Anti-Inflammatory Salidroside Delivery from Chitin Hydrogels for NIR-II Image-Guided Therapy of Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Quaternized Chitin (QC) and Oxidized Dextran (OD)
2.3. Preparation and Characterization of QCOD Hydrogels
2.4. Preparation of QCOD@Sal Hydrogels
2.5. The Controlled Release of Salidroside In Vitro
2.6. MTT Cytotoxicity Assay
2.7. Hemolysis Assay
2.8. Animal Models and In Vivo Therapy
2.9. Antioxidant Activity of the Hydrogel
2.10. NIR-II Imaging and Image-Guided Therapy of AD
2.11. Measurement of IL-6 and TNF-α Release and Blood Routine Examination
2.12. Histological Analysis
2.13. Statistical Analysis
3. Results and Discussions
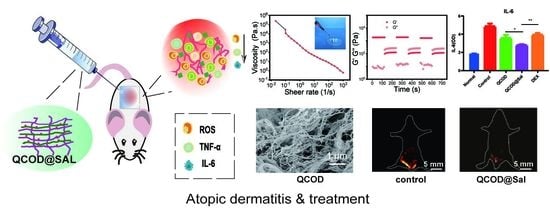
3.1. Preparation and Characterization of QCOD and QCOD@Sal Hydrogels
3.2. NIR-II Imaging and Image-Guided Therapy of DNFB-Induced AD Mice
3.3. Mechanism of QCOD@Sal for Ameliorating AD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fuxench, Z.C.C.; Block, J.K.; Boguniewicz, M.; Boyle, J.; Fonacier, L.; Gelfand, J.M.; Grayson, M.H.; Margolis, D.J.; Mitchell, L.; Silverberg, J.I.; et al. Atopic dermatitis in america study: A cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J. Investig. Dermatol. 2019, 139, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Halling, A.-S.; Loft, N.; Silverberg, J.I.; Guttman-Yassky, E.; Thyssen, J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; Nemolizumab, J.P.S.G. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immun. 2019, 143, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021, 371, 6500. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernandez-Nogueras, M.-V.; Perez-Rodriguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernandez-Gonzalez, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tools to assess disease severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Brit. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faiz, S.; Giovannelli, J.; Podevin, C.; Jachiet, M.; Bouaziz, J.-D.; Reguiai, Z.; Nosbaum, A.; Lasek, A.; Le Bouedec, M.-C.F.; Du Thanh, A.; et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J. Am. Acad. Dermatol. 2019, 81, 143–151. [Google Scholar] [CrossRef]
- Norris, M.R.; Bielory, L. Cosmetics and ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, D.; Park, E.; Jang, S.Y.; Cheon, S.Y.; Han, S.; Koo, H. Rhamnolipid-coated W/O/W double emulsion nanoparticles for efficient delivery of doxorubicin/erlotinib and combination chemotherapy. J. Anobiotechnol. 2021, 19, 411. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, Q.; Zeng, X.; Xiao, Y.J.C.S. Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm. Chem. Sci. 2020, 11, 2621. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Zhao, B.; Lei, J.; Lu, S.; Gong, W.; Liang, K.; Wu, J.; Hong, X.; Xiao, Y. A single-molecular ruthenium(ii) complex-based NIR-II fluorophore for enhanced chemo-photothermal therapy. Chem. Commun. 2022, 58, 6546–6549. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Wu, J.; Zhou, W.; Hong, X.J.C.S. All-in-one mitochondria-targeted NIR-II fluorophores for cancer therapy and imaging. Chem. Sci. 2021, 12, 1843–1850. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: From strategic design toward molecular imaging and theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xue, L.; Yang, M.; Wang, J.; Li, Y.; Jiang, Y.; Hong, X.; Wu, M.; Xiao, Y. NIR-II fluorescence/photoacoustic imaging of ovarian cancer and peritoneal metastasis. Nano Res. 2022, 15, 9183–9191. