Ketoprofen-Based Polymer-Drug Nanoparticles Provide Anti-Inflammatory Properties to HA/Collagen Hydrogels
Abstract
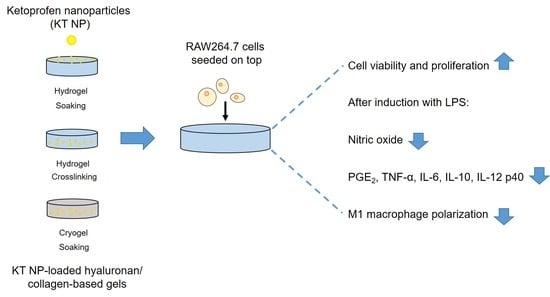
:1. Introduction
2. Materials and Methods
2.1. Hyaluronan Methacrylate Preparation
2.2. Nanoparticle Synthesis
2.3. NP Characterization
2.4. Scaffold Fabrication
2.4.1. Preparation of Hydro- and Cryogels
2.4.2. Loading of the Gels with NP
2.5. NP Release Studies from Hydro- and Cryogels
2.6. Determination of NP Concentration
2.7. NP Distribution in Hydro- and Cryogels
2.8. Hydro- and Cryogel Morphology
2.9. Mechanical Characterization of the Hydro- and Cryogels
2.10. Porosity of the Hydro- and Cryogels
2.11. Cell Culture
2.12. Direct Assays
2.12.1. Micro-Mass Cell Seeding
2.12.2. Cell Proliferation
2.12.3. Cell Viability
2.13. Anti-Inflammatory Activity of Hydrogels
2.13.1. Nitric Oxide Production
2.13.2. Macrophages Spreading and Multinucleated Cells Evaluation by Fluorescence Microscopy
2.13.3. ELISA for Anti- and Pro-Inflammatory Markers
2.14. Indirect Assays
2.14.1. Extract Collection from Hydrogels
2.14.2. Indirect Cell Proliferation Assay
2.14.3. Indirect Nitric Oxide (NO) Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Hydrodynamic Characterization of Ketoprofen NP
3.2. Binding and Release of Ketoprofen NP from HA/Coll Hydro- and Cryogels
3.3. Controlled Loading of Hydro- and Cryogels with NP
3.4. NP Distribution in Hydro- and Cryogels
3.5. Morphology and Mechanical Properties of Hydro- and Cryogels


3.6. Proliferation and Viability of a Macrophage Cell Line
3.7. Anti-Inflammatory Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wicke, C.; Bachinger, A.; Coerper, S.; Beckert, S.; Witte, M.B.; Königsrainer, A. Aging Influences Wound Healing in Patients with Chronic Lower Extremity Wounds Treated in a Specialized Wound Care Center. Wound Repair Regen. 2009, 17, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy: PERSPECTIVE ARTICLE. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting Tissue Regeneration by Modulating the Immune System. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Koh, T.J. Dysregulation of Monocyte/Macrophage Phenotype in Wounds of Diabetic Mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Weinheimer-Haus, E.M.; Ennis, W.J.; Koh, T.J. Sustained Inflammasome Activity in Macrophages Impairs Wound Healing in Type 2 Diabetic Humans and Mice. Diabetes 2014, 63, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Torregrossa, M.; Kakpenova, A.; Simon, J.C.; Franz, S. Modulation of Macrophage Functions by ECM-Inspired Wound Dressings—A Promising Therapeutic Approach for Chronic Wounds. Biol. Chem. 2021, 402, 1289–1307. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds: Chronic Wound Care and Management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Pallaske, F.; Pallaske, A.; Herklotz, K.; Boese-Landgraf, J. The Significance of Collagen Dressings in Wound Management: A Review. J. Wound Care 2018, 27, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Tittelbach, J.; Hipler, U.-C.; Elsner, P. Clinical Efficacy of Dressings for Treatment of Heavily Exuding Chronic Wounds. CWCMR Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Powers, J.G.; Morton, L.M.; Phillips, T.J. Dressings for Chronic Wounds. Dermatol. Ther. 2013, 26, 197–206. [Google Scholar] [CrossRef]
- Rangaraj, Aravindan and Harding, Keith and Leaper, David Role of Collagen in Wound Management. Wounds Uk 2011, 7, 54–63.
- Dressings.Org—Dressings and Datacards for Woundcare. Available online: http://www.dressings.org/ (accessed on 3 March 2022).
