone). Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 73–79. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Huong, P.T.L.; Van Son, T.; Phan, V.N.; Tam, L.T.; Le, A.-T. Microstructure and Chemo-Physical Characterizations of Functional Graphene Oxide-Iron Oxide-Silver Ternary Nanocomposite Synthesized by One-Pot Hydrothermal Method. J. Nanosci. Nanotechnol. 2018, 18, 5591–5599. [Google Scholar] [CrossRef]
- Rodríguez-González, B.; Sánchez-Iglesias, A.; Giersig, M.; Liz-Marzán, L.M. AuAg Bimetallic Nanoparticles: Formation, Silica-Coating and Selective Etching. Faraday Discuss. 2004, 125, 133–144. [Google Scholar] [CrossRef]
- Lee, H.K.; Talib, Z.A.; Mamat @ Mat Nazira, M.S.; Wang, E.; Lim, H.N.; Mahdi, M.A.; Ng, E.K.; Yusoff, N.M.; AL-Jumaili, B.E.; Liew, J.Y.C. Effect of Sodium Hydroxide Concentration in Synthesizing Zinc Selenide/Graphene Oxide Composite via Microwave-Assisted Hydrothermal Method. Materials 2019, 12, 2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Antimicrobial Activity of Silver/Starch/Polyacrylamide Nanocomposite. Int. J. Biol. Macromol. 2014, 68, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Loyola-Rodríguez, J.P.; Niño-Martínez, N.; Ruiz, F.; Zavala-Alonso, N.V.; Lara, R.H.; Reyes-López, S.Y. Bovine Serum Albumin and Chitosan Coated Silver Nanoparticles and Its Antimicrobial Activity against Oral and Nonoral Bacteria. J. Nanomater. 2015, 2015, 420853. [Google Scholar] [CrossRef] [Green Version]
- Tuan, T.Q.; Van Son, N.; Dung, H.T.K.; Luong, N.H.; Thuy, B.T.; Van Anh, N.T.; Hoa, N.D.; Hai, N.H. Preparation and Properties of Silver Nanoparticles Loaded in Activated Carbon for Biological and Environmental Applications. J. Hazard. Mater. 2011, 192, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Kaler, A.; Jain, S.; Banerjee, U.C. Green and Rapid Synthesis of Anticancerous Silver Nanoparticles by Saccharomyces Boulardii and Insight into Mechanism of Nanoparticle Synthesis. BioMed Res. Int. 2013, 2013, 872940. [Google Scholar] [CrossRef] [Green Version]
- Prema, P.; Veeramanikandan, V.; Rameshkumar, K.; Gatasheh, M.K.; Hatamleh, A.A.; Balasubramani, R.; Balaji, P. Statistical Optimization of Silver Nanoparticle Synthesis by Green Tea Extract and Its Efficacy on Colorimetric Detection of Mercury from Industrial Waste Water. Environ. Res. 2022, 204, 111915. [Google Scholar] [CrossRef]
- Sirisha, P.; Gayathri, G.; Dhoom, S.; Amulya, K. Antimicrobial Effect of Silver Nanoparticles Synthesised with Ocimum Sanctum Leaf Extract on Periodontal Pathogens. J. Oral Health Dent. Sci. 2017, 1, 106. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions OnEscherichia Coli AndStaphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef]
- Soares, G.M.S.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of Action of Systemic Antibiotics Used in Periodontal Treatment and Mechanisms of Bacterial Resistance to These Drugs. J. Appl. Oral Sci. 2012, 20, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic Susceptibility of Periodontal Enterococcus Faecalis. J. Periodontol. 2013, 84, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral Biofilm Architecture on Natural Teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [Green Version]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The Oral Microbiome in Health and Disease and the Potential Impact on Personalized Dental Medicine. Oral Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ghazeeri, G.; Abdullah, L.; Abbas, O. Immunological Differences in Women Compared with Men: Overview and Contributing Factors. Am. J. Reprod. Immunol. 2011, 66, 163–169. [Google Scholar] [CrossRef]