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Gu, M.; Lu, D.; Xiong, X.; Zhang, Z.; Pan, Y.; Liao, Y.; Ding, Q.; Gong, W.; et al. A second near-infrared Ru(II) polypyridyl complex for synergistic chemo-photothermal therapy. J. Med. Chem. 2022, 65, 2225–2237. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, X.; Li, A.; Zhou, W.; Tang, L.; Hu, W.; Fan, Q.; Meng, X.; Deng, H.; Duan, L.; et al. Upconversion NIR-II fluorophores for mitochondria-targeted cancer imaging and photothermal therapy. Nat. Commun. 2020, 11, 6183. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, M.; Ding, Q.; Zhang, Z.; Gong, W.; Yuan, Y.; Miao, X.; Ma, H.; Hong, X.; Hu, W.; et al. Highly twisted conformation thiopyrylium photosensitizers for in vivo near infrared-ii imaging and rapid inactivation of coronavirus. Angew. Chem. Int. Ed. 2022, e202214875. [Google Scholar]
- Cheng, X.; Zhang, C.; Shen, K.; Liu, H.; Bai, C.; Ding, Q.; Guan, M.; Wu, J.; Tian, Z.; Chen, D.; et al. Novel diketopyrrolopyrrole NIR-II fluorophores and DDR inhibitors for in vivo chemo-photodynamic therapy of osteosarcoma. Chem. Eng. J. 2022, 446, 136929. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Wang, S.; Li, S.; Hou, X.; Pan, Y.; Gao, J.; Qiao, X.; Tian, Z.; Chen, D.; et al. Organic NIR-II dyes with ultralong circulation persistence for image-guided delivery and therapy. J. Control Release 2022, 342, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Wang, X.; Yan, C.; Gu, L.; Hou, X.; Guan, M.; Wu, J.; Xiao, Y.; Xiong, X.; et al. Small-molecule fluorophores for NIR-IIb imaging and image-guided therapy of vascular diseases. CCS Chem. 2022, 4, 3735–3750. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Xu, Y.; Liang, Z.; Hu, H.; Wang, S.; Wang, Y. Quality evaluation of randomized controlled trials of Rhodiola species: A systematic review. Evid-Based Compl. Alt. 2021, 2021, 17. [Google Scholar] [CrossRef]
- Pu, W.-L.; Zhang, M.-Y.; Bai, R.-Y.; Sun, L.-K.; Li, W.-H.; Yu, Y.-L.; Zhang, Y.; Song, L.; Wang, Z.-X.; Peng, Y.-F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Bio. Pharma. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Brinckmann, J.A.; Cunningham, A.B.; Harter, D.E.V. Running out of time to smell the roseroots: Reviewing threats and trade in wild Rhodiola rosea L. J. Ethnopharmacol. 2021, 269, 113710. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, B.; Xu, L.; Liu, H.; Song, Y.; Lin, F. Effects of Rhodiola Rosea supplementation on exercise and sport: A systematic review. Front. Nutr. 2022, 9, 856287. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Yan, T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-B and MAPK signaling pathways. Int. J. Mol. Sci. 2019, 20, 1103. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Ding, Q.; Lai, S.; Jiang, F.; Song, Q.; Zhao, X.; Fu, A.; Moustaid-Moussad, N.; Su, D.; Li, S. Salidroside alleviates lipotoxicity-induced cell death through inhibition of TLR4/MAPKs pathway, and independently of AMPK and autophagy in AML-12 mouse hepatocytes. J. Funct. Foods. 2020, 65, 103691. [Google Scholar] [CrossRef]
- Deciga-Campos, M.; Eva Gonzalez-Trujano, M.; Ventura-Martinez, R.; Mariana Montiel-Ruiz, R.; Esther Angeles-Lopez, G.; Brindis, F. Antihyperalgesic activity of rhodiola rosea in a diabetic rat model. Drug Develop. Res. 2016, 77, 29–36. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsu, C.C.; Lin, S.W.; Hsu, M.C. Rhodiola rosea does not reduce in vivo inflammatory activity after continuous endurance exercise. Sci. Sport 2019, 34, E155–E158. [Google Scholar] [CrossRef]