- Schreml, S.; Klein, S.M.; Babilas, P.; Karrer, S. Wundauflagen in der Therapie chronischer Wunden. Phlebologie 2013, 42, 189–196. [Google Scholar] [CrossRef]
- Matsumura, H.; Imai, R.; Ahmatjan, N.; Ida, Y.; Gondo, M.; Shibata, D.; Wanatabe, K. Removal of Adhesive Wound Dressing and Its Effects on the Stratum Corneum of the Skin: Comparison of Eight Different Adhesive Wound Dressings. Int. Wound J. 2014, 11, 50–54. [Google Scholar] [CrossRef]
- Westby, M.J.; Dumville, J.C.; Soares, M.O.; Stubbs, N.; Norman, G. Dressings and Topical Agents for Treating Pressure Ulcers. Cochrane Database Syst. Rev. 2017, 12, CD009362. [Google Scholar] [CrossRef] [Green Version]
- Zamboni, F.; Vieira, S.; Reis, R.L.; Miguel Oliveira, J.; Collins, M.N. The Potential of Hyaluronic Acid in Immunoprotection and Immunomodulation: Chemistry, Processing and Function. Prog. Mater. Sci. 2018, 97, 97–122. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants—A Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Andorko, J.I.; Jewell, C.M. Designing Biomaterials with Immunomodulatory Properties for Tissue Engineering and Regenerative Medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular Matrix Hydrogels from Decellularized Tissues: Structure and Function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dziki, J.L.; Huleihel, L.; Scarritt, M.E.; Badylak, S. Extracellular Matrix Bioscaffolds as Immunomodulatory Biomaterials. Tissue Eng. Part A 2017, 23, 1152–1159. [Google Scholar] [CrossRef]
- Frenkel, J.S. The Role of Hyaluronan in Wound Healing. Int. Wound J. 2014, 11, 159–163. [Google Scholar] [CrossRef]
- Muto, J.; Sayama, K.; Gallo, R.L.; Kimata, K. Emerging Evidence for the Essential Role of Hyaluronan in Cutaneous Biology. J. Dermatol. Sci. 2019, 94, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Gravante, G.; Sorge, R.; Merone, A.; Tamisani, A.M.; Lonardo, A.D.; Scalise, A.; Doneddu, G.; Melandri, D.; Stracuzzi, G.; Onesti, M.G.; et al. Hyalomatrix PA in Burn Care Practice: Results From a National Retrospective Survey, 2005 to 2006. Ann. Plast. Surg. 2010, 64, 69–79. [Google Scholar] [CrossRef]
- Rother, S.; Galiazzo, V.D.; Kilian, D.; Fiebig, K.M.; Becher, J.; Moeller, S.; Hempel, U.; Schnabelrauch, M.; Waltenberger, J.; Scharnweber, D.; et al. Hyaluronan/Collagen Hydrogels with Sulfated Hyaluronan for Improved Repair of Vascularized Tissue Tune the Binding of Proteins and Promote Endothelial Cell Growth. Macromol. Biosci. 2017, 17, 1700154. [Google Scholar] [CrossRef]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gómez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/Collagen Hydrogels Containing Sulfated Hyaluronan Improve Wound Healing by Sustained Release of Heparin-Binding EGF-like Growth Factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Hauck, S.; Zager, P.; Halfter, N.; Wandel, E.; Torregrossa, M.; Kakpenova, A.; Rother, S.; Ordieres, M.; Räthel, S.; Berg, A.; et al. Collagen/Hyaluronan Based Hydrogels Releasing Sulfated Hyaluronan Improve Dermal Wound Healing in Diabetic Mice via Reducing Inflammatory Macrophage Activity. Bioact. Mater. 2021, 6, 4342–4359. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cano, E.; Aguilar, M.R.; Portilla, Y.; Barber, D.F.; San Román, J. Polymeric Nanoparticles That Combine Dexamethasone and Naproxen for the Synergistic Inhibition of Il12b Transcription in Macrophages. Macromol. Biosci. 2020, 20, 2000002. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cano, E.; Aguilar, M.R.; Portilla, Y.; Barber, D.F.; San Román, J. Anti-Inflammatory Polymeric Nanoparticles Based on Ketoprofen and Dexamethasone. Pharmaceutics 2020, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Botting, R.M. Inhibitors of cyclooxygenases: Mechanisms, selectivity and uses. J. Physiol. Pharmacol. 2006, 57, 113–124. [Google Scholar]
- Paik, J.H.; Ju, J.H.; Lee, J.Y.; Boudreau, M.D.; Hwang, D.H. Two Opposing Effects of Non-Steroidal Anti-Inflammatory Drugs on the Expression of the Inducible Cyclooxygenase: MEDIATION THROUGH DIFFERENT SIGNALING PATHWAYS. J. Biol. Chem. 2000, 275, 28173–28179. [Google Scholar] [CrossRef] [Green Version]