- Nakaya, M.; Tachibana, H.; Yamada, K. Effect of Estrogens on the Interferon-Gamma Producing Cell Population of Mouse Splenocytes. Biosci. Biotechnol. Biochem. 2006, 70, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Valerio, M.S.; Basilakos, D.S.; Kirkpatrick, J.E.; Chavez, M.; Hathaway-Schrader, J.; Herbert, B.A.; Kirkwood, K.L. Sex-Based Differential Regulation of Bacterial-Induced Bone Resorption. J. Periodontal Res. 2017, 52, 377–387. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bhardwaj, S. Effect of Menopause on Women′s Periodontium. J. Midlife Health 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Mahler, D.; Sanduri, H.; Peled, M. The Effect of Menstrual Cycle on Periodontal Health. J. Periodontol. 2004, 75, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, M.S.; Su, S.; Crespo, C.J.; Hung, M. Men and Oral Health: A Review of Sex and Gender Differences. Am. J. Mens. Health 2021, 15, 155798832110163. [Google Scholar] [CrossRef] [PubMed]
- Krejci, C.B.; Bissada, N.F. Women’s Health Issues and Their Relationship to Periodontitis. J. Am. Dent. Assoc. 2002, 133, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Kim, K.-J.; Lee, D.G. A Novel Mechanism for the Antibacterial Effect of Silver Nanoparticles on Escherichia Coli. BioMetals 2014, 27, 1191–1201. [Google Scholar] [CrossRef]
- Qin, G.; Tang, S.; Li, S.; Lu, H.; Wang, Y.; Zhao, P.; Li, B.; Zhang, J.; Peng, L. Toxicological Evaluation of Silver Nanoparticles and Silver Nitrate in Rats Following 28 Days of Repeated Oral Exposure. Environ. Toxicol. 2017, 32, 609–618. [Google Scholar] [CrossRef]
- Venugopal, A.; Muthuchamy, N.; Tejani, H.; Gopalan, A.-I.; Lee, K.-P.; Lee, H.-J.; Kyung, H.M. Incorporation of Silver Nanoparticles on the Surface of Orthodontic Microimplants to Achieve Antimicrobial Properties. Korean J. Orthod. 2017, 47, 3. [Google Scholar] [CrossRef] [Green Version]
- Sweet, M.J.; Chessher, A.; Singleton, I. Review: Metal-Based Nanoparticles; Size, Function, and Areas for Advancement in Applied Microbiology. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2012; pp. 113–142. ISBN 9780123943811. [Google Scholar]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic Aspects in the Biogenic Synthesis of Extracellular Metal Nanoparticles by Peptides, Bacteria, Fungi, and Plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Kailasa, S.K.; Park, T.-J.; Rohit, J.V.; Koduru, J.R. Antimicrobial Activity of Silver Nanoparticles. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128165041. [Google Scholar]
- Loyola-Rodriguez, J.P.; Ponce-Diaz, M.E.; Loyola-Leyva, A.; Garcia-Cortes, J.O.; Medina-Solis, C.E.; Contreras-Ramire, A.A.; Serena-Gomez, E. Determination and Identification of Antibiotic-Resistant Oral Streptococci Isolated from Active Dental Infections in Adults. Acta Odontol. Scand. 2018, 76, 229–235. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Holguín-Meráz, C.; Zaragoza-Contreras, E.A.; Martínez-Martínez, R.E.; Donohue-Cornejo, A.; Loyola-Rodríguez, J.P.; Cuevas-González, J.C.; Reyes-López, S.Y. Antimicrobial and Substantivity Properties of Silver Nanoparticles against Oral Microbiomes Clinically Isolated from Young and Young-Adult Patients. J. Nanomater. 