- Recio, M.-C.; Giner, R.-M.; Manez, S. Immunmodulatory and antiproliferative properties of Rhodiola species. Planta Med. 2016, 82, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Skopinska-Rozewska, E.; Malinowski, M.; Wasiutynski, A.; Sommer, E.; Furmanowa, M.; Mazurkiewicz, M.; Siwicki, A.K. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol. J. Vet. Sci. 2008, 11, 97–104. [Google Scholar]
- Skopinska-Rozewska, E.; Sokolnicka, I.; Siwicki, A.K.; Stankiewicz, W.; Dabrowski, M.P.; Buchwald, W.; Krajewska-Patan, A.; Mielcarek, S.; Mscisz, A.; Furmanowa, M. Dose-dependent in vivo effect of Rhodiola and Echinacea on the mitogen-induced lymphocyte proliferation in mice. Pol. J. Vet. Sci. 2011, 14, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albadawy, R.; Hasanin, A.H.; Agwa, S.H.A.; Hamady, S.; Aboul-Ela, Y.M.; Raafat, M.H.; Kamar, S.S.; Othman, M.; Yahia, Y.A.; Matboli, M. Rosavin ameliorates hepatic inflammation and fibrosis in the NASH rat model via targeting hepatic cell death. Int. J. Mol. Sci. 2022, 23, 10148. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, D.; Xu, H.; Zhang, Y.; Shi, H.; Huang, X.; Wang, X.; Wu, Y.; Qi, Z. Salidroside activates the AMP-activated protein kinase pathway to suppress nonalcoholic steatohepatitis in mice. Hepatology 2021, 74, 3056–3073. [Google Scholar] [CrossRef]
- Hu, R.; Wang, M.-Q.; Ni, S.-H.; Wang, M.; Liu, L.-Y.; You, H.-Y.; Wu, X.-H.; Wang, Y.-J.; Lu, L.; Wei, L.-B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappa B/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharm. 2020, 867, 172797. [Google Scholar] [CrossRef]
- Huang, Y.; Han, X.; Tang, J.; Long, X.; Wang, X. Salidroside inhibits endothelial-mesenchymal transition via the KLF4/eNOS signaling pathway. Mol. Med. Rep. 2021, 24, 692. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Mondal, P.; Chatterjee, K. Injectable and self-healing double network polysaccharide hydrogel as a minimally-invasive delivery platform. Carbohydr. Polym. 2022, 291, 119585. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Y.; Song, S.; Tong, C.; Shi, X.; Zhao, Y.; Zhang, J.; Hou, M. Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr. Polym. 2019, 205, 312–321. [Google Scholar] [CrossRef]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable multifunctional hyaluronic acid/methylcellulose hydrogels for chronic wounds repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.J.; Wei, P.D.; Zhong, Y.; Chen, C.J.; Cai, J. Extremely strong and tough chitosan films mediated by unique hydrated chitosan crystal structures. Mater. Today. 2021, 51, 27–38. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Zhong, Y.; Wei, P.; Yu, X.; Huang, J.; Cai, J. Super-strong and super-stiff chitosan filaments with highly ordered hierarchical structure. Adv Funct Mater. 2021, 31, 2104368. [Google Scholar] [CrossRef]
- Zhong, Y.; Cai, J.; Zhang, L.N. A review of chitin solvents and their dissolution mechanisms. Chin. J. Pol. Sci. 2020, 38, 1047–1060. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhong, Y.; Zhang, L.N.; Cai, J. Distinctive viewpoint on the rapid dissolution mechanism of alpha-chitin in aqueous potassium hydroxide-urea solution at low temperatures. Macromolecules 2020, 53, 5588–5598. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhong, Y.; Wei, P.D.; Cai, J. Rapid dissolution of beta-chitin and hierarchical self-assembly of chitin chains in aqueous KOH/urea solution. Green Chem. 2021, 23, 3048–3060. [Google Scholar] [CrossRef]