- Becher, J.; Moeller, S.; Schnabelrauch, M. Building Blocks for Artificial Extracellular Matrices Based on Cross-Linkable Polysaccharide and Glycosaminglycan Sulfates. Mater. Sci. Forum 2016, 879, 1270–1275. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lozinsky, V. Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, F.; Wei, Y.; Zhang, H. Freeze–Thaw-Induced Gelation of Hyaluronan: Physical Cryostructuration Correlated with Intermolecular Associations and Molecular Conformation. Macromolecules 2017, 50, 6647–6658. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, Y.; Wei, Y.; Wang, P.; Zhang, H. Physically Cross-Linked Hyaluronan-Based Ultrasoft Cryogel Prepared by Freeze–Thaw Technique as a Barrier for Prevention of Postoperative Adhesions. Biomacromolecules 2021, 22, 4967–4979. [Google Scholar] [CrossRef]
- Achterberg, V.F.; Buscemi, L.; Diekmann, H.; Smith-Clerc, J.; Schwengler, H.; Meister, J.-J.; Wenck, H.; Gallinat, S.; Hinz, B. The Nano-Scale Mechanical Properties of the Extracellular Matrix Regulate Dermal Fibroblast Function. J. Investig. Dermatol. 2014, 134, 1862–1872. [Google Scholar] [CrossRef] [Green Version]
- Samani, A.; Zubovits, J.; Plewes, D. Elastic Moduli of Normal and Pathological Human Breast Tissues: An Inversion-Technique-Based Investigation of 169 Samples. Phys. Med. Biol. 2007, 52, 1565–1576. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent Advances in Hyaluronic Acid Hydrogels for Biomedical Applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, S.; Wang, J. Photo-Crosslinkable Hydrogel and Its Biological Applications. Chin. Chem. Lett. 2021, 32, 1603–1614. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- McNally, A.K.; Anderson, J.M. Macrophage Fusion and Multinucleated Giant Cells of Inflammation. In Cell Fusion in Health and Disease; Advances in Experimental Medicine and Biology; Dittmar, T., Zänker, K.S., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 97–111. ISBN 978-94-007-0763-4. [Google Scholar]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage Responses to Implants: Prospects for Personalized Medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.; Adcock, I.M.; Ahmed, B.Y.; Punchard, N.A. Modulation of LPS Stimulated NF-KappaB Mediated Nitric Oxide Production by PKCε and JAK2 in RAW Macrophages. J. Inflamm. 2007, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular Mechanisms Underlying Prostaglandin E2-Exacerbated Inflammation and Immune Diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef] [PubMed]







| Label | c HA-MAC 1 [mg/mL] | c Coll 1 [mg/mL] | Gel Type | Loading Method | Target NP c [µg/HG] |
|---|---|---|---|---|---|
| Binding and release studies of NPs | |||||
| 10HA max NP | 10 | 0.5 | HG | Soaking 2 | - |
| 30HA max NP | 30 | 0.5 | HG | Soaking 2 | - |
| cryo 10HA max NP | 10 | 0.5 | CG | Soaking 2 | - |
| Cell experiments and determination of the properties | |||||
| 10HA | 10 | 0.5 | HG | - | - |
| 10HA 40NP | 10 | 0.5 | HG | Soaking | 40 |
| 10HA 120NP | 10 | 0.5 | HG | Soaking | 120 |
| CL-10HA 40NP | 10 | 0.5 | HG | Crosslinking | 40 1 |
| 30HA | 30 | 0.5 | HG | - | - |
| 30HA 40NP | 30 | 0.5 | HG | Soaking | 40 |
| cryo 10HA | 10 | 0.5 | CG | - | - |
| cryo 10HA 40NP | 10 | 0.5 | CG | Soaking | 40 |
| Gel | NP Content | Incubation Temperature [°C] | Incubation Time [d] | Change of Supernatant |
|---|---|---|---|---|
| 10HA | Max NP, 40NP, and 120NP | 37 | 1–2 | - |
| 30HA | Max NP and 40NP | 4–6 | 2× | |
| cryo 10HA | Max NP and 40NP | 4–6 | 2× |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halfter, N.; Espinosa-Cano, E.; Pontes-Quero, G.M.; Ramírez-Jiménez, R.A.; Heinemann, C.; Möller, S.; Schnabelrauch, M.; Wiesmann, H.-P.; Hintze, V.; Aguilar, M.R. Ketoprofen-Based Polymer-Drug Nanoparticles Provide Anti-Inflammatory Properties to HA/Collagen Hydrogels. J. Funct. Biomater. 2023, 14, 160. https://doi.org/10.3390/jfb14030160
Halfter N, Espinosa-Cano E, Pontes-Quero GM, Ramírez-Jiménez RA, Heinemann C, Möller S, Schnabelrauch M, Wiesmann H-P, Hintze V, Aguilar MR. Ketoprofen-Based Polymer-Drug Nanoparticles Provide Anti-Inflammatory Properties to HA/Collagen Hydrogels. Journal of Functional Biomaterials. 2023; 14(3):160. https://doi.org/10.3390/jfb14030160
Chicago/Turabian StyleHalfter, Norbert, Eva Espinosa-Cano, Gloria María Pontes-Quero, Rosa Ana Ramírez-Jiménez, Christiane Heinemann, Stephanie Möller, Matthias Schnabelrauch, Hans-Peter Wiesmann, Vera Hintze, and Maria Rosa Aguilar. 2023. "Ketoprofen-Based Polymer-Drug Nanoparticles Provide Anti-Inflammatory Properties to HA/Collagen Hydrogels" Journal of Functional Biomaterials 14, no. 3: 160. https://doi.org/10.3390/jfb14030160