2019, 2019, 3205971. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Espinosa-Cristobal, L.F.; Martinez-Castañon, G.A.; Loyola-Rodriguez, J.P.; Patiño-Marin, N.; Reyes-Macías, J.F.; Vargas-Morales, J.M.; Ruiz, F. Toxicity, Distribution, and Accumulation of Silver Nanoparticles in Wistar Rats. J. Nanopart. Res. 2013, 15, 1702. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; El-Rafie, M.H.; EL-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial Wound Dressing and Anti-Inflammatory Efficacy of Silver Nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef] [PubMed]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; et al. Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In Vivo Human Time-Exposure Study of Orally Dosed Commercial Silver Nanoparticles. Nanomedicine 2014, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]






| AgNP | DLS (nm) | Shape | Concentration (µg/mL) | Zeta Potential (mV) |
|---|---|---|---|---|
| 5.4 nm | 5.4 ± 1.3 | Spherical | 1070 | −38.2 ± 5.8 |
| 17.5 nm | 17.5 ± 3.4 | Spherical | 1070 | −32.6 ± 5.4 |
| Periodontal Disease n = 30 Subjects (%) | Control (Healthy) n = 30 Subjects (%) | |
|---|---|---|
| Age (years old) | 39 ± 6.9 | 28 ± 8.9 |
| Women | 37 ± 7.7 | 28.3 ± 9.4 |
| Men | 40.5 ± 5.8 | 27.5 ± 8.6 |
| Gender | ||
| Women | 14 (47) | 14 (47) |
| Men | 16 (53) | 16 (53) |
| Variable | Periodontal Disease (r) | p-Value | Healthy (r) | p-Value | Total (r) | p-Value |
|---|---|---|---|---|---|---|
| OD | 0.179 | 0.345 | 0.087 | 0.647 | 0.501 | 0.000 ** |
| AgNP 5.4 nm | 0.021 | 0.914 | −0.248 | 0.186 | 0.223 | 0.087 |
| AgNP 17.5 nm | 0.122 | 0.521 | −0.042 | 0.825 | 0.223 | 0.074 |
| Groups | No Periodontal Disease | Periodontal Disease | ||||
|---|---|---|---|---|---|---|
| Subjects | 1 | 2 | 3 | 4 | 5 | 6 |
| Age (years) | 32 | 31 | 34 | 32 | 35 | 31 |
| Gender | F | M | F | F | M | F |
| Bacterial strains | ||||||
| P.gingivalis | + | − | + | + | + | + |
| T. forsythia | − | − | + | + | + | + |
| T. denticola | + | − | + | + | + | + |
| P. intermedia | + | + | + | + | + | + |
| F. nucleatum | + | + | + | + | + | + |
| A. actinomycetemcomitans | − | − | − | − | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Venegas, P.A.; Martínez-Martínez, R.E.; Zaragoza-Contreras, E.A.; Domínguez-Pérez, R.A.; Reyes-López, S.Y.; Donohue-Cornejo, A.; Cuevas-González, J.C.; Molina-Frechero, N.; Espinosa-Cristóbal, L.F. Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease. J. Funct. Biomater. 2023, 14, 311. https://doi.org/10.3390/jfb14060311
Hernández-Venegas PA, Martínez-Martínez RE, Zaragoza-Contreras EA, Domínguez-Pérez RA, Reyes-López SY, Donohue-Cornejo A, Cuevas-González JC, Molina-Frechero N, Espinosa-Cristóbal LF. Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease. Journal of Functional Biomaterials. 2023; 14(6):311. https://doi.org/10.3390/jfb14060311
Chicago/Turabian StyleHernández-Venegas, Perla Alejandra, Rita Elizabeth Martínez-Martínez, Erasto Armando Zaragoza-Contreras, Rubén Abraham Domínguez-Pérez, Simón Yobanny Reyes-López, Alejandro Donohue-Cornejo, Juan Carlos Cuevas-González, Nelly Molina-Frechero, and León Francisco Espinosa-Cristóbal. 2023. "Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease" Journal of Functional Biomaterials 14, no. 6: 311. https://doi.org/10.3390/jfb14060311