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-flexibility, high-toughness double-cross-linked chitin hydrogels by sequential chemical and physical cross-linkings. Adv. Mater. 2016, 28, 5844–5849. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, Y.; Zhang, L.; Cai, J. Extremely strong and transparent chitin films: A high-efficiency, energy-saving, and “green” route using an aqueous KOH/Urea solution. Adv. Funct. Mater. 2017, 27, 1701100. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Cai, J. Injectable, self-healing, β-chitin-based hydrogels with excellent cytocompatibility, antibacterial activity, and potential as drug/cell carriers. ACS Appl. Energy Mater. 2018, 2, 196–204. [Google Scholar] [CrossRef]
- Jung, H.; Son, G.M.; Lee, J.J.; Park, H.S. Therapeutic effects of tonsil-derived mesenchymal stem cells in an atopic dermatitis mouse model. In Vivo 2021, 35, 845–857. [Google Scholar] [CrossRef]
- Kong, N.; Tao, W.; Ling, X.; Wang, J.; Xiao, Y.; Shi, S.; Ji, X.; Shajii, A.; Gan Silvia, T.; Kim Na, Y.; et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019, 11, 1565. [Google Scholar] [CrossRef]
- Shi, H.-j.; Song, H.-b.; Gao, Q.; Si, J.-w.; Zou, Q. Combination of oxymatrine and diammonium glycyrrhizinate significantly mitigates mice allergic contact dermatitis induced by dinitrofluorobenzene. Exp. Biol. Med. 2019, 244, 1111–1119. [Google Scholar] [CrossRef]
- Ji, X.; Kang, Y.; Ouyang, J.; Chen, Y.; Artzi, D.; Zeng, X.; Xiao, Y.; Feng, C.; Qi, B.; Kim, N.Y.; et al. Synthesis of ultrathin biotite nanosheets as an intelligent theranostic platform for combination cancer therapy. Adv. Sci. 2019, 6, 1901211. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Jiang, L.; Xiao, X.; Lu, Y.; Liu, R.; Jiang, W.; Cai, J. Quaternized polysaccharide-based cationic micelles as a macromolecular approach to eradicate multidrug-resistant bacterial infections while mitigating antimicrobial resistance. Small 2022, 18, 2104885. [Google Scholar] [CrossRef]
- Hoque, J.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Haldar, J. Biocompatible injectable hydrogel with potent wound healing and antibacterial properties. Mol. Pharm. 2017, 14, 1218–1230. [Google Scholar] [CrossRef]
- Li, H.; Du, Y.; Wu, X.; Zhan, H. Effect of molecular weight and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids Surfaces A 2004, 242, 1–8. [Google Scholar] [CrossRef]
- Raymond, L.; Morin, F.G.; Marchessault, R.H. Degree of deacetylation of chitosan using conductometric titration and solid-state NMR. Carbohyd. Res. 1993, 246, 331–336. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, H.K.; Ndeh, K.P.U.; Choi, Y.-J.; Fan, M.; Kim, E.-k.; Chung, K.-H.; An, J.-H. Inhibitory effect of centella asiatica extract on DNCB-induced atopic dermatitis in HaCaT cells and BALB/c mice. Nutrients 2020, 12, 411. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Xie, F.; Su, W.; Luo, H.; Chen, D.; Cai, J.; Hong, X. Anti-Inflammatory Salidroside Delivery from Chitin Hydrogels for NIR-II Image-Guided Therapy of Atopic Dermatitis. J. Funct. Biomater. 2023, 14, 150. https://doi.org/10.3390/jfb14030150
He S, Xie F, Su W, Luo H, Chen D, Cai J, Hong X. Anti-Inflammatory Salidroside Delivery from Chitin Hydrogels for NIR-II Image-Guided Therapy of Atopic Dermatitis. Journal of Functional Biomaterials. 2023; 14(3):150. https://doi.org/10.3390/jfb14030150
Chicago/Turabian StyleHe, Shengnan, Fang Xie, Wuyue Su, Haibin Luo, Deliang Chen, Jie Cai, and Xuechuan Hong. 2023. "Anti-Inflammatory Salidroside Delivery from Chitin Hydrogels for NIR-II Image-Guided Therapy of Atopic Dermatitis" Journal of Functional Biomaterials 14, no. 3: 150. https://doi.org/10.3390/jfb